Abstract
Genetic background plays an important role in the development of Dupuytren’s disease. A genome-wide association study (GWAS) showed that nine loci are associated with the disease, six of which contain genes that are involved in Wnt signaling (WNT2, WNT4, WNT7B, RSPO2, SFRP4, SULF1). To obtain insight in the role of these genes, we performed expression studies on affected and unaffected patient’s tissues. Surgically obtained nodules and cords from eight Dupuytren’s patients were compared to patient-matched control tissue (unaffected transverse palmar fascia). The Wnt-related genes found in the GWAS, the classical Wnt-downstream protein β-catenin, as well as (myo)fibroblast markers were analyzed using real-time qPCR and immunohistochemical stainings for mRNA levels and protein levels, respectively. The collagen-coding genes COL1A1 and COL3A1 were highly upregulated on mRNA level, both in cords and nodules. Three Wnt-related genes were found to be differently regulated compared to control tissue: WNT2 was downregulated in nodules, WNT7B was upregulated in nodules, and SFRP4 was upregulated in nodules and cords. Immunohistochemistry revealed significantly less staining of Wnt2 in cords, but significantly more staining for Wnt7b in nodules. There was significantly more staining of α-SMA in nodules and cord and β-catenin in nodules than in control tissue. We found differences in expression, both at mRNA and protein level, in several Wnt-related genes found earlier to be associated with Dupuytren’s disease. Of these, Wnt7b was upregulated and found in close association with both α-SMA and β-catenin expressing cells, making it a candidate pro-fibrotic mediator in Dupuytren’s disease.
Electronic supplementary material
The online version of this article (doi:10.1007/s12079-015-0312-8) contains supplementary material, which is available to authorized users.
Keywords: β-catenin, Dupuytren’s disease, Fibrosis, Wnt signaling
Introduction
Dupuytren’s disease is a benign fibroproliferative disorder of the hand, which causes the formation of nodules and cords in the palm and fingers. It may eventually lead to the inability to fully extend the fingers. The prevalence varies from 1 % to 32 % in Western countries (Lanting et al. 2013). The disease is more common in people of European ancestry, in older persons and in males (Gudmundsson et al. 2000; Hindocha et al. 2009). The main treatment option has until recently been open surgery, but use of less invasive methods, such as percutaneous needle fasciotomy and collagenase injections in the cords, is becoming more popular (van Rijssen et al. 2012; Hurst et al. 2009). However, there is no definitive cure and recurrences are frequent (van Rijssen et al. 2012).
Pathophysiologically, both contraction and matrix deposition caused by uncontrolled myofibroblast activity in and around the palmar fascia of the hand are key features (Tomasek et al. 1987). The development of myofibroblasts in general depends on a number of different environmental cues, including tension in the matrix and exposure to a variety of different mediators, such as transforming growth factor-β1 (Hinz 2007). Myofibroblasts have been suggested to make up the majority of nodular cells, with cords being less cellular and more tendon-like (Verjee et al. 2009).
Several causes have been proposed for Dupuytren’s disease, and a genetic component is one of them. Concerning this genetic predisposition, in a genome-wide association study (GWAS), nine chromosomal loci were found to be associated with susceptibility to Dupuytren’s disease (Dolmans et al. 2011). Six of these loci contain genes involved in the Wnt signaling pathway. The canonical pathway of Wnt signaling is the most extensively studied and has been shown to promote cell proliferation and survival via β-catenin (Moon et al. 2004; Rao and Kuhl 2010). Alternatively, Wnt proteins may signal via the non-canonical Wnt pathway, defined as all Wnt signaling activities that operate independently of β-catenin.
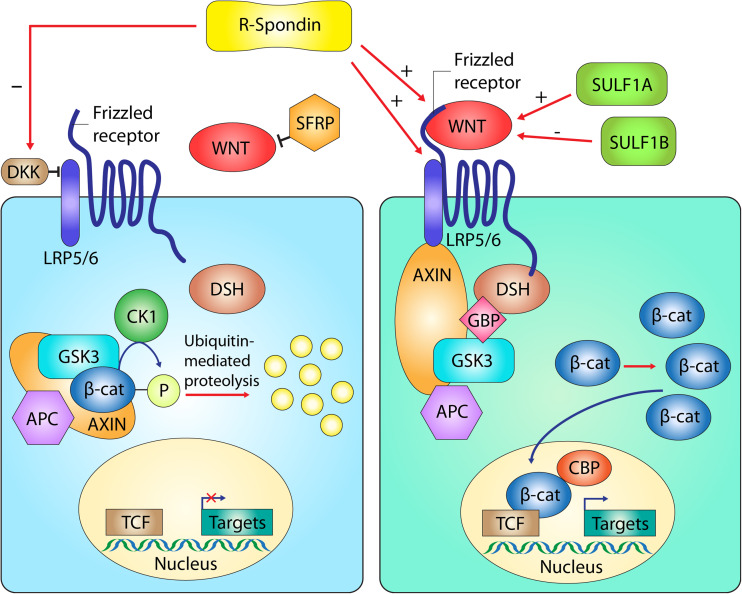
The Wnt-related genes that have been identified in the GWAS are WNT2, WNT4, WNT7B, RSPO2, SFRP4 and SULF1 (Fig. 1). Of these, three Wnt proteins exert their effect through binding to Frizzled receptors, causing a nuclear translocation of β-catenin via the canonical pathway. They may also activate the non-canonical pathway via Frizzled and other receptors. Secreted Frizzled-related proteins (Sfrp) bind to Wnt proteins, thereby inhibiting normal Wnt-Frizzled interactions. R-Spondin activates the pathway by interacting with Frizzled receptors, Lrp5/6 and Dkk proteins (Rao and Kuhl 2010). Sulf1 is a member of the sulfatase gene family that also interacts with canonical Wnt signaling, although the mechanism is unclear: both activation and inhibition of Wnt signaling have been described (Sahota and Dhoot 2009).
Fig. 1.
The tested Wnt-related genes and proteins and their functions in the canonical Wnt signaling pathway are shown here. On the left, the Wnt pathway is inhibited: β-catenin (β-cat) is degraded in the abcense of a Wnt protein. On the right, the Wnt pathway is activated: a translocation of β-catenin to the nucleus when a Wnt protein binds to the Frizzled receptor
Although the association between the Wnt pathway and Dupuytren’s disease, as described by Dolmans et al., has been confirmed in three independent association studies (Dolmans et al. 2011; Shih et al. 2012; Anderson et al. 2014). the expression/involvement of Wnt pathway members in diseased Dupuytren’s tissue is unclear. An increased protein expression and nuclear translocation of β-catenin has repeatedly been reported (Varallo et al. 2003; Howard et al. 2003; Montgomery and Folpe 2005; O’Gorman et al. 2006; Degreef et al. 2009; Vi et al. 2009), but reports on mRNA expression and protein localization data on the Wnt pathway are rare and seem to be contradictory (O’Gorman et al. 2006; Degreef et al. 2009). In this study we have investigated the expression and protein localization of the six genes identified in the GWAS in affected nodules and cords of eight Dupuytren’s patients, and used unaffected fascia of the same donors as control tissue. By doing so, we were able to investigate the presence of mRNA levels of several Wnt-related genes and their corresponding proteins in affected and non-affected tissues. Collagen type I and III and alfa-smooth muscle actin (α-SMA) were measured to obtain an impression of the diseased state of the cords and nodules.
Material and methods
Primary tissues
Dupuytren’s nodules, cords and unaffected transverse ligaments of the palmar aponeurosis (TLPA) were obtained from Dupuytren’s patients with primary disease that underwent limited fasciectomy or dermofasciectomy by plastic surgeons in the University Medical Center Groningen. Nodules and cords were considered affected, and TLPA was considered unaffected (control). All affected and control tissues were patient-matched.
Quantitative RT-PCR
Up to 30 mg of tissue per sample was cut into small pieces and disrupted using an ultra-turrax. RNA extraction was performed using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. RNA was quantified using a NanoDrop-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Complementary DNA was synthesized from RNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Rockford, IL). Primers were ordered from Sigma-Aldrich (Zwijndrecht, The Netherlands) (sequences: Table 1). Samples were analyzed in triplo (Wnt-related) or duplo (others) and pipetted onto MicroAmp Optical 384-Well Reaction Plates with Barcode (Applied Biosystems, Foster City, CA). Plates were run on the ViiA 7 Real-Time PCR system (Applied Biosystems). The relative amount of product was calculated using the ΔΔCt method, normalizing for the averaged expression of the household genes GAPDH and YWHAZ and related to the control tissue.
Table 1.
Primers for the genes used for qPCR
| Gene | Forward | Reverse |
|---|---|---|
| WNT2 | atgggagcatcagtatgcaa | tccctgaatgtcatcttttgg |
| WNT4 | gcagagccctcatgaacct | cCacccgcatgtgtgtcag |
| WNT7B | cacagaaactttcgcaagtgg | ggctaggccaggaatcttgtt |
| RSPO2 | cccacgtgctaaccaagc | catctctccgccacgaac |
| SFRP4 | gcctgaagccatcgtcac | ccatcatgtctggtgtgatgt |
| SULF1 | gcgttcatcatacatcataccttt | tcatgccaagaaaaccaaaa |
| ACTA2 | ctgttccagccatccttcat | tcatgatgctgttgtaggtggt |
| COL1A1 | gcctcaaggtattgctggac | accttgtttgccaggttcac |
| COL3A1 | ctggaccccagggtcttc | catctgatccagggtttcca |
| GAPDH | agccacatcgctcagacac | gcccaatacgaccaaatcc |
| YWHAZ | gatccccaatgcttcacaag | tgcttgttgtgactgatcgac |
Immunohistochemistry
Frozen tissue on Tissue-Tek (Sakura, Zoeterwoude, The Netherlands) was cut into 5–10 μm coupes and put onto microscope slides (Starfrost, Waldemar Knittel GmbH, Brunschweig, Germany). Sections were fixed for 10 min with acetone (stainings for Wnt2, Wnt7b, Sfrp4, α-SMA), or 4 % paraformaldehyde in PBS with permeabilization using 0.1 % Triton X (stainings for Wnt4, Rspo2, Sulf1, β-catenin, Collagen-I, Collagen-III). The sections were incubated with a primary antibody for 60 min at room temperature, with 1 % bovine serum albumin (antibody information: see Table 2). Endogenous peroxidases were blocked using 0.1 % H2O2 during 15 min. For all biotinylated secondary antibodies, avidin and biotin were blocked for 15 min using the Biotin Blocking System (Dako, Glosstrup, Denmark). Secondary antibodies (Dako and Southern Biotech, Birmingham, AL) diluted 1:100 with 2 % human serum, were incubated during 30 min. The stainings were visualized using 3-amino-9-ethylcarbazole (AEC; Sigma-Aldrich) or Vector Red (Vector Laboratories, Burlingame, CA). Hematoxylin (Merck, Darmstadt, Germany) was used as counterstaining and all slides were mounted in Kaiser’s glycerin-gelatin (Merck).
Table 2.
Primary antibodies used for immunohistochemical stainings
| Antibody | Manufacturer | Product Code | Dilution |
|---|---|---|---|
| Wnt2 | R&D, Minneapolis, MN | AF3464 | 1:50 |
| Wnt4 | Sigma-Aldrich, Zwijndrecht, The Netherlands | HPA011397 | 1:50 |
| Wnt7b | Sigma-Aldrich, Zwijndrecht, The Netherlands | SAB2104506 | 1:200 |
| R-Spondin2 | Santa Cruz Biotechnology, Santa Cruz, CA | SC-74,883 | 1:100 |
| Sfrp4 | Sigma-Aldrich, Zwijndrecht, The Netherlands | HPA009712 | 1:25 |
| Sulf1 | Santa Cruz Biotechnology, Santa Cruz, CA | SC-98,325 | 1:100 |
| β-catenin | BD Transduction Laboratories, San Diego, CA | 610,153 | 1:100 |
| α-SMA | Dako, Glosstrup, Denmark | M0851 | 1:100 |
| Collagen-I | Abcam, Cambridge, MA | 6308 | 1:5000 |
| Collagen-III | Abcam, Cambridge, MA | 6310 | 1:5000 |
Quantification of stainings
Stainings were analyzed using a Leica DM 2000 microscope (Leica, Wetzlar, Germany). For quantification, three to five representative photomicrographs (40× magnification) were taken per tissue section, and analyzed using Nuance 3.0 software (PerkinElmer, Waltham, MA), allowing detection of a specific signal without interfering background noise. For each specific staining, stained areas were quantified as μm2/high power field.
Statistics
Statistical analysis was performed using GraphPad Prism version 5 (GraphPad Software, La Jolla, CA). A Kruskal-Wallis test was used to determine whether there were differences in ranks between the three groups. A post-hoc Dunn’s Multiple Comparisons test compared controls with cords and controls with nodules. P values <0.05 were considered to be statistically significant. Graphs show individual sample values, accompanied by a mean with SEM whisker plot per group. Significances are shown as * (P < 0.05); ** (P < 0.01) and *** (P < 0.001).
Results
Characterization of tissue: collagen and α-smooth muscle actin
Tissues were characterized by real-time qPCR and immunohistochemical stainings on nodules, cords and control tissue in terms of expression of ACTA2/α-SMA, COL1A1/collagen type I and COL3A1/collagen type III. We did not find significant differences in mRNA expression of ACTA2 between affected tissues and control, but observed a high upregulation of both COL1A1 and COL3A1 in both cords and nodules (Supplemental Fig. 1a). At protein level we found significantly more α-SMA, the main marker for myofibroblasts, in cord and nodules than in control tissue (Supplemental Fig. 1b). These stainings clearly showed a pattern of different zones of high intensity within the affected tissues. Both collagens were highly upregulated in cords at protein level, while collagen type III only showed a significant upregulation in nodules (Supplemental Fig. 1c-e).
MRNA expression of Wnt-related genes
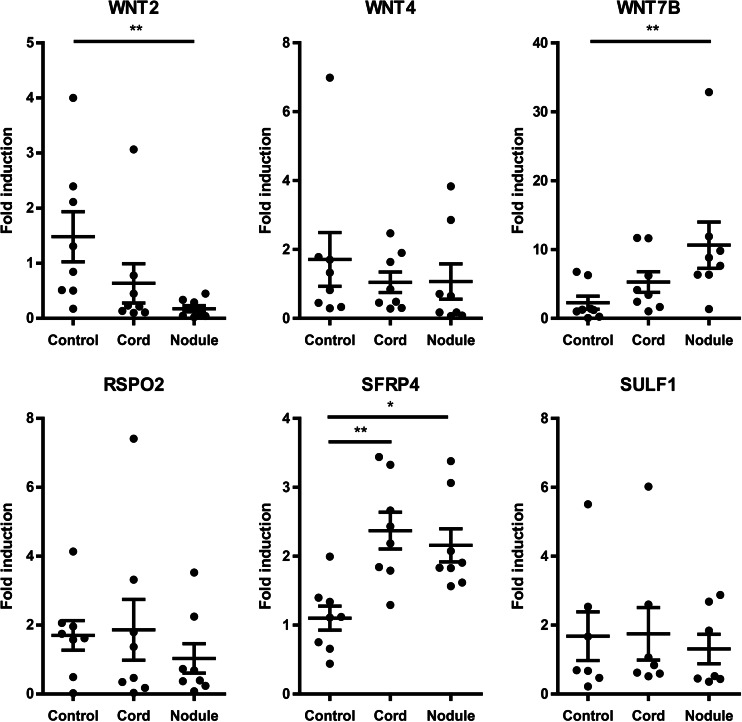
The six Wnt-related genes that were found to be associated with Dupuytren’s disease in the GWAS (Dolmans et al. 2011) were analyzed in eight (SULF1: seven) affected cords and nodules and in unaffected TLPA by performing real-time qPCR. Significant expression differences in three of these genes were found in affected tissue compared to control tissue: a nine-fold downregulation of WNT2 in nodules (P < 0.01), a five-fold upregulation of WNT7B in nodules (P < 0.01), and a two-fold upregulation of SFRP4 in cords as well as nodules (p < 0.01). (Fig. 2) We did not find any significant differences in expression of WNT4, RSPO2 and SULF1.
Fig. 2.
mRNA levels of WNT-related genes in Dupuytren’s disease tissue (cord, nodule) as compared to control tissue (unaffected transverse ligaments of the palmar aponeurosis). Upper panel: WNT2, WNT4, WNT7B; lower panel: RSPO2, SFRP4 and SULF1. * P < 0.05, ** P < 0.01 by Kruskal-Wallis test, followed by post-hoc Dunn’s Multiple Comparisons test
Immunohistochemistry
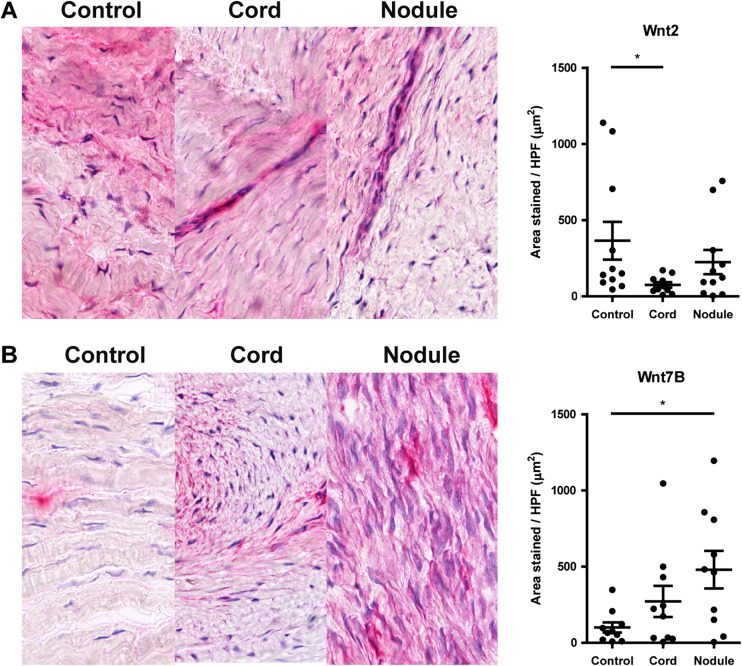
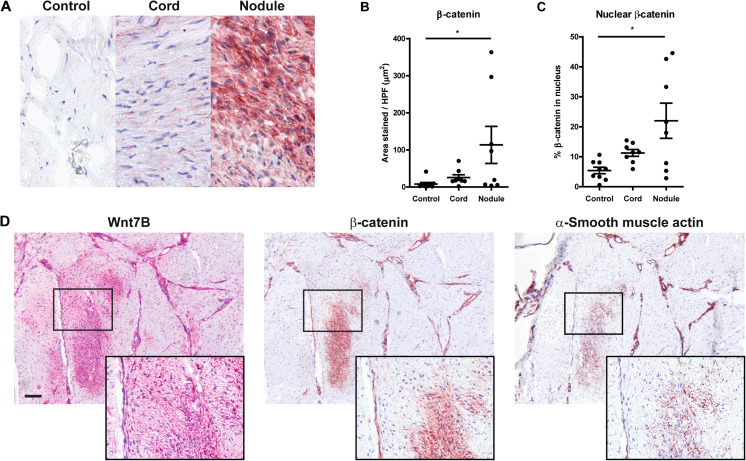
Significantly less staining of the Wnt2 protein was observed in cords than in controls (P < 0.05). Also, we found more Wnt7b staining (P < 0.05) in nodules than in controls (Fig. 3). Both results are in agreement with the qPCR results (Fig. 2). There were no significant differences in staining for Wnt4, Rspo2, Sfrp4 and Sulf1 (data not shown). β-catenin, which is the common downstream target of the canonical Wnt pathway, showed significantly more staining in nodules than in controls (P < 0.05) (Fig. 4a,b). A larger percentage of this staining was associated with the nucleus compared to controls (Fig. 4c). Finally, using staining of serial sections, we found that stainings for Wnt7b and β-catenin clustered in or around the zones with a positive α-SMA staining (Fig. 4d).
Fig. 3.
Representative pictures and quantification of staining of selected Wnt-proteins in Dupuytren’s disease tissue (cords, nodule) as compared to control tissue (unaffected transverse ligaments of the palmar aponeurosis). a Wnt2. b Wnt7b. * P < 0.05 by Kruskal-Wallis test, followed by post-hoc Dunn’s Multiple Comparisons test
Fig. 4.
Representative pictures and quantification of staining of β-catenin in Dupuytren’s disease tissue (cord, nodule) as compared to control tissue (unaffected transverse ligaments of the palmar aponeurosis). a photomicrographs. b quantification of staining for β-catenin. * P < 0.05 by Kruskal-Wallis test, followed by post-hoc Dunn’s Multiple Comparisons test. c percentage of nuclear localization measured as colocalization of β-catenin positive signal with hematoxylin signal. * P < 0.05 by Kruskal-Wallis test, followed by post-hoc Dunn’s Multiple Comparisons test. d) Staining for Wnt7b, β-catenin and α-smooth muscle actin (scale bar represents 100 μm) in serial sections of a Dupuytren’s nodule
Discussion
We examined the expression of the Wnt-related genes found to be associated with Dupuytren’s disease in the GWAS by Dolmans et al. (2011). as well as the corresponding proteins and the Wnt downstream target β-catenin. We found that several Wnt-related genes were differentially regulated in affected tissue compared to control tissue. Specifically, we report upregulation of WNT7B and SFRP4 and downregulation of WNT2. We also found a clear increase in expression of Wnt-downstream protein β-catenin in Dupuytren’s nodules, especially in nuclei, strongly suggesting an overall activation of the canonical Wnt signaling pathway in Dupuytren’s disease.
Dolmans et al. (2011) found single nucleotide polymorphisms in six areas where several Wnt genes are coded to be associated with Dupuytren’s. They concluded that these genes could be relevant in the occurrence and development of the disease. In accordance with this, we show three of these genes to be differentially expressed between normal and affected tissues within patients, extending the findings of the GWAS study. Not all Wnt-related genes found in the GWAS showed an altered regulation in our study. Our limited study size, as well as the different study design, since we used internal instead of external controls, might be responsible for this. A previous study showed that differences between affected fibroblasts and fibroblasts from unaffected fascia in Dupuytren’s patients are smaller than those between palmar fascia fibroblasts from healthy persons and affected fibroblasts from Dupuytren’s patients (Satish et al. 2012). In line with these findings, we did indeed find less differences, however, these can all be ascribed to the disease process, since inter-patient variation does not play a role in our study set-up. Besides, TLPA may seem unaffected during surgery, but contain microscopical spurs of disease that influence the outcome of PCR and immunohistochemical examinations. Expression profiles of ACTA2/α-SMA, COL1A1/collagen type I and COL3A1/collagen type III on mRNA and/or protein level confirmed that the affected tissues were fibrotic, as compared to the control TLPA. These data contribute to the validation of our findings regarding Wnt signaling and β-catenin in Dupuytren’s tissues.
Of the 19 Wnt-ligand genes in the human genome (Clevers and Nusse 2012) we studied three: WNT2, WNT4 and WNT7B. Next to these Wnt ligands, we examined three genes that influence Wnt signaling: SFRP4, RSPO2 and SULF1. The Wnt-related genes that we analyzed have different functions in the Wnt signaling pathway. We found the WNT2 gene to be lower expressed in Dupuyten’s nodules, and the Wnt2 protein to have less staining in cords compared to controls. In view of the general profibrotic role ascribed to the Wnt pathway this is a remarkable finding. Wnt2 has been specifically linked to fibrosis before, for instance by Bayle et al., who found an increased mRNA expression of WNT2 in a mouse model of skin fibrosis (Bayle et al. 2008). Possibly, a negative feedback mechanism in which the WNT2 gene is being suppressed as a reaction to excessive activation of the Wnt signaling pathway, plays a role in Dupuytren’s disease. In this context, it is of interest to note that WNT2 was also found to be a susceptibility locus in the related Peyronie’s disease, in which the penis is affected (Dolmans et al. 2012). However, it is not known whether in this disease WNT2 is up- or downregulated. Further study is needed to prove a similar downregulation in Peyronie’s disease.
In contrast to the lower expression of the WNT2 gene and protein in Dupuytren’s tissue, we found a significantly higher expression of the WNT7B gene in nodules compared to control tissue, and more Wnt7b positive cells in nodules. This gene has been reported to play a role in several pulmonary conditions. A higher expression of WNT7B was found in lung tissue from patients with idiopathic pulmonary fibrosis, on mRNA (Konigshoff et al. 2008) and protein level. The increased immunostaining of the Wnt7b protein was found especially in regions with fibrotic changes (Meuten et al. 2012). which is in accordance with our findings in nodule, where Wnt7b staining was found in α-SMA positive areas. Overall, these and our findings seem to pinpoint WNT7B as an important effector molecule in fibrosis. If so, a higher expression of WNT7B in affected tissue of Dupuytren’s patients seems a likely candidate to be connected with the disease process, either as a cause or consequence.
We found a significantly (two-fold) higher expression of the SFRP4 gene in cords and nodules, but no differences in protein staining. SFRP4 codes for one of the secreted Frizzled-related proteins, a group of Wnt-binding proteins that inhibit interactions between Wnt and Wnt receptors (Clevers and Nusse 2012). but it has also been reported to activate Wnt signaling (Mahdi et al. 2012). Shih et al. found a higher mRNA expression of SFRP4 in Dupuytren’s tissues (Shih et al. 2012). In humans with systemic sclerosis, an upregulation of SFRP4 on mRNA and protein level was found in fibrotic skin (Bayle et al. 2008). On the other hand, Sfrp4 administration in rats reduces fibrosis after ischemic injury to the heart, and a therapeutical effect of recombinant Sfrp4 on renal fibrosis in mice has been described (Matsushima et al. 2010; Surendran et al. 2005). Our findings showed a higher transcriptional expression of SFRP4, but – in the absence of a change in protein levels – the effect of this remains unclear.
Several studies have reported increased β-catenin in Dupuytren’s disease and discussed a dysregulation of the Wnt signaling pathway as an upstream regulator of β-catenin (Varallo et al. 2003; Montgomery and Folpe 2005; O’Gorman et al. 2006; Degreef et al. 2009). Varallo et al. described higher levels of β-catenin in diseased palmar fascia as compared to patient-matched normal fascia, with both cytoplasmic and nuclear staining. They suggested that Wnt signaling might contribute to these findings, but a role of TGF-β and the extracellular matrix was also suggested (Varallo et al. 2003). O’Gorman et al. found that changes in Wnt expression are unlikely to be the cause of dysregulation of β-catenin expression in Dupuytren’s disease. They reported that overall Wnt expression, with the exception of WNT10B, was unchanged between normal and disease palmar fascia on mRNA level, but they did not examine protein expression (O’Gorman et al. 2006). Degreef et al. found evident staining of β-catenin in involutional zones of Dupuytren’s nodules, without comparing it to control tissue. They found no differences between nodules of patients with recurrent and non-recurrent disease (Degreef et al. 2009). We found significantly more staining of β-catenin in nodules than in control tissue, and the relative amount of staining associated with nuclei was much higher than in controls, confirming activation of the Wnt signaling cascade. In view of the high expression of Wnt7b in the same region as nuclear β-catenin, this protein might be among the Wnt proteins causing the activation of the Wnt pathway in this tissue.
Based on our results, the Wnt signaling pathway is (over)activated in Dupuytren’s disease, which would make inhibition of this pathway an interesting possible treatment target. Although systemic therapy influencing the Wnt pathway might not be a realistic solution, in view of its ubiquitous importance in cellular processes, local injections with Wnt-blocking agents as a preventive measure may be a possibility. However, our study does not address the question whether the activated Wnt pathway is the cause or the result of Dupuytren’s disease. In either case, inhibition of the Wnt pathway will have to be tested further in vitro and in vivo, before it would make a feasible treatment.
Conclusion
In this study we report dysregulation of several Wnt-related genes and proteins, although not all genes found in the GWAS were changed. We found an increase in Wnt-target β-catenin in affected tissue, indicating activation of the canonical Wnt-signaling pathway. In addition, we found a substantial upregulation of Wnt7b, which might be a mediator of pro-fibrotic Wnt signaling in Dupuytren’s disease.
Electronic supplementary material
Characterization of Dupuytren’s disease tissue (cord, nodule) as compared to control tissue (unaffected transverse ligaments of the palmar aponeurosis). A) mRNA levels of ACTA2, COL1A1 and COL3A1. * P < 0.05, *** P < 0.001 by Kruskal-Wallis test, followed by post-hoc Dunn’s Multiple Comparisons test. B) Representative pictures of α-smooth muscle actin staining in control, cord and nodule tissue of a Dupuytren’s disease patient (scale bar represents 50 μm). C) Representative pictures of collagen type I staining in control, cord and nodule tissue of a Dupuytren’s disease patient (scale bar represents 50 μm). D) Representative pictures of collagen type III staining in control, cord and nodule tissue of a Dupuytren’s disease patient (scale bar represents 50 μm). E) Quantification of stainings for α-smooth muscle actin, collagen type I and collagen type III, * P < 0.05 by Kruskal-Wallis test, followed by post-hoc Dunn’s Multiple Comparisons test. (GIF 449 kb)
Abbreviations
- GWAS
Genome-wide association study
- TLPA
Transverse ligaments of the palmar aponeurosis
- PBS
Phosphate buffered saline
- q (RT)PCR
Quantitative (real-time) polymerase chain reaction
Compliance with ethical standards
Tissue samples were collected after informed written consent and approval of the Medical Ethics Committee (METc) of the University Medical Center Groningen (2007/067).
References
- Anderson ER, Ye Z, Caldwell MD, Burmester JK. SNPs previously associated with Dupuytren’s disease replicated in a north American cohort. Clin Med Res. 2014;12:133–137. doi: 10.3121/cmr.2013.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle J, Fitch J, Jacobsen K, Kumar R, Lafyatis R, Lemaire R. Increased expression of Wnt2 and SFRP4 in Tsk mouse skin: role of Wnt signaling in altered dermal fibrillin deposition and systemic sclerosis. J Invest Dermatol. 2008;128:871–881. doi: 10.1038/sj.jid.5701101. [DOI] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Degreef I, De Smet L, Sciot R, Cassiman JJ, Tejpar S. Beta-catenin overexpression in Dupuytren’s disease is unrelated to disease recurrence. Clin Orthop Relat Res. 2009;467:838–845. doi: 10.1007/s11999-008-0590-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmans GH, Werker PM, Hennies HC, Furniss D, Festen EA, Franke L, Becker K, van der Vlies P, Wolffenbuttel BH, Tinschert S, Toliat MR, Nothnagel M, Franke A, Klopp N, Wichmann HE, Nurnberg P, Giele H, Ophoff RA, Wijmenga C, Group Dutch Dupuytren Study, Group German Dupuytren Study, Study LifeLines Cohort, Consortium BSSH-GODD. Wnt signaling and Dupuytren’s disease. N Engl J Med. 2011;365:307–317. doi: 10.1056/NEJMoa1101029. [DOI] [PubMed] [Google Scholar]
- Dolmans GH, Werker PM, de Jong IJ, Nijman RJ, Study LifeLines Cohort, Wijmenga C, Ophoff RA. WNT2 locus is involved in genetic susceptibility of peyronie’s disease. J Sex Med. 2012;9:1430–1434. doi: 10.1111/j.1743-6109.2012.02704.x. [DOI] [PubMed] [Google Scholar]
- Gudmundsson KG, Arngrimsson R, Sigfusson N, Bjornsson A, Jonsson T. Epidemiology of Dupuytren’s disease: clinical, serological, and social assessment. The Reykjavik Study. J Clin Epidemiol. 2000;53:291–296. doi: 10.1016/S0895-4356(99)00145-6. [DOI] [PubMed] [Google Scholar]
- Hindocha S, McGrouther DA, Bayat A. Epidemiological evaluation of Dupuytren’s disease incidence and prevalence rates in relation to etiology. Hand (N Y) 2009;4:256–269. doi: 10.1007/s11552-008-9160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- Howard JC, Varallo VM, Ross DC, Roth JH, Faber KJ, Alman B, Gan BS. Elevated levels of beta-catenin and fibronectin in three-dimensional collagen cultures of Dupuytren’s disease cells are regulated by tension in vitro. BMC Musculoskelet Disord. 2003;4:16. doi: 10.1186/1471-2474-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst LC, Badalamente MA, Hentz VR, Hotchkiss RN, Kaplan FT, Meals RA, Smith TM, Rodzvilla J, Study Group CORD I. Injectable collagenase Clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting R, van den Heuvel ER, Westerink B, Werker PM. Prevalence of Dupuytren disease in The Netherlands. Plast Reconstr Surg. 2013;132:394–403. doi: 10.1097/PRS.0b013e3182958a33. [DOI] [PubMed] [Google Scholar]
- Mahdi T, Hanzelmann S, Salehi A, Muhammed SJ, Reinbothe TM, Tang Y, Axelsson AS, Zhou Y, Jing X, Almgren P, Krus U, Taneera J, Blom AM, Lyssenko V, Esguerra JL, Hansson O, Eliasson L, Derry J, Zhang E, Wollheim CB, Groop L, Renstrom E, Rosengren AH. Secreted frizzled-related protein 4 reduces insulin secretion and is overexpressed in type 2 diabetes. Cell Metab. 2012;16:625–633. doi: 10.1016/j.cmet.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Matsushima K, Suyama T, Takenaka C, Nishishita N, Ikeda K, Ikada Y, Sawa Y, Jakt LM, Mori H, Kawamata S. Secreted frizzled related protein 4 reduces fibrosis scar size and ameliorates cardiac function after ischemic injury. Tissue Eng Part A. 2010;16:3329–3341. doi: 10.1089/ten.tea.2009.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuten T, Hickey A, Franklin K, Grossi B, Tobias J, Newman DR, Jennings SH, Correa M, Sannes PL (2012) WNT7B in fibroblastic foci of idiopathic pulmonary fibrosis. Respir Res 13:62–9921-13-62. doi:10.1186/1465–9921-13-62 [DOI] [PMC free article] [PubMed]
- Montgomery E, Folpe AL. The diagnostic value of beta-catenin immunohistochemistry. Adv Anat Pathol. 2005;12:350–356. doi: 10.1097/01.pap.0000194628.58501.71. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- O’Gorman DB, Wu Y, Seney S, Zhu RD, Gan BS. Wnt expression is not correlated with beta-catenin dysregulation in Dupuytren’s disease. J Negat Results Biomed. 2006;5:13. doi: 10.1186/1477-5751-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- Sahota AP, Dhoot GK. A novel SULF1 splice variant inhibits Wnt signalling but enhances angiogenesis by opposing SULF1 activity. Exp Cell Res. 2009;315:2752–2764. doi: 10.1016/j.yexcr.2009.06.029. [DOI] [PubMed] [Google Scholar]
- Satish L, LaFramboise WA, Johnson S, Vi L, Njarlangattil A, Raykha C, Krill-Burger JM, Gallo PH, O’Gorman DB, Gan BS, Baratz ME, Ehrlich GD, Kathju S. Fibroblasts from phenotypically normal palmar fascia exhibit molecular profiles highly similar to fibroblasts from active disease in Dupuytren’s Contracture. BMC Med Genomics. 2012;5:15–8794-5-15. doi: 10.1186/1755-8794-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih B, Tassabehji M, Watson JS, Bayat A. DNA copy number variations at chromosome 7p14.1 and chromosome 14q11.2 are associated with Dupuytren’s disease: potential role for MMP and Wnt signaling pathway. Plast Reconstr Surg. 2012;129:921–932. doi: 10.1097/PRS.0b013e3182442343. [DOI] [PubMed] [Google Scholar]
- Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Schultz RJ, Haaksma CJ. Extracellular matrix-cytoskeletal connections at the surface of the specialized contractile fibroblast (myofibroblast) in Dupuytren disease. J Bone Joint Surg Am. 1987;69:1400–1407. doi: 10.2106/00004623-198769090-00013. [DOI] [PubMed] [Google Scholar]
- van Rijssen AL, ter Linden H, Werker PM. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129:469–477. doi: 10.1097/PRS.0b013e31823aea95. [DOI] [PubMed] [Google Scholar]
- Varallo VM, Gan BS, Seney S, Ross DC, Roth JH, Richards RS, McFarlane RM, Alman B, Howard JC. Beta-catenin expression in Dupuytren’s disease: potential role for cell-matrix interactions in modulating beta-catenin levels in vivo and in vitro. Oncogene. 2003;22:3680–3684. doi: 10.1038/sj.onc.1206415. [DOI] [PubMed] [Google Scholar]
- Verjee LS, Midwood K, Davidson D, Essex D, Sandison A, Nanchahal J. Myofibroblast distribution in Dupuytren’s cords: correlation with digital contracture. J Hand Surg Am. 2009;34:1785–1794. doi: 10.1016/j.jhsa.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Vi L, Njarlangattil A, Wu Y, Gan BS, O’Gorman DB (2009) Type-1 Collagen differentially alters beta-catenin accumulation in primary Dupuytren’s Disease cord and adjacent palmar fascia cells. BMC Musculoskelet Disord 10:72–2474-10-72. doi:10.1186/1471-2474-10-72 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of Dupuytren’s disease tissue (cord, nodule) as compared to control tissue (unaffected transverse ligaments of the palmar aponeurosis). A) mRNA levels of ACTA2, COL1A1 and COL3A1. * P < 0.05, *** P < 0.001 by Kruskal-Wallis test, followed by post-hoc Dunn’s Multiple Comparisons test. B) Representative pictures of α-smooth muscle actin staining in control, cord and nodule tissue of a Dupuytren’s disease patient (scale bar represents 50 μm). C) Representative pictures of collagen type I staining in control, cord and nodule tissue of a Dupuytren’s disease patient (scale bar represents 50 μm). D) Representative pictures of collagen type III staining in control, cord and nodule tissue of a Dupuytren’s disease patient (scale bar represents 50 μm). E) Quantification of stainings for α-smooth muscle actin, collagen type I and collagen type III, * P < 0.05 by Kruskal-Wallis test, followed by post-hoc Dunn’s Multiple Comparisons test. (GIF 449 kb)