Abstract
The aim of this study was to investigate the effects of homocysteine (Hcy), a risk factor for cardiovascular diseases, hypertension, stroke and obesity, on expression of CD36 that regulates uptake of oxidized low-density lipoprotein (Ox-LDL) by adipocytes and differentiation of 3T3-L1 cells to adipocytes. Cell viability was determined using MTT assay, and density of triglycerides were measured with Oil Red O staining. The expression levels of CD36 were analyzed using SYBR green assay by quantitative RT-PCR. Our results showed that the addition of Hcy inhibited differentiation of 3T3-L1 preadipocytes in a dose-dependent manner without a significant cell toxicity (p < 0.05). Percentage CD36 gene expression increased in the Hcy treatment groups, but not statistically significantly (p > 0.05) compared to differentiated adipocytes. Hcy reduced adipocyte differentiation, but had no effect on the expression level of CD36 in vitro conditions. The effect of Hcy on uptake and clearance of Ox-LDL by adipose tissue now needs to be investigated in vivo.
Keywords: Adipocytes, CD36, Homocysteine, Oxidized low-density lipoprotein, 3T3-L1 cells
Introduction
CD36, an 88 kDa glycoprotein, is a class B scavenger receptor. It is expressed in many cells such as, platelets, monocytes, macrophages, capillary endothelial cells and adipocytes. Studies with CD36 transferred human epithelial kidney cells have demonstrated that CD36 is an oxidized LDL receptor by revealing a specific Ox-LDL binding capacity of CD36 (Endemann et al. 1993). It has a high affinity for Ox-LDL. The Ox-LDL binding and uptake capacity of CD36 is thought to regulate transformation of macrophages to foam cells. CD36 is expressed in adipocytes, but mechanisms and factors that regulate uptake and degradation of Ox-LDL by adipocytes have not been fully identified (Zhao et al. 2004). Adipocytes take and reduce Ox-LDLs by CD36 from circulation under hypercholesterolemic conditions. Adipose tissue is described as a buffering pool for circulating cholesterol. This makes adipose tissue a potential target for the treatment of atherosclerosis (Wu and Zhao 2006).
Homocysteine is a sulfur containing amino acid produced during methionine metabolism and not seen in protein structure. Hyperhomocysteinemia is a known independent risk factor for cardiovascular diseases. Although the mechanism is not fully understood, Hcy has been reported to contribute to atherosclerosis development and foam cell formation (Li et al. 2008). Homocysteine has been shown to be related to endothelial dysfunction, proliferation of vascular smooth cells, increased lipoprotein oxidation and platelet activation. Serum Hcy levels have been found to be elevated in obese humans (Vayá et al. 2011). However, the relation between Hcy and CD36 in adipose tissue has not been studied.
The aim of this study was the in vitro investigation of the effects of Hcy, involved in pathologies including cardiovascular diseases, hypertension, stroke and obesity, on expression of CD36 that regulates Ox-LDL uptake and differentiation of 3T3-L1 cells.
Materials and methods
Compounds
DL-homocysteine, insulin, dexamethasone, isobutylmethyl xanthine (IBMX), biotin, [3-(4,5-dimetiltiazol-2-yl)]-2,5-difeniltetrazolyum bromide (MTT), Oil red O were obtained from Sigma (St. Louis, MO, USA). Fetal bovine serum was purchased from Biochrom (Berlin, Germany), Dulbecco’s Modified Eagle’s Medium (DMEM) from American Type Culture Collection (ATCC, Manassas, VA, USA), 2-propanol from J.T. Baker (PA, USA), TriPure Isolation Reagent and SYBR Green from Roche (Mannheim, Germany) and cDNA synthesis kit from Promega (Madison, WI, USA).
Cell culture and differentiation
3T3-L1 cells were purchased from American Type Culture Collection and were grown in DMEM containing 10 % fetal bovine serum. For adipocyte differentiation, cells were grown in 6-well plates to full confluence for 2 days and then in differentiation medium (DM) containing 10 μg/mL insulin, 0.25 μM dexamethasone, 0.5 mM IBMX and 100 ng/mL biotin was added to the culture. After 4 days’ induction, the medium was changed to DMEM with 10 % fetal bovine serum, 10 μg/mL insulin and different concentrations of Hcy for differentiation at 37 °C and 5 % CO2. After 6 days’ induction, the medium was changed to DMEM with 10 % fetal bovine serum, 10 μg/mL insulin and 100 ng/mL biotin. Hcy was dissolved in DMEM and was added to the medium at the indicated concentration.
Oil Red O staining
Staining procedure was conducted according to the previously described method (Ramirez-Zacarias et al. 1992) with slight modifications. In brief, the cells were washed twice with PBS and fixed with 10 % formalin at room temperature for 1 h. After incubation, the wells were washed with 60 % 2-propanol twice and stained with oil red O at room temperature for 10 min. After incubation, the wells were washed with pure water five times. Pictures of cells were taken using an inverted microscope (Nikon Eclipse TS100, Tokyo, Japan). 2-propanol of 100 % was added to wells and oil red-O was added to the 2-propanol phase. One milliliter of solution was taken, and absorbances were read using a spectrophotometer (Shimadzu UV1601, Australia) at 520 nm.
Assessment of cell viability
Cell viability was determined using MTT assay (Mosmann 1983). Cells were plated in flat-bottomed 96-well microplates. After 24 h, different concentrations (0, 10, 50, 100, 250, 500, and 750 μM) of Hcy were added to cells and left to incubate for 72 h. Next, 10 μL of MTT solution (final concentration of 0.25 mg/mL) was added to each well and the composed crystals were then dissolved in DMSO. Finally, absorbance was measured at 570 nm using a microplate reader (Versamax, Molecular Devices, California, USA). Optical densities were used to determine % cell viabilities using the formula [(OD of treated group/OD of control group) × 100] in treatment cells compared to control cells with no compound exposure (Xuan et al. 2014).
Gene expression studies
Total RNA was isolated using TriPure Isolation Reagent. Purities of RNAs were determined with NanoDrop (Thermo Scientific, Waltham, MA, USA) by measuring the OD260/OD280 ratio. RNA samples were converted to complementary DNA (cDNA) using a first strand cDNA synthesis kit following the manufacturer’s recommendations.
Expression levels of CD36 were analyzed by SYBR green assay with quantitative RT-PCR conducted using LightCycler 480 II (Roche, Rotkreuz, Switzerland). The primers used in the experiments were shown in Table 1 according to previously described (Lefrere et al. 2002) and were manufactured by Metabion International AG (Martinsried, Germany). Initial incubation was performed for 5 min at 95 °C, followed by 50 cycles of PCR (95 °C 10s, 72 °C, 10 s) and finally at 40 °C for 10 s. Results were calculated with an advance relative quantification module compared to ß-actin expression of undifferentiated cells (cells with no test compound).
Table 1.
Primer pair sets and parameters used in the real time polymerase chain reaction analysis
| Name | Primer Sequence | |
|---|---|---|
| β-aktin | Forward | 5’-GAG ACC TTC AAC ACC CC- 3’ |
| Reverse | 5’-GTG GTG GTG AAG CTG TAG CC- 3’ | |
| CD 36 | Forward | 5’- GAT GTG GAA CCC ATA ACT GGA TTC AC- 3’ |
| Reverse | 5’- GGT CCC AGT CTC ATT TAG CCA CAG TA- 3’ |
Statistical analysis
All experiments were carried out in triplicate, and all results were expressed as mean ± standard deviation (mean ± S.D). Statistical analysis was performed using the paired-samples T test. p < 0.05 was regarded as significant.
Results
Effect of Hcy on the cell viability
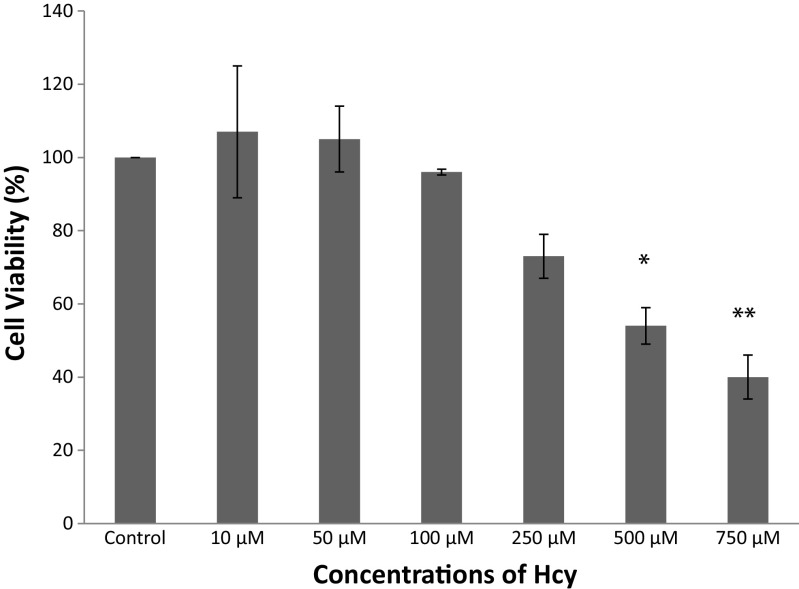
3T3-L1 cells were incubated with Hcy at several doses (0–750 μM) for 72 h and cell viabilities were detected using MTT assay (Fig. 1). Concentrations of Hcy up to 100 μM were found to have no cytotoxic effect in 3T3-L1 cells, but significantly decreased cell viability was observed at concentrations as high as 250–750 μM (p < 0.05). So, Hcy was used in further experiments at the concentrations between 10 and 100 μM.
Fig. 1.
Cell viability of 3T3-L1 cells treated with different concentrations of Hcy for 72 h. Data are mean values ± SD (n = 3) *p < 0.05, **p < 0.0001 compared to control
Differentiation of 3T3-L1 cells to adipocytes
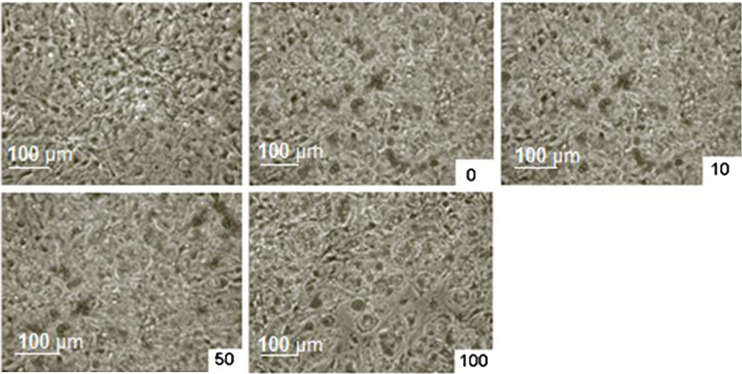
The representative images of Oil Red O staining shown in Fig. 2 and it suggests that successful differentiation procedure of preadipocytes to mature adipocytes was achieved.
Fig. 2.
Effects of Hcy on intracellular lipid accumulation in 3T3-L1 cells. Microscopic images of undifferentiated, differentiated, differentiated and treated with several doses of Hcy (10, 50, and 100 μM) after Oil Red O staining, respectively
Hcy inhibits adipocyte differentiation of 3T3-L1 preadipocytes
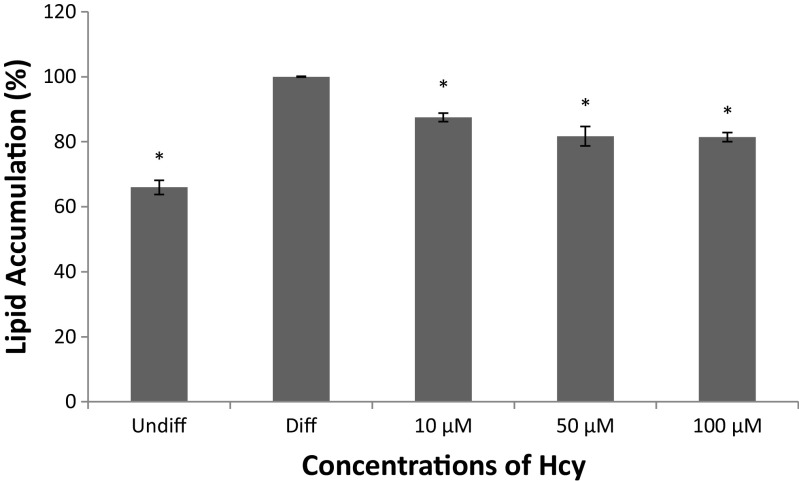
Our results showed that Hcy inhibited differentiation of 3T3-L1 preadipocytes without a significant cell toxicity (p < 0.0001), (Figs. 2 and 3). It is evident from the data shown in Fig. 3 that as the concentration of Hcy is increased, there is a proportional decrease in the level of differentiation of 3T3-L1 preadipocytes; the differentiation rate at 10, 50 and 100 μM declined by 12.5 %, 18.3 % and 18.6 % respectively.
Fig. 3.
Quantitative analysis of lipid accumulation. After Oil red O staining, lipid and Oil red O were dissolved in isopropanol and absorbance was measured at the wavelength of 520 nm. Undiff means undifferentiation; diff means differentiation control. Data are mean values ± SD (n = 3), *p < 0.0001 compared to differentiation control
Effects of Hcy on gene expression of CD36 receptor in differentiated adipocytes from 3T3-L1 preadipocytes
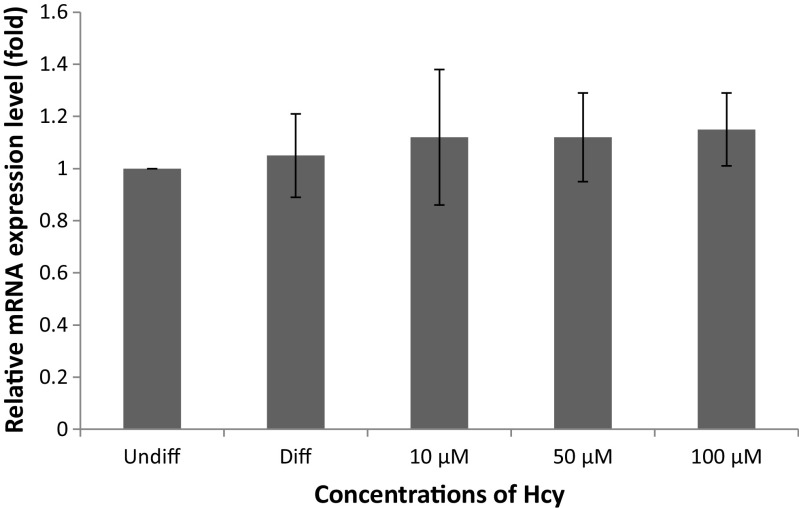
To further explore the mechanism underlying the suppression of adipocyte differentiation by Hcy, the mRNA level of CD36 was examined. Although a 5 % increase in CD36 gene expression was determined in differentiated cells compared with undifferentiated cells, this was not significant (p = 0.532). CD36 gene expression was increased by 10 % at the concentration of 100 μM Hcy treatments compared to differentiated cells, but this increase was not significantly (p > 0.05). There is no statistical difference among treatment groups (p > 0.05) (Fig. 4).
Fig. 4.
Effect of Hcy on the expression of CD36 in 3T3-L1 adipocytes. Undiff means undifferentiation; diff means differentiation control. Data are mean values ± SD (n = 3)
Discussion
Hyperhomocysteinemia is an independent risk factor for atherosclerotic vascular disease and atherothrombosis. It has been shown to induce several functional changes in vascular cells, leading to endothelial cell dysfunction and activation, smooth-muscle cell proliferation, monocyte attraction, and monocyte adhesion to endothelial cells (Ide et al. 2006). Also, several mechanisms have been proposed to explain the atherogenic properties of Hcy. In particular, it has been shown that Hcy promotes activation of the protein kinase C (PKC)/c-fos signaling and coagulation pathway, and induces platelet aggregation and lipoprotein oxidation (Beauchamp and Renier 2002). We demonstrated that Hcy could decrease the viability of 3T3-L1 cells in a dose-dependent manner. So, we have not used greater than the concentrations of 100 μM in following experiments. Consistent with our study, there are many papers about cytotoxic effect of Hcy on various different cell lines (Sung et al. 2002; Nakanishi et al. 2005; Liu et al. 2009; Lin et al. 2014) and the cytotoxic effect of Hcy is attributed to its potential of forming reactive oxygen species (Poddar et al. 2001; Sung et al. 2002; Li et al. 2008; Wang et al. 2011a). It might been suggested that the cytotoxic effect of Hcy, at least partially, mediated by the induction of oxidative stress.
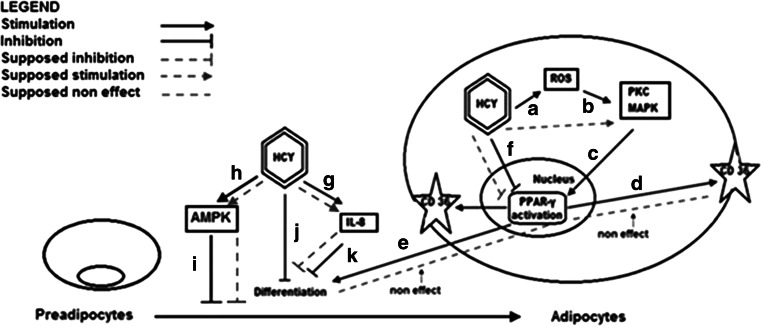
Obesity is accepted an independent risk factor for coronary heart diseases (Bray 2004; Lavie et al. 2009; Lovren et al. 2015). However, the relation between atherosclerosis and obesity is not fully understood. Excessive uptake of Ox-LDL by adipocytes seems to be useful for preventing atherosclerosis, but it causes over-accumulation of lipid in adipocytes. Adipose tissue therefore releases cytokines, such as resistin and PAI-1, in association with this. This may cause atherosclerosis and insulin resistance (Kuniyasu et al. 2002). Adipose tissue plays a central role in nutrient sensing and energy homeostasis. It stores surplus calories and secretes peptide hormones or adipokines that regulate food intake and energy use (Hajri et al. 2007). In the present study, a statistically significant difference was determined between mean absorbance values after staining of undifferentiated 3T3-L1 cells and mature adipocytes (p < 0.05). This finding demonstrated successful differentiation of preadipocytes to mature adipocytes. When we compared Hcy treated and untreated cells we found that Hcy significantly reduced adipocyte differentiation (p < 0.05). PPARγ and C/EBPα are known to be expressed in early stages of differentiation (Ntambi and Young-Cheul 2000). PPARγ is required for adipocyte differentiation and is the main transcriptional regulator of differentiation. It also activates many genes associated with adipocyte lipid storage and is expressed abundantly in white adipose tissue (Chui et al. 2005). Knockout of PPARγ gene caused drastic suppression of the formation and function of both brown and white adipose tissues (Jones et al. 2005). In the differentiation of 3T3-L1 preadipocytes, PPARγ is quickly induced after induction of differentiation (Ntambi and Young-Cheul 2000). A negative correlation has been reported between increased Hcy levels and PPARγ (Mujumdar et al. 2002; Wang et al. 2011a). In this study, we observed that increasing Hcy concentrations reduced differentiation of preadipocytes. Consistent with our results Wang et al. reported that Hcy inhibits adipogenesis in 3T3-L1 cells via suppression of the gene expressions of PPARγ and its downstream target adipocyte protein 2 (AP2) (Wang et al. 2011a). Moreover, Hcy is considered to be a potent pro-inflammatory factor (Li et al. 2008). Poddar et al. shown that Hcy induces both mRNA expression and protein secretion of the pro-inflammatory cytokines monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) in cultured human aortic endothelial cells (Poddar et al. 2001) and Zhou et al. reported that IL-8 inhibits the differentiation of 3T3-L1 preadipocytes by decreasing the clonal expansion of the cells (Zhou et al. 2008). Endogenous stimuli such as β-adrenergic stimulators, HDLs (high-density lipoproteins), homocysteine and eicosapentaenoic acid have also been reported to stimulate AMP-activated protein kinase (AMPK) activity in adipocytes (Wang et al. 2011b; Bijland et al. 2013). Proliferation and adipogenesis are anabolic processes that multiple lines of evidence suggest are inhibited by AMPK. AMPK activation has been suggested to inhibit adipogenesis via the mitotic clonal expansion phase, with reduced expression of C/EBPβ (which is essential for initiation of the adipogenic transcriptional cascade), and subsequent inhibition of PPARγ, C/EBPα and late adipogenic markers such as FAS, aP2 and SREBP-1c (Bijland et al. 2013). We concluded that Hcy may reduced adipocyte differentiation by inhibiting PPARγ and increasing IL-8 expressions and activating AMPK (Fig. 5).
Fig. 5.
Possible mechanism of Hcy on preadipocytes differantiation. a: Poddar et al. 2001; Mujumdar et al. 2002; Sung et al. 2002; Li et al. 2008; Wang et al. 2011a. b: Mujumdar et al. 2002. c: Mujumdar et al. 2002. d: Christiaens et al. 2012. e: Ntambi and Young-Cheul 2000; Chui et al. 2005. f: Mujumdar et al. 2002; Yideng et al. 2008; Wang et al. 2011a. g: Poddar et al. 2001. h: Wang et al. 2011b; Bijland et al. 2013. ı: Bijland et al. 2013. j: Wang et al. 2011a. k: Zhou et al. 2008. Hcy may reduces adipocyte differentiation by inhibiting PPARγ and increasing IL-8 expressions and activating AMPK. Hcy may have a non-functional role on the expression of CD36 in adipogenesis via affect simultaneously PKC and PPARγ
CD36 is a class B scavenger receptor that is involved in metabolism, atherosclerosis, angiogenesis, immunity and behavior. It is a membrane glycoprotein present on platelets, mononuclear phagocytes, adipocytes, hepatocytes, myocytes, and some epithelia (Christiaens et al. 2012). CD36 is a target gene of PPARγ and its expression is regulated by the PPARγ pathway (Christiaens et al. 2012). In the present study we have evaluated the hypothesis that whether the scavenger receptor CD36 may play a functional role in adipocyte differentiation and adipogenesis or not. In hypercholesterolemic conditions, adipose tissue CD36 uptakes Ox-LDL and reduces its circulation concentration (Li et al. 2008). In monocytes, phorbol 12-myristate 13-acetate (PMA), IL-4, macrophage colony stimulating factor induces CD36 expression via PPARγ activation. Protein kinase agonists such as PMA stimulate mitogen activated protein (MAP) kinase signal pathway. Activation of PKC is regulated by Hcy in macrophages. Hcy has also been reported to induce free radical production. Thus oxidative stress that causes an increase in reactive oxygen species and activation of the PKC-MAP kinase pathway via PPARγ activation enhances CD36 activation of Hcy, and therefore Ox-LDL uptake. It has been demonstrated that Hcy induces PKC and reduces PPARγ (Mujumdar et al. 2002). Yideng et al. reported that Hcy also could decreases mRNA and protein levels of PPARα and γ in monocytes (Yideng et al. 2008). Another study reported that Ox-LDL increases level of CD36 by PKC and PPARγ pathway in macrophages (Ide et al. 2006). Our in vitro data support a non-functional role for CD36 in adipogenesis. This situation may arisen from simultaneously effected of CD36 gene expression from both inducing PKC and reducing PPARγ activities in the presence of Hcy (Fig. 5). In opposite with our results, Ide et al. reported that the concentrations of 50 and 200 μM Hcy increases the CD36 expression in human macrophages by 18 % and 41 %, respectively (Ide et al. 2006). This discrepancy may be arisen from type of used cell line.
In conclusion, we demonstared that Hcy reduces adipocyte differentiation but had no effect on CD36 gene expression in vitro conditions in 3T3-L1 adipocytes. The effect of Hcy on uptake and clearance of Ox-LDL by adipose tissue now needs to be investigated in vivo.
Acknowledgments
This study was supported by the Karadeniz Technical University Research Fund.
Abbreviations
- Hcy
Homocysteine
- Ox-LDL
Oxidized low-density lipoprotein
- IBMX
Isobutylmethyl xanthine
- PPARγ
Peroxisome proliferator-activated receptor gamma
- C/EBPα
CCAAT/enhancer binding protein alpha
Compliance with ethical standards
Conflicts of Interest
None of the authors had any financial or personal relationships with other individuals or organizations that might inappropriately influence their work during the submission process.
References
- Beauchamp MC, Renier G. Homocysteine induces protein kinase C activation and stimulates c-Fos and lipoprotein lipase expression in macrophages. Diabetes. 2002;51:1180–1187. doi: 10.2337/diabetes.51.4.1180. [DOI] [PubMed] [Google Scholar]
- Bijland S, Mancini SJ, Salt IP. Role of AMP-activated protein kinase in adipose tissue metabolism and inflammation. Clin Sci. 2013;124:491–507. doi: 10.1042/CS20120536. [DOI] [PubMed] [Google Scholar]
- Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89(6):2583–2589. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- Christiaens V, Van Hul M, Lijnen HR, Scroyen I. CD36 promotes adipocyte differentiation and adipogenesis. Biochim Biophys Acta. 2012;1820:949–956. doi: 10.1016/j.bbagen.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Chui PC, Guan HP, Lehrke M, Lazar MA. PPARgamma regulates adipocyte cholesterol metabolism via oxidized LDL receptor 1. J Clin Invest. 2005;115(8):2244–2256. doi: 10.1172/JCI24130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268(16):11811–11816. [PubMed] [Google Scholar]
- Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes. 2007;56:1872–1880. doi: 10.2337/db06-1699. [DOI] [PubMed] [Google Scholar]
- Ide N, Keller C, Weiss N. Aged garlic extract inhibits homocysteine-induced CD36 expression and foam cell formation in human macrophages. J Nutr. 2006;136(3 Suppl):755S–758S. doi: 10.1093/jn/136.3.755S. [DOI] [PubMed] [Google Scholar]
- Jones JR, Barrick C, Kim KA, Lindner J, Blondeau B, Fujimoto Y, Shiota M, Kesterson RA, Kahn BB, Magnuson MA. Deletion of PPAR gamma in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci U S A. 2005;102:6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniyasu A, Hayashi S, Nakayama H. Adipocytes recognize and degrade oxidized low density lipoprotein through CD36. Biochem Biophys Res Commun. 2002;295(2):319–323. doi: 10.1016/S0006-291X(02)00666-6. [DOI] [PubMed] [Google Scholar]
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- Lefrere I, De Coppet P, Camelin JC, Le Lay S, Mercier N, Elshourbagy N, Bril A, Berrebi-Bertrand I, Feve B, Krief S. Neuropeptide AF and FF modulation of adipocyte metabolism. Primary insights from functional genomics and effects on beta-adrenergic responsiveness. J Biol Chem. 2002;277(42):39169–39178. doi: 10.1074/jbc.M205084200. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang C, Xu G, Wang N, Zhu Y, Tang C, Wang X. Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes. 2008;57(4):817–827. doi: 10.2337/db07-0617. [DOI] [PubMed] [Google Scholar]
- Lin N, Qin S, Luo S, Cui S, Huang G, Zhang X. Homocysteine induces cytotoxicity and proliferation inhibition in neural stem cells via DNA methylation in vitro. FEBS J. 2014;281:2088–2096. doi: 10.1111/febs.12764. [DOI] [PubMed] [Google Scholar]
- Liu CC, Ho WY, Leu KL, Tsai HM, Yang TH. Effects of S-Adenosylhomocysteine and Homocysteine on DNA Damage and Cell Cytotoxicity in Murine Hepatic and Microglia Cell Lines. J Biochem Mol Toxicol. 2009;23(5):349–356. doi: 10.1002/jbt.20298. [DOI] [PubMed] [Google Scholar]
- Lovren F, Teoh H, Verma S. Obesity and atherosclerosis: Mechanistic insights. Can J Cardiol. 2015;31(2):177–183. doi: 10.1016/j.cjca.2014.11.031. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mujumdar VS, Tummalapalli CM, Aru GM, Tyagi SC. Mechanism of constrictive vascular remodeling by homocysteine: role of PPAR. Am J Physiol Cell Physiol. 2002;282(5):C1009–C1015. doi: 10.1152/ajpcell.00353.2001. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Akabane ER, Nanami M, Kiyobayashi Y, Moriguchi R, Hasuike Y, Otaki Y, Miyagawa K, Itahana R, Izumi M. Comparison of cytotoxicity of cysteine and homocysteine for renal epithelial cells. Nephron Exp Nephrol. 2005;100(1):e11–e20. doi: 10.1159/000084108. [DOI] [PubMed] [Google Scholar]
- Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130(12):3122S–3126S. doi: 10.1093/jn/130.12.3122S. [DOI] [PubMed] [Google Scholar]
- Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells. Circulation. 2001;103:2717–2723. doi: 10.1161/01.CIR.103.22.2717. [DOI] [PubMed] [Google Scholar]
- Ramirez-Zacarias JL, Castro-Munozledo F, Kuri-Harcuch W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil Red O. Histochemistry. 1992;97:493–497. doi: 10.1007/BF00316069. [DOI] [PubMed] [Google Scholar]
- Sung JJ, Kim HJ, Choi-Kwon S, Lee JH, Kim M, Lee KW. Homocysteine induces oxidative cytotoxicity in Cu, Zn-superoxide dismutase mutant motor neuronal cell. Neuroreport. 2002;13(4):377–381. doi: 10.1097/00001756-200203250-00003. [DOI] [PubMed] [Google Scholar]
- Vayá A, Ejarque I, Tembl J, Corella D, Laiz B. Hyperhomocysteinemia, obesity and cryptogenic stroke. Clin Hemorheol Microcirc. 2011;47(1):53–58. doi: 10.3233/CH-2010-1365. [DOI] [PubMed] [Google Scholar]
- Wang Z, Dou X, Yao T, Song Z. Homocysteine inhibits adipogenesis in 3T3-L1 preadipocytes. Exp Biol Med. 2011;236:1379–1388. doi: 10.1258/ebm.2011.011234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Pini M, Yao T, Zhou Z, Sun C, Fantuzzi G, Song Z. Homocysteine suppresses lipolysis in adipocytes by activating the AMPK pathway. Am J Physiol Endocrinol Metab. 2011;30:E703–E712. doi: 10.1152/ajpendo.00050.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZH, Zhao SP. Adipocyte: a potential target for the treatment of atherosclerosis. Med Hypotheses. 2006;67(1):82–86. doi: 10.1016/j.mehy.2005.12.042. [DOI] [PubMed] [Google Scholar]
- Xuan H, Li Z, Yan H, Sang Q, Wang K, He Q, Wang Y, Hu F (2014). Antitumor activity of Chinese propolis in human breast cancer MCF-7 and MDA-MB-231 cells. Evid Based Complement Alternat Med. Article ID 280120, 11 pages [DOI] [PMC free article] [PubMed]
- Yideng J, Zhihong L, Jiantuan X, Jun C, Guizhong L, Shuren W. Homocysteine-mediated PPARα,γ DNA methylation and its potential pathogenic mechanism in monocytes. DNA Cell Biol. 2008;27(3):143–150. doi: 10.1089/dna.2007.0658. [DOI] [PubMed] [Google Scholar]
- Zhao SP, Wu J, Zhang DQ, Ye HJ, Liu L, Li JQ. Fenofibrate enhances CD36 mediated endocytic uptake and degradation of oxidized low density lipoprotein in adipocytes from hypercholesterolemia rabbit. Atherosclerosis. 2004;177(2):255–262. doi: 10.1016/j.atherosclerosis.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Zhou H, Yang X, Zhang YO, Cai GP. Interleukin-8 inhibits clonal expansion of 3T3-L1 preadipocyte during differentiation. Chin J Appl Physiol. 2008;24(2):243–247. [PubMed] [Google Scholar]