Abstract
This paper reviews the simulations of catheter ablation in computer models of the atria, from the first attempts to the most recent anatomical models. It describes how postulated substrates of atrial fibrillation can be incorporated into mathematical models, how modelling studies can be designed to test ablation strategies, what their current trade‐offs and limitations are, and what clinically relevant lessons can be learnt from these simulations. Drawing a parallel between clinical and modelling studies, six ablation targets are considered: pulmonary vein isolation, linear ablation, ectopic foci, complex fractionated atrial electrogram, rotors and ganglionated plexi. The examples presented for each ablation target illustrate a major advantage of computer models, the ability to identify why a therapy is successful or not in a given atrial fibrillation substrate. The integration of pathophysiological data to create detailed models of arrhythmogenic substrates is expected to solidify the understanding of ablation mechanisms and to provide theoretical arguments supporting substrate‐specific ablation strategies.
Abbreviations
- ACh
acetylcholine
- AF
atrial fibrillation
- CFAE
complex fractionated atrial electrogram
- CT
computed tomography
- CV
conduction velocity
- ECG
electrocardiogram
- MRI
magnetic resonance imaging
- PVI
pulmonary vein isolation
Introduction
Atrial fibrillation (AF) is a major cardiac arrhythmia in terms of prevalence and cost of health care. Clinical AF classification is based on patient history (Levy et al. 2003). AF is marked by an insidious progression from its paroxysmal form, which appears to be driven from the pulmonary veno‐atrial junctions and may occur in a structurally normal heart, to persistent and permanent AF characterised by electrical and structural remodelling, including atrial dilatation and fibrosis. The pathophysiological mechanisms of persistent and permanent AF are highly complex and involve pro‐arrhythmogenic processes on multiple temporal and spatial scales (notably caused by successive AF episodes) inducing alterations to the substrate and the triggers (Andrade et al. 2014).
Catheter ablation is a therapy of choice for patients with AF refractory to anti‐arrhythmic drug treatment (Calkins et al. 2012). This therapy consists in creating lesions with a catheter using radiofrequency energy or cryoablation, thus forming barriers for impulse propagation. Ablations may isolate triggers, target structurally remodelled regions or trap fibrillation waves in a maze. Current AF management reflects the clinical history of AF. Pulmonary vein isolation (PVI) is the cornerstone of most strategies. Effective in paroxysmal AF patients, this approach was found to be insufficient to treat persistent AF, thus requiring further ablation targets (e.g. linear ablation, ectopic sources, fractionated electrograms, rotors or ganglionated plexi). However, due to conflicting outcomes and promising strategies that were less successful in large multicentre studies, there is no consensus on what to ablate after PVI in persistent AF patients and when to stop (Verma et al. 2015). A rational, mechanistic approach is needed to better optimise ablation strategy.
Computer models of atrial electrophysiology have been developed to contribute to the understanding of AF mechanisms, the design of diagnosis techniques, and the optimisation of therapeutic approaches (Jacquemet et al. 2008; Dossel et al. 2012; Virag et al. 2012; Trayanova, 2014; Zhao et al. 2015). The strengths of these models are the following: (1) they provide a theoretical basis supporting the design of clinical protocols; (2) hypotheses can be tested in a fully controllable environment (access to all variables everywhere); (3) different effects can be isolated or discriminated (e.g. imperfect ablation vs. inadequate ablation site); (4) the efficacy of many therapies can be assessed in the same AF model. As a result, modelling is emerging as a complementary approach to animal experiments and clinical trials to help design more effective therapies (Winslow et al. 2012).
While recent work emphasises the construction of patient‐specific anatomical models, this paper concentrates on the design of modelling studies to explore the mechanisms of AF ablation. The different electrophysiological targets for ablation are presented and their implementation in computer models is discussed through examples of recently developed models. The differences between clinical and modelling studies are highlighted, as well as their strengths and limitations.
Targets for ablation
This section outlines the mechanistic basis and clinical evidences of the ablation approaches that aim to be simulated. Complete discussions about the procedures are found in clinical reviews (Calkins et al. 2012; Magnani et al. 2015).
Pulmonary vein isolation (PVI)
Triggers that initiate AF have been identified in the pulmonary veins (Haissaguerre et al. 1998). Electric isolation of the pulmonary veins by creating ablation lines encircling the veins was shown to be effective at preventing AF recurrence in paroxysmal AF patients (Ganesan et al. 2013). Success rates were lower in persistent or permanent AF patients (Lin et al. 2013). Incomplete or non‐transmural isolation may lead to AF recurrence and require another intervention. PVI is the basis of most strategies and may be supplemented by one of the techniques described below used as adjuvants, particularly in patients with persistent AF.
Linear ablation
Usually combined with PVI, this approach creates a predefined set of additional linear lesions (Calkins et al. 2012), typically a roof line and a mitral isthmus line, to block the pathways for anatomical reentries and form a maze in which wavelets are trapped, which may cause AF termination (Ernst et al. 2003). The extreme example of such an approach is the Cox Maze III (Cox et al. 1991). Not recommended for paroxysmal AF (Calkins et al. 2012), adjuvant linear ablation has been included in stepwise approaches (O'Neill et al. 2009), but remains controversial for persistent AF (Verma et al. 2015).
Ectopic foci
Non‐pulmonary vein triggers have been identified, for instance in the left posterior wall and near the venae cavae (Lee et al. 2005; Miyazaki et al. 2014). A stepwise ablation strategy may include successively eliminating these focal sources (Calkins et al. 2012). This approach has been successful in a sub‐group of paroxysmal AF patients (Lin et al. 2003).
Complex fractionated atrial electrograms (CFAEs)
Electrical mapping during AF has revealed regions where atrial electrograms have multiple high‐frequency low voltage deflections with very short cycle lengths (Konings et al. 1997). It has been hypothesised that these CFAEs identify sites of AF reentry and provide targets for catheter ablation (Nademanee et al. 2004). Others have argued that CFAEs are a signature of structural substrates that contribute to the progression from paroxysmal to permanent AF (Stiles et al. 2009). Despite promising outcomes reported for CFAE‐guided ablation of high risk AF patients (Nademanee et al. 2008), multicentre clinical trials have not demonstrated any improvement in sustained reversal of persistent AF when this approach is used in addition to PVI (Li et al. 2011; Verma et al. 2015).
Rotors
Instantaneous electroanatomic maps acquired with intracardiac basket catheters (Narayan et al. 2012) and from body surface potential recordings (Haissaguerre et al. 2014) have been used to identify the presence of rotors (defined as stable reentry) in the atria during AF. The location of these rotors tended to be consistent over time, although a wide range of rotor lifespan has been reported, from a few cycles (Cuculich et al. 2010; Haissaguerre et al. 2014) to thousands (Swarup et al. 2014). The location of rotors is believed to coincide with fibrotic regions that provide stable pathways for reentry. Late gadolinium‐enhanced magnetic resonance imaging (MRI) provides the technology to assess fibrotic structural remodelling non‐invasively (McGann et al. 2014). Narayan et al. (2014) showed that ablation of rotor cores markedly reduced long‐term recurrence of AF in a group of patients, most with persistent AF. However, a recent multicentre study failed to reproduce these positive results and advocated randomised studies (Buch et al. 2015). While panoramic, low‐resolution electrical mapping and phase analysis identifies rotors during persistent AF, high‐resolution mapping provides evidence of a highly complex disorganised multiple‐wavelet activity under these circumstances (Lee et al. 2014). How these two conflicting views might be resolved remains a subject of controversy (Zaman & Peters, 2014).
Ganglionated plexi
AF is expected to also have a neurogenic origin (Coumel, 1994; Efimov & Fedorov, 2005). Evidence of the involvement of atrial ganglionated plexi in pulmonary vein ectopy supports the idea of ablating ganglionated plexi to treat AF (Lu et al. 2009; Lim et al. 2011). A retrospective meta‐analysis demonstrated the efficacy of this approach at preventing AF recurrence when combined with PVI (Zhou et al. 2011).
Modelling framework
This section presents the elements composing an atrial model as well as the challenges encountered during its construction.
Mathematical formulation
To simulate AF episodes and catheter ablation in a computer model, knowledge about atrial electrophysiology and anatomy is translated into mathematical equations. The bidomain model comprises two partial differential equations that describe the propagation of the electrical impulse within the cardiac muscle (Plonsey & Barr, 2000). The monodomain approximation decouples these two equations, resulting in faster computation that may be traded for finer spatial resolution; accuracy remains appropriate in the absence of external stimulation (Potse et al. 2006). The formulation relies on a model of cellular electrophysiology that integrates the contributions of different types of ion channels, ion exchangers and pumps and computes the resulting variations in ion concentrations, including the intracellular calcium dynamics. Coupling with neighbouring cells through gap junctions is incorporated as a diffusive process representing current flow in a continuous, homogenised intracellular medium. This coarse‐scale continuous representation of current flow enables the simulation of whole‐organ models but does not capture the effects of discontinuities at the microstructure level (Jacquemet & Henriquez, 2009; Hubbard & Henriquez, 2014).
Geometry
During AF, depolarisation wavelets exploit the complex atrial structure to propagate and create reentrant circuits. Although the basic properties of reentry in the presence of ablation lines can be studied in two‐dimensional geometries (Spector et al. 2012; Spector, 2013), realistic atrial size and topology are generally required. The duration of non‐sustained AF depends on the relation between tissue area and the number and lengths of the obstacles (veins, valves), which is very relevant to the investigation of ablation efficacy (Qu, 2006). Tremendous effort has been spent in streamlining the construction of patient‐specific geometries based on imaging modalities (Aslanidi et al. 2011; Gonzales et al. 2013; Krueger et al. 2013; Hwang et al. 2014; Trayanova, 2014). Some challenges persist. Spatial resolution (typically at most 0.5 mm for clinical MRI) is limited relative to atrial wall thickness. Some anatomical structures are barely visible on the images (rim of the valves, fibre bundles, discrete left–right connections). A solution is to register the geometry with a validated atlas atrial model (Neher et al. 2011).
Conduction properties
The conductivity tensor is determined by the local fibre orientation and the longitudinal and transverse conductivities. Fibre identification in the atria through high resolution imaging has been limited so far to animal models: Zhao et al. (2012) used structure tensor analysis of volume images acquired using serial block face imaging in the sheep; Aslanidi et al. (2013) used contrast‐enhanced micro‐CT in the dog. The problem is challenging due to the thinness of the atrial wall and possible transmural fibre rotation resulting from the superposition of multiple fibre bundles and dissociation of endo‐ and epicardial layers (Verheule et al. 2014; Hansen et al. 2015). For human atria, fibre orientation is currently either rule‐based, that is, interpolated from a set of lines generated from landmark points (Krueger et al. 2011), or atlas‐based, which consists in mapping a validated dataset of fibre orientation onto a newly acquired geometry (McDowell et al. 2012). These operations may be difficult or ill‐posed due to interpatient variability in anatomy, e.g. five pulmonary veins, absence of Bachmann's bundle (Platonov et al. 2002). Then, the longitudinal and transverse conductivities are adjusted to reproduce measured activation maps; different values are assigned in the fast conducting system such as Bachmann's bundle, terminal crest and pectinate muscles (Harrild & Henriquez, 2000). Clinical maps may be obtained using electrical mapping catheters. Spatial resolution is, however, limited; this enables the optimisation of conduction properties at the macroscale only. Additional validation may come from the comparison of body surface potential signals (Jacquemet et al. 2006). Some insights into local structural heterogeneity and fibrosis density may be inferred using late gadolinium‐enhanced MRI and hypotheses about the nature and the electrophysiological impact of fibrosis (McDowell et al. 2012).
Cellular properties
Detailed ionic models describing the membrane kinetics of human atrial cells have been developed and validated using cellular measurements and patch clamp data (Wilhelms et al. 2012). Variants of these models have been created to reproduce pathological conditions, notably electrical remodelling. Target ionic current responsible for regional changes in action potential morphology have been identified (Ramirez et al. 2000). The major challenge remains determining the spatial distribution of repolarisation properties in a patient and modifying the target ion channels accordingly. Repolarisation gradients play an important role in the occurrence of conduction blocks (Fareh et al. 1998). Clinically, effective refractory periods and possibly monophasic action potentials may be measured at selected locations. But hypotheses and extrapolations about local repolarisation properties remain unavoidable.
Modelling ablations
The lesions created by catheter ablation are intended to be non‐conductive. Ablation is therefore simulated by setting tissue conductivity to zero in the ablated zone, usually defined by a fixed diameter of 3 mm (ablation lines; Reumann et al. 2008) to 7 mm (single points; McDowell et al. 2015) around the ablation catheter. To simulate reconnection of ablation lines or imperfect ablation, a gap is introduced along the line (Dang et al. 2005) or the line is made non‐transmural (Reumann et al. 2008). The location of ablation lines may be obtained using a catheter localisation system or designed based on clinician's conceptual drawings.
Design of virtual ablation experiments
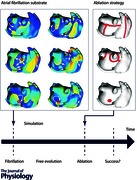
This section highlights the differences in the design of clinical and modelling studies of AF ablation. A summary is provided in Table 1 and an illustration in the Abstract figure.
Table 1.
Summary of the differences between clinical and modelling studies (examples of study design)
| Clinical study | Modelling study | |
|---|---|---|
| Patient selection | Group of patients satisfying inclusion/exclusion criteria | Multiple variants of one or several models (modification of the substrate) |
| Indications for ablation | Drug‐resistant paroxysmal or persistent AF | AF episode of duration >10–30 s |
| Study design | Same therapy in many patients | Several therapies in the same model (or a few different models) |
| Statistics over patients | Statistics over simulations in the same substrate | |
| The substrate is sometimes characterised | The substrate is specified | |
| AF mechanism is studied | AF mechanism is postulated and is an input to the model | |
| Ablation procedure | Step‐wise ablation | Instantaneous application of all ablation lines |
| Procedure time | Up to a few hours | 2–30 s of simulation |
| Side effects | Inflammation, risk of complications | None simulated |
| Criteria for AF termination | No AF after the procedure and until patient release | AF termination within 2–30 s |
| Criteria for AF prevention | No symptomatic AF episodes (48 h or several months of follow‐up) | All induction attempts failed (ectopic in the pulmonary vein) |
| Access to physiological data | ECG, electrical mapping | All variables everywhere all the time |
| Reproducibility | Limited by inter‐patient variability; AF episodes may vary in the same patient | Yes; variability can be controlled |
| Unique features | Real patient, real heart | Validation is critical |
| Ablation is irreversible | Possibility to ‘undo’ an ablation line and try another one | |
| Risks for the patients, including mortality and morbidity | No human life at risk |
AF, atrial fibrillation.
Patient selection
To test an ablation strategy clinically, the same ablation pattern is performed in a group of selected patients. Inclusion criteria typically include having drug‐resistant paroxysmal or persistent AF episodes for months. In a modelling study, one or a limited number of atrial models are used. Parameters can be changed within the physiological range and sensitivity analysis can be performed. Multiple therapies can be tested in the same model, even for identical AF episodes, thus enabling the determination of the optimal therapy for a given AF mechanism, instead of searching for the therapy that is effective in most patients. However, the statistics of simulated interventions may not be the same as the patient population.
Arrhythmogenic substrate
In a clinical study, the atrial substrate can be controlled through inclusion–exclusion criteria, mostly based on patient history, symptoms, concomitant heart disease, electrical signal interpretation and failure of other therapies. Advances in electrocardiogram (ECG) signal processing may eventually help non‐invasively identify AF aetiology and refine the selection of AF substrates (Bonizzi et al. 2015). In modelling studies, the AF substrate is an input to the model and has to be postulated a priori. These substrates, some of which are presented in the next section, reproduce atrial dilatation, electrical or structural remodelling and originate from hypotheses about AF mechanisms. Clean, well‐defined AF mechanisms can be specified, controlled, modified and combined. The results of modelling studies are therefore substrate specific.
AF episodes
In paroxysmal AF patients, AF episodes may occur spontaneously. In computer models, a set of episodes have to be generated to enable statistical analysis of ablation success rate. The first approach is to apply several initiation protocols, for example by varying the sites of programmed stimulation or ectopic foci (Virag et al. 2002; Vigmond et al. 2004; Gong et al. 2007; McDowell et al. 2015). The second approach consists in running a very long AF simulation (up to 10 min), extracting the state of the atrial tissue at regularly distributed time instants and running simulations of AF ablation from each these states (Dang et al. 2005). Finally, fibrillatory initial conditions may be created de novo from the locations of reentrant circuits (Herlin & Jacquemet, 2011; Matene & Jacquemet, 2012).
Ablation
Clinical ablation procedures may last up to a couple of hours depending on the complexity and possible complications (Calkins et al. 2012). In contrast, all ablation lines are generally applied simultaneously in computer models. Even when a stepwise approach is simulated, the time interval between each ablation step is in the order of a few seconds only. Since computational requirements impose restrictions on the duration of the simulations, often limited to 10–30 s, computer studies investigate in detail acute effects of ablation (or reconnection) in critical time windows where the mechanisms of AF maintenance or termination are in action.
Definition of success
The clinical objective of ablation is to terminate any ongoing AF episode and to prevent short‐term or long‐term AF recurrence. The therapy is considered successful when the patient is free of symptomatic AF episodes for a short period (e.g. a few months) or over the long term (years of follow‐up). The detection of AF recurrence may not be perfect as some asymptomatic episodes may remain unnoticed. In modelling studies, AF termination and prevention are studied separately. Ablation is considered successful if AF termination is observed within 2–30 s, depending on the studies. One may wonder whether the AF episode would have self‐terminated anyway, since it is difficult to establish that a computer model of AF is persistent, except in the case of a regular form of AF perpetuated by a stable rotor anchored around a functional or structural obstacle. In general, simulated AF is said to be persistent when it lasts over some predefined duration, 30 s for instance, due to computational time. To assess the prevention of AF recurrence by ablation, the mechanism of AF initiation has to be postulated. Modelling studies measure the vulnerability to a certain type of AF trigger, typically ectopic foci in the pulmonary veins. This is similar to clinical attempts to electrically induce AF to assess the vulnerability of the substrate after an intervention. A limitation is that failure to induce AF might result from too low a number of attempts. In the months following an ablation procedure, a patient may have many more atrial ectopic beats than one could possibly simulate in a model; unlikely but possible events may occur at some point.
Unique features
Simulated ablations take computational time, but never cost human life. Many ablation strategies (including emerging techniques not yet proven safe) can be compared in the same modelling study, while conducting a randomised clinical study with 10–20 groups would necessitate too many patients to reach statistical significance.
Lessons from simulations of ablation
This section presents, for each ablation target, modelling studies attempting to reproduce the ablation approach and the lessons that have been learnt from these simulations. The substrate properties of these models are recapitulated in Table 2 and the types of ablation patterns compared are listed in Fig. 1.
Table 2.
Atrial substrates in simulation studies of catheter ablation
| Reference | Geometry | Cell model | Conduction | AF dynamics |
|---|---|---|---|---|
| Virag et al. (2001) | Monolayer, both atria, idealised geometry | Simplified ionic model | Uniform isotropic, reduced CV | Multiple wavelets |
| Dang et al. (2005); Ruchat et al. (2007 a,b,c); Rotter et al. (2007) | Monolayer, both atria, MRI‐derived geometry | Simplified ionic model | Uniform isotropic, reduced CV | Multiple wavelets |
| Haissaguerre et al. (2007) | 3‐D, both atria, MRI‐derived geometry | Modified Courtemanche et al. (1998) model, repolarisation heterogeneity | Isotropic + fast conducting bundles | Focal AF |
| Reumann et al. (2008) | 3‐D, both atria, visible female | Advanced cellular automaton | Anisotropic, reduced CV | Focal AF |
| Hwang et al. (2014) | Monolayer, 20 patient‐specific CT‐derived left atrial geometries | Modified Courtemanche et al. (1998) model | Uniform isotropic, reduced CV | Multiple wavelets |
| McDowell et al. (2015) | 3‐D, 4 patient‐specific MRI‐derived left atrial geometries | Krummen et al. (2012) model + fibroblasts | Anisotropic, LGE‐MRI‐based structural remodelling | Rotors |
AF, atrial fibrillation; CT, computed tomography; CV, conduction velocity; LGE, late gadolinium‐enhanced; MRI, magnetic resonance imaging.
Figure 1. Schematic recapitulation of the types of ablation patterns tested and compared in computer models .
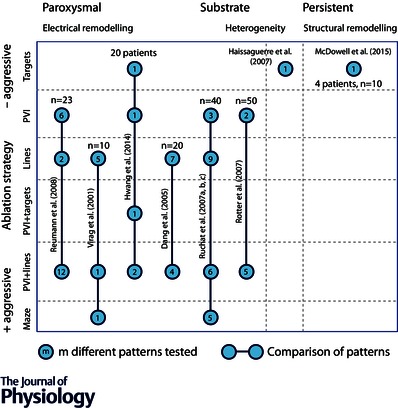
The horizontal axis symbolises the evolution of the substrate from paroxysmal (left) to persistent/permanent AF (right) and the vertical axis corresponds to the type of ablation patterns with increasing complexity (from top to bottom). Each circle represents a family of tested patterns. The number of patterns in each family is written within the circle. The lines connecting the circles indicate that a statistical comparison between the patterns has been performed. The value of n is the number of AF episodes simulated per group. The number of patients is reported in the case of patient‐specific models. PVI: pulmonary vein isolation.
Pulmonary vein isolation (PVI)
Ectopic foci can be simulated by periodically injecting current in a group of cells (Gong et al. 2007). Reumann et al. (2008) investigated the prevention of AF recurrence by three variants of PVI in a model with ectopic foci. As expected, perfect isolation of the sources prevented AF initiation (up to 100% success rate), while non‐transmural lesion created exit points for wavelets. This illustrates the difficulty in achieving a modelling equivalent of a double‐blind study. The modeller necessarily knows the exact location of the foci and atrial wall thickness, and of course in the model no new source could emerge elsewhere later. The outcome depended a lot on how much anatomical information was used to design ablation lines (location and depth). The lesson is that clinical success might be improved if these data were available.
AF termination by PVI was assessed in multiple‐wavelet AF models simulated in a structurally uniform substrate with remodelled membrane properties (Virag et al. 2002; Gong et al. 2007; Kharche et al. 2007). Ionic channels targeted for remodelling were typically I CaL, I to, I Kr and I Kur (Courtemanche et al. 1999; Pandit et al. 2005). Regional or fibre bundle‐specific repolarisation heterogeneities may be introduced to provide additional pathways for functional reentry (Jacquemet et al. 2005; Seemann et al. 2006; Colman et al. 2013). In these models, PVI terminated AF in 25% of the cases in Hwang et al. (2014), 20–55% in Ruchat et al. (2007 c) and 28–50% in Rotter et al. (2007), with higher success when isolated regions were larger. AF prevention was not studied. These low success rates suggest that AF termination and prevention of recurrence should be investigated separately since independent mechanisms may be involved. This also questions the use of multiple‐wavelet AF as a paradigm for paroxysmal AF. Note that success rates might have been better if longer periods had been simulated (instead of 3.5–30 s).
Linear ablation
The first simulation studies of ablation used a multiple‐wavelet AF model to test over a dozen clinically used patterns resulting from successive addition of lines to PVI until it formed a complete maze (Virag et al. 2001; Dang et al. 2005; Ruchat et al. 2007 c). These studies consistently indicated the superiority of more extensive ablation patterns and the importance of combining right and left lines. The addition of a roof line and a posterior mitral line after PVI improved AF termination rates from 25% to 55% (Hwang et al. 2014) and decreased the conversion to left atrial flutter (Rotter et al. 2007). Right lines were needed where AF originated from the right atrium. This occurs in a minority of patients (Vincenti et al. 2006) and was explained in the model by an AF perpetuation mechanism relying on short‐lived reentries in the free wall of the right atrium. Incomplete ablation lines sometimes led to atypical flutter as in the clinic (Jais et al. 2000). The lessons are that if the substrate is uniform, (1) more aggressive strategies have higher success rates, and (2) ablation is more effective when ablation lines uniformly cover the whole atrial surface. In a two‐dimensional simulation study, Spector et al. (2012) attributed this effect to the increased border length to tissue area ratio. Note that simulation results are relevant only to patients with AF mechanisms and atrial anatomical structure similar to the model used, which may be a small subgroup of AF patients.
The results of initial model‐based analyses of linear ablation attracted the attention of clinicians who used the approach to test the idea of simplifying the Maze III procedure (Ruchat et al. 2007 b) and then validated the computer‐derived reduced maze clinically (Ruchat et al. 2007 a). This was the first translational example where modelling stimulated the brain‐storming phase of the design of a new ablation pattern.
A limitation in the design of these studies is that a wide range of patterns from PVI to complete maze were tested in the same substrate, while these patterns are clinically applied to different types of AF (paroxysmal or persistent).
Ectopic foci
Provided that ectopic sources not isolated by PVI can be localised, they can be successively ablated. In a computer model of AF with repolarisation heterogeneities driven by eight active focal sources, Haissaguerre et al. (2007) monitored the AF cycle length along the progression of stepwise ablation of these sources. Each source ablation tended to prolong the cycle length until sinus rhythm was restored. The simulation study demonstrated that after suppression of a high frequency source, a previously masked lower frequency source may take control. The same time course of cycle length was observed in clinical recordings, although clinicians cannot guarantee that a source has effectively been removed (Haissaguerre et al. 2007). The lesson is that computer models can be used to determine what the outcome would be if the ablation strategy was perfectly executed, reinforcing the need for improved signal processing tools for ectopic focus detection.
Complex fractionated atrial electrograms
Complex electrogram waveforms can be reproduced by introducing structural heterogeneities (Ellis et al. 1995; Jacquemet & Henriquez, 2009; Campos et al. 2013). In contrast, Hwang et al. (2014) identified CFAE in a uniform model of the left atrium. In one of the very few attempts to simulate a CFAE ablation protocol, they ablated the CFAE regions in the model after PVI. Consistent with recent clinical findings (Verma et al. 2015), this additional intervention did not improve success rate. The nature of the substrate (uniform conduction) might have taken precedence over the apparent electrical disturbances caused by the complex AF dynamics. The lesson is that the signal processing techniques used to identify the targets (both clinical and simulated) add another level of complexity and further uncertainties that may interfere with the interpretation of AF mechanisms. This study should be repeated in a structurally remodelled substrate to improve clinical relevance.
Rotors
Atrial models have been used by a number of groups to investigate the effects of fibrosis and myofibre disarray on rotor formation (McDowell et al. 2012; Gonzales et al. 2014; Krueger et al. 2014). All incorporated patient‐specific atrial geometry and representative myofibre arrangement, but fibrosis was either introduced explicitly from late gadolinium‐enhanced MRI (McDowell et al. 2012; Krueger et al. 2014) or simulated as inexcitable regions (Gonzales et al. 2014). In these studies, rotors were sustained and stabilised by fibrosis. However, McDowell et al. (2013) needed to include electrical coupling between myofibroblasts and myocytes, in addition to the conduction barriers created by collagen deposition, to replicate arrhythmogenesis. They also demonstrated that when cores of stable rotors were ablated, AF was no longer inducible (McDowell et al. 2015). This suggests that the distribution of fibrosis determines the location of rotors as well as targets for ablation. It remains to be seen whether the optimal target might be identified from imaging data alone (without simulations). An advantage of such models of structurally remodelled tissue is that the key parameter (the distribution of fibrosis) can be extracted to some extent from patient data.
Ganglionated plexi
The incorporation of an acetylcholine (ACh)‐modulated K+ current (Kneller et al. 2002) enabled the simulation of ACh‐induced repolarisation heterogeneity and its influence on AF vulnerability (Vigmond et al. 2004). A new atrial membrane model has been proposed to simulate ACh and β‐adrenergic challenges (Grandi et al. 2011). Matene et al. (2014) investigated the changes in atrial dynamics resulting from progressive time‐dependent variations in local ACh concentration, including the occurrence of self‐termination of a reentry following the elimination of vagal stimulation. This study may be relevant to the ablation of neurogenic targets, but much remains to be done in this area in terms of modelling.
Discussion and perspectives
Current state of research
The years 2000–2007 may be viewed as a successful first iteration of AF ablation modelling research. With the development of more realistic atrial models, it became possible to address specific clinical questions about the efficacy of ablation patterns. During this period, however, AF models based on the multiple wavelets hypothesis in a uniform tissue were challenged by new data and concepts (Efimov & Fedorov, 2005). A second iteration has followed. This has been built on more realistic representations of pathophysiological atrial substrates, including arrhythmogenic ion‐channel remodelling (Colman et al. 2013), anisotropic conduction (Krueger et al. 2013), as well as structural remodelling and fibrosis (McDowell et al. 2012). Meanwhile, AF treatment has evolved (Calkins et al. 2012), with the establishment of PVI as a basic approach and the successive advent of sometimes controversial new targets such as CFAE and rotors. Modelling research is now slowly catching up with state‐of‐the‐art AF management and has the capacity to contribute to it.
Future directions
Although most ablation strategies have now been modelled, Fig. 1 reveals a large knowledge gap (bottom right). The majority of simulation studies have used models that relate best to paroxysmal AF, because they do not incorporate heterogeneous electrical properties and structural remodelling. However, both factors intensify during persistent AF and PVI alone is least successful in this patient group. This calls for a systematic comparison of all strategies (PVI and adjuvant targets) in terms of both AF termination and prevention in a sequence of substrates that correspond to the progression of AF. Despite the limited spatial resolution of clinical late‐gadolinium MRI, the Utah staging system for fibrosis provides a tool to move in that direction (McDowell et al. 2015). Computational ablation analysis has two major advantages in this setting: the capacity to compare many ablation patterns and the ability to specify the substrate and draw substrate‐specific or mechanism‐specific conclusions.
In parallel, further advances in modelling the arrhythmogenic substrate of AF are needed, notably on the effect of the autonomic nervous system in paroxysmal AF and the effect of fibrosis, microstructure and layer dissociation in persistent AF. The triggering mechanisms are of particular importance to appropriately simulate the prevention of AF recurrence. These advances in substrate modelling will contribute to the debate on rotors vs. multiple wavelets by determining the tissue conditions under which each type of AF dynamics can occur and by identifying the critical pathways of reentry in relation to the substrate. An ensemble of models with random distribution of parameters around pathophysiological values may be used to reproduce inter‐patient variability and assess the robustness of the results (Sanchez et al. 2014).
The disappointing outcome of the Star AF II trial (Verma et al. 2015) and poor correlation between CFAE and AF sources (Narayan et al. 2013) suggest that the criteria for electrogram morphology analysis should be at least rethought. Modelling electrical mapping systems (Sabouri et al. 2014) during AF might contribute to better signal processing techniques for characterising regions in which reentry occurs. In combination with imaging‐based fibrosis detection, this could further improve the identification of ablation targets.
Obstacles to patient‐specific modelling
The ultimate goal would be to create patient‐specific models and determine in silico the optimal treatment for each patient (Winslow et al. 2012). Efficient construction of atrial models with patient‐specific geometry (Trayanova, 2014) is an important step toward this end in AF ablation. Nevertheless, one may humbly argue against overuse of the expression ‘patient‐specific modelling’. Limited accuracy in conduction properties determination corresponds to inaccurate geometry reconstruction. Indeed, a local reduction in conductivity by a factor k is equivalent to a local dilatation by a factor √k. What matters is the timing of depolarisation (Young & Panfilov, 2010) and repolarisation, which are known at only limited spatial resolution. Any uncertainty in action potential duration becomes an uncertainty in wavelength, the critical pathway length for reentry (Jacquemet et al. 2005; Krogh‐Madsen et al. 2012). Another problem is accounting for the role of microstructure in arrhythmogenesis (Hubbard & Henriquez, 2014). It is difficult to identify structural heterogeneities at scales <1 mm using existing clinical imaging modalities, but emerging technologies might alleviate this limitation (Lasher et al. 2009; Xu et al. 2014). An interim approach is to create retrospective patient‐specific models from explanted human hearts. Integrated 3‐D structural and functional mapping of ex vivo human atria at the sub‐millimetre scale (Hansen et al. 2015) may open the way to more accurate, high‐resolution modelling of diseased human tissue (including action potential waveforms derived from optical mapping, but lacking autonomic regulation) and potentially fill the gap between clinical and basic research.
Conclusion
Atrial models are not intended to predict the population‐based success rate of a therapy, but rather to discover whether (and why) the therapy succeeds or fails in a specific AF substrate. In the case of failure, it is possible to ‘click on the cancel button’ and try another strategy until a successful one is found. Another key advantage is the ability to differentiate between ‘incompletely burning the target’, ‘missing the target’ and ‘trying to burn the wrong target’, which all result in a similar clinical failure although the actual problem may implicate catheter technology, signal processing or cardiac pathophysiology. Modelling research is expected to reduce the ‘learning by burning’ approach by providing an objective basis for the design of ablation patterns. Most of the learning so far has been by the scientists and engineers developing models; hopefully, future lessons will inform clinical cardiac electrophysiologists.
Additional information
Competing interests
None declared.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC grant RGPIN‐2015‐05658).
Biography
Vincent Jacquemet obtained an MSc in physics and a PhD in biomedical engineering at École Polytechnique Fédérale de Lausanne in Switzerland. After postdoctoral studies at Duke University, he moved to Montreal where he is currently Associate Professor at Université de Montréal in the Department of Molecular and Integrative Physiology and in the research centre of the Sacré‐Coeur Hospital. His research interests include biophysical modelling, cardiac electrophysiology and signal processing.

This review was presented at the symposium “Cardiac Arrhythmias: Challenges for Diagnosis and Treatment. A symposium in honour of George Ralph Mines (1886–1914)”, which took place at McGill University, Montreal, QC, Canada, between 6–7 November 2014.
References
- Andrade J, Khairy P, Dobrev D & Nattel S (2014). The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 114, 1453–1468. [DOI] [PubMed] [Google Scholar]
- Aslanidi OV, Colman MA, Stott J, Dobrzynski H, Boyett MR, Holden AV & Zhang H (2011). 3D virtual human atria: A computational platform for studying clinical atrial fibrillation. Prog Biophys Mol Biol 107, 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanidi OV, Nikolaidou T, Zhao J, Smaill BH, Gilbert SH, Holden AV, Lowe T, Withers PJ, Stephenson RS, Jarvis JC, Hancox JC, Boyett MR & Zhang H (2013). Application of micro‐computed tomography with iodine staining to cardiac imaging, segmentation, and computational model development. IEEE Trans Med Imaging 32, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi P, Zeemering S, Karel JM, Di Marco LY, Uldry L, Van Zaen J, Vesin JM & Schotten U (2015). Systematic comparison of non‐invasive measures for the assessment of atrial fibrillation complexity: a step forward towards standardization of atrial fibrillation electrogram analysis. Europace 17, 318–325. [DOI] [PubMed] [Google Scholar]
- Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J, Mandapati R, Ellenbogen KA & Shivkumar K (2015). Long‐term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: A multicenter experience. Heart Rhythm DOI: 10.1016/j.hrthm.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM & Wilber D; Heart Rhythm Society Task Force on Catheter and Surgical Ablation of Atrial Fibrillation (2012). 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 9, 632–696 e621. [DOI] [PubMed] [Google Scholar]
- Campos FO, Wiener T, Prassl AJ, dos Santos RW, Sanchez‐Quintana D, Ahammer H, Plank G & Hofer E (2013). Electroanatomical characterization of atrial microfibrosis in a histologically detailed computer model. IEEE Trans Biomed Eng 60, 2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman MA, Aslanidi OV, Kharche S, Boyett MR, Garratt C, Hancox JC & Zhang H (2013). Pro‐arrhythmogenic effects of atrial fibrillation‐induced electrical remodelling: insights from the three‐dimensional virtual human atria. J Physiol 591, 4249–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coumel P (1994). Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur Heart J 15 (Suppl. A), 9–16. [DOI] [PubMed] [Google Scholar]
- Courtemanche M, Ramirez RJ & Nattel S (1998). Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol 275, H301–H321. [DOI] [PubMed] [Google Scholar]
- Courtemanche M, Ramirez RJ & Nattel S (1999). Ionic targets for drug therapy and atrial fibrillation‐induced electrical remodeling: insights from a mathematical model. Cardiovasc Res 42, 477–489. [DOI] [PubMed] [Google Scholar]
- Cox JL, Schuessler RB, D'Agostino HJ Jr, Stone CM, Chang BC, Cain ME, Corr PB & Boineau JP (1991). The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 101, 569–583. [PubMed] [Google Scholar]
- Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ Jr, Li L & Rudy Y (2010). Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation 122, 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, Virag N, Ihara Z, Jacquemet V, Vesin JM, Schlaepfer J, Ruchat P & Kappenberger L (2005). Evaluation of ablation patterns using a biophysical model of atrial fibrillation. Ann Biomed Eng 33, 465–474. [DOI] [PubMed] [Google Scholar]
- Dossel O, Krueger MW, Weber FM, Wilhelms M & Seemann G (2012). Computational modeling of the human atrial anatomy and electrophysiology. Med Biol Eng Comput 50, 773–799. [DOI] [PubMed] [Google Scholar]
- Efimov IR & Fedorov VV (2005). Chessboard of atrial fibrillation: reentry or focus? Single or multiple source(s)? Neurogenic or myogenic? Am J Physiol Heart Circ Physiol 289, H977–979. [DOI] [PubMed] [Google Scholar]
- Ellis WS, Auslander DM & Lesh MD (1995). Fractionated electrograms from a computer model of heterogeneously uncoupled anisotropic ventricular myocardium. Circulation 92, 1619–1626. [DOI] [PubMed] [Google Scholar]
- Ernst S, Ouyang F, Lober F, Antz M & Kuck KH (2003). Catheter‐induced linear lesions in the left atrium in patients with atrial fibrillation: an electroanatomic study. J Am Coll Cardiol 42, 1271–1282. [DOI] [PubMed] [Google Scholar]
- Fareh S, Villemaire C & Nattel S (1998). Importance of refractoriness heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia‐induced atrial electrical remodeling. Circulation 98, 2202–2209. [DOI] [PubMed] [Google Scholar]
- Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts‐Thomson KC & Sanders P (2013). Long‐term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta‐analysis. J Am Heart Assoc 2, e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Xie F, Stein KM, Garfinkel A, Culianu CA, Lerman BB & Christini DJ (2007). Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: a simulation study. Circulation 115, 2094–2102. [DOI] [PubMed] [Google Scholar]
- Gonzales MJ, Sturgeon G, Krishnamurthy A, Hake J, Jonas R, Stark P, Rappel WJ, Narayan SM, Zhang Y, Segars WP & McCulloch AD (2013). A three‐dimensional finite element model of human atrial anatomy: new methods for cubic Hermite meshes with extraordinary vertices. Med Image Anal 17, 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales MJ, Vincent KP, Rappel WJ, Narayan SM & McCulloch AD (2014). Structural contributions to fibrillatory rotors in a patient‐derived computational model of the atria. Europace 16 (Suppl. 4), iv3–iv10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J & Bers DM (2011). Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res 109, 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P & Dubois R (2014). Driver domains in persistent atrial fibrillation. Circulation 130, 530–538. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P & Clementy J (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339, 659–666. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Lim KT, Jacquemet V, Rotter M, Dang L, Hocini M, Matsuo S, Knecht S, Jais P & Virag N (2007). Atrial fibrillatory cycle length: computer simulation and potential clinical importance. Europace 9 (Suppl. 6), vi64–70. [DOI] [PubMed] [Google Scholar]
- Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD & Fedorov VV (2015). Atrial fibrillation driven by micro‐anatomic intramural re‐entry revealed by simultaneous sub‐epicardial and sub‐endocardial optical mapping in explanted human hearts. Eur Heart J 36, 2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrild D & Henriquez C (2000). A computer model of normal conduction in the human atria. Circ Res 87, 25. [DOI] [PubMed] [Google Scholar]
- Herlin A & Jacquemet V (2011). Eikonal‐based initiation of fibrillatory activity in thin‐walled cardiac propagation models. Chaos 21, 043136. [DOI] [PubMed] [Google Scholar]
- Hubbard ML & Henriquez CS (2014). A microstructural model of reentry arising from focal breakthrough at sites of source‐load mismatch in a central region of slow conduction. Am J Physiol Heart Circ Physiol 306, H1341–H1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang M, Kwon SS, Wi J, Park M, Lee HS, Park JS, Lee YS, Shim EB & Pak HN (2014). Virtual ablation for atrial fibrillation in personalized in‐silico three‐dimensional left atrial modeling: comparison with clinical catheter ablation. Prog Biophys Mol Biol 116, 40–47. [DOI] [PubMed] [Google Scholar]
- Jacquemet V & Henriquez CS (2009). Genesis of complex fractionated atrial electrograms in zones of slow conduction: a computer model of microfibrosis. Heart Rhythm 6, 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemet V, Kappenberger L & Henriquez CS (2008). Modeling atrial arrhythmias: Impact on clinical diagnosis and therapies. IEEE Rev Biomed Eng 1, 94–114. [DOI] [PubMed] [Google Scholar]
- Jacquemet V, van Oosterom A, Vesin JM & Kappenberger L (2006). Analysis of electrocardiograms during atrial fibrillation. A biophysical model approach. IEEE Eng Med Biol Mag 25, 79–88. [DOI] [PubMed] [Google Scholar]
- Jacquemet V, Virag N & Kappenberger L (2005). Wavelength and vulnerability to atrial fibrillation: Insights from a computer model of human atria. Europace 7 (Suppl. 2), 83–92. [DOI] [PubMed] [Google Scholar]
- Jais P, Shah DC, Haissaguerre M, Hocini M, Peng JT, Takahashi A, Garrigue S, Le Metayer P & Clementy J (2000). Mapping and ablation of left atrial flutters. Circulation 101, 2928–2934. [DOI] [PubMed] [Google Scholar]
- Kharche S, Seemann G, Leng J, Holden AV, Garatt CJ & Zhang H (2007). Scroll waves in 3D virtual human atria: A computational study In Functional Imaging and Modeling of the Heart, ed. Sachse FB. & Seemann G, pp. 129–138. Springer, Berlin/Heidelberg. [Google Scholar]
- Kneller J, Zou R, Vigmond EJ, Wang Z, Leon LJ & Nattel S (2002). Cholinergic atrial fibrillation in a computer model of a two‐dimensional sheet of canine atrial cells with realistic ionic properties. Circ Res 90, 73. [DOI] [PubMed] [Google Scholar]
- Konings KT, Smeets JL, Penn OC, Wellens HJ & Allessie MA (1997). Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation 95, 1231–1241. [DOI] [PubMed] [Google Scholar]
- Krogh‐Madsen T, Abbott GW & Christini DJ (2012). Effects of electrical and structural remodeling on atrial fibrillation maintenance: a simulation study. PLoS Comput Biol 8, e1002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger M, Schmidt V, Tobón C, Weber F, Lorenz C, Keller D, Barschdorf H, Burdumy M, Neher P, Plank G, et al (2011). Modeling atrial fiber orientation in patient‐specific geometries: A semi‐automatic rule‐based approach In Functional Imaging and Modeling of the Heart, ed. Metaxas DN. & Axel L, pp. 223–232. Springer, Berlin/Heidelberg. [Google Scholar]
- Krueger MW, Rhode KS, O'Neill MD, Rinaldi CA, Gill J, Razavi R, Seemann G & Doessel O (2014). Patient‐specific modeling of atrial fibrosis increases the accuracy of sinus rhythm simulations and may explain maintenance of atrial fibrillation. J Electrocardiol 47, 324–328. [DOI] [PubMed] [Google Scholar]
- Krueger MW, Seemann G, Rhode K, Keller DU, Schilling C, Arujuna A, Gill J, O'Neill MD, Razavi R & Dossel O (2013). Personalization of atrial anatomy and electrophysiology as a basis for clinical modeling of radio‐frequency ablation of atrial fibrillation. IEEE Trans Med Imaging 32, 73–84. [DOI] [PubMed] [Google Scholar]
- Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, Trayanova NA & Narayan SM (2012). Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ Arrhythm Electrophysiol 5, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasher RA, Hitchcock RW & Sachse FB (2009). Towards modeling of cardiac micro‐structure with catheter‐based confocal microscopy: a novel approach for dye delivery and tissue characterization. IEEE Trans Med Imaging 28, 1156–1164. [DOI] [PubMed] [Google Scholar]
- Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM & Kalman JM (2014). Epicardial wave mapping in human long‐lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J 35, 86–97. [DOI] [PubMed] [Google Scholar]
- Lee SH, Tai CT, Hsieh MH, Tsao HM, Lin YJ, Chang SL, Huang JL, Lee KT, Chen YJ, Cheng JJ & Chen SA (2005). Predictors of non‐pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation: implication for catheter ablation. J Am Coll Cardiol 46, 1054–1059. [DOI] [PubMed] [Google Scholar]
- Levy S, Camm AJ, Saksena S, Aliot E, Breithardt G, Crijns H, Davies W, Kay N, Prystowsky E, Sutton R, Waldo A & Wyse DG; Working Group on Arrhythmias, Working Group on Cardiac Pacing of the European Society of Cardiology, North American Society of Pacing and Electrophysiology (2003). International consensus on nomenclature and classification of atrial fibrillation; a collaborative project of the Working Group on Arrhythmias and the Working Group on Cardiac Pacing of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Europace 5, 119–122. [DOI] [PubMed] [Google Scholar]
- Li WJ, Bai YY, Zhang HY, Tang RB, Miao CL, Sang CH, Yin XD, Dong JZ & Ma CS (2011). Additional ablation of complex fractionated atrial electrograms after pulmonary vein isolation in patients with atrial fibrillation: a meta‐analysis. Circ Arrhythm Electrophysiol 4, 143–148. [DOI] [PubMed] [Google Scholar]
- Lim PB, Malcolme‐Lawes LC, Stuber T, Wright I, Francis DP, Davies DW, Peters NS & Kanagaratnam P (2011). Intrinsic cardiac autonomic stimulation induces pulmonary vein ectopy and triggers atrial fibrillation in humans. J Cardiovasc Electrophysiol 22, 638–646. [DOI] [PubMed] [Google Scholar]
- Lin G, Lu HH, Shen Y, Huang JF, Shi LS & Guo YN (2013). Meta‐analysis of the therapeutic effects of various methods for the treatment of chronic atrial fibrillation. Exp Ther Med 6, 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WS, Tai CT, Hsieh MH, Tsai CF, Lin YK, Tsao HM, Huang JL, Yu WC, Yang SP, Ding YA, Chang MS & Chen SA (2003). Catheter ablation of paroxysmal atrial fibrillation initiated by non‐pulmonary vein ectopy. Circulation 107, 3176–3183. [DOI] [PubMed] [Google Scholar]
- Lu Z, Scherlag BJ, Lin J, Yu L, Guo JH, Niu G, Jackman WM, Lazzara R, Jiang H & Po SS (2009). Autonomic mechanism for initiation of rapid firing from atria and pulmonary veins: evidence by ablation of ganglionated plexi. Cardiovasc Res 84, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KS, Vadakkumpadan F, Blake R, Blauer J, Plank G, MacLeod RS & Trayanova NA (2012). Methodology for patient‐specific modeling of atrial fibrosis as a substrate for atrial fibrillation. J Electrocardiol 45, 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KS, Vadakkumpadan F, Blake R, Blauer J, Plank G, Macleod RS & Trayanova NA (2013). Mechanistic inquiry into the role of tissue remodeling in fibrotic lesions in human atrial fibrillation. Biophys J 104, 2764–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KS, Zahid S, Vadakkumpadan F, Blauer J, MacLeod RS & Trayanova NA (2015). Virtual electrophysiological study of atrial fibrillation in fibrotic remodeling. PLoS One 10, e0117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, Wilson B, Cates J, Harrison A, Ranjan R, Burgon NS, Greene T, Kim D, Dibella EV, Parker D, Macleod RS & Marrouche NF (2014). Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 7, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnani S, Muser D, Chik W & Santangeli P (2015). Adjunct ablation strategies for persistent atrial fibrillation–beyond pulmonary vein isolation. J Thorac Dis 7, 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matene E & Jacquemet V (2012). Fully automated initiation of simulated episodes of atrial arrhythmias. Europace 14 (Suppl. 5), v17–v24. [DOI] [PubMed] [Google Scholar]
- Matene E, Vinet A & Jacquemet V (2014). Dynamics of atrial arrhythmias modulated by time‐dependent acetylcholine concentration: a simulation study. Europace 16 (Suppl. 4), iv11–iv20. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Takigawa M, Kusa S, Kuwahara T, Taniguchi H, Okubo K, Nakamura H, Hachiya H, Hirao K, Takahashi A & Iesaka Y (2014). Role of arrhythmogenic superior vena cava on atrial fibrillation. J Cardiovasc Electrophysiol 25, 380–386. [DOI] [PubMed] [Google Scholar]
- Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C & Ngarmukos T (2004). A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 43, 2044–2053. [DOI] [PubMed] [Google Scholar]
- Nademanee K, Schwab MC, Kosar EM, Karwecki M, Moran MD, Visessook N, Michael AD & Ngarmukos T (2008). Clinical outcomes of catheter substrate ablation for high‐risk patients with atrial fibrillation. J Am Coll Cardiol 51, 843–849. [DOI] [PubMed] [Google Scholar]
- Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K & Miller JM (2014). Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow‐up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol 63, 1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Krummen DE & Rappel WJ (2012). Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol 23, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Shivkumar K, Krummen DE, Miller JM & Rappel WJ (2013). Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation: stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ Arrhythm Electrophysiol 6, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher P, Barschdorf H, Dries S, Weber FM, Krueger MW, Dössel O & Lorenz C (2011). Automatic segmentation of cardiac CTs ‐ Personalized atrial models augmented with electrophysiological structures In Functional Imaging and Modeling of the Heart, ed. Metaxas DN. & Axel L, pp. 80–87. Springer, Berlin/Heidelberg. [Google Scholar]
- O'Neill MD, Wright M, Knecht S, Jais P, Hocini M, Takahashi Y, Jonsson A, Sacher F, Matsuo S, Lim KT, Arantes L, Derval N, Lellouche N, Nault I, Bordachar P, Clementy J & Haissaguerre M (2009). Long‐term follow‐up of persistent atrial fibrillation ablation using termination as a procedural endpoint. Eur Heart J 30, 1105–1112. [DOI] [PubMed] [Google Scholar]
- Pandit SV, Berenfeld O, Anumonwo JMB, Zaritski RM, Kneller J, Nattel S & Jalife J (2005). Ionic determinants of functional reentry in a 2‐D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J 88, 3806–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platonov PG, Mitrofanova LB, Chireikin LV & Olsson SB (2002). Morphology of inter‐atrial conduction routes in patients with atrial fibrillation. Europace 4, 183–192. [DOI] [PubMed] [Google Scholar]
- Plonsey R & Barr RC (2000). Bioelectricity: A Quantitative Approach. Kluwer Academic Plenum Publishers, New York. [Google Scholar]
- Potse M, Dube B, Richer J, Vinet A & Gulrajani RM (2006). A comparison of monodomain and bidomain reaction‐diffusion models for action potential propagation in the human heart. IEEE Trans Biomed Eng 53, 2425–2435. [DOI] [PubMed] [Google Scholar]
- Qu Z (2006). Critical mass hypothesis revisited: role of dynamical wave stability in spontaneous termination of cardiac fibrillation. Am J Physiol Heart Circ Physiol 290, H255–H263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez RJ, Nattel S & Courtemanche M (2000). Mathematical analysis of canine atrial action potentials: rate, regional factors, and electrical remodeling. Am J Physiol Heart Circ Physiol 279, 1767. [DOI] [PubMed] [Google Scholar]
- Reumann M, Bohnert J, Seemann G, Osswald B & Dossel O (2008). Preventive ablation strategies in a biophysical model of atrial fibrillation based on realistic anatomical data. IEEE Trans Biomed Eng 55, 399–406. [DOI] [PubMed] [Google Scholar]
- Rotter M, Dang L, Jacquemet V, Virag N, Kappenberger L & Haissaguerre M (2007). Impact of varying ablation patterns in a simulation model of persistent atrial fibrillation. Pacing Clin Electrophysiol 30, 314–321. [DOI] [PubMed] [Google Scholar]
- Ruchat P, Dang L, Schlaepfer J, Virag N, von Segesser LK & Kappenberger L (2007. a). Use of a biophysical model of atrial fibrillation in the interpretation of the outcome of surgical ablation procedures. Eur J Cardiothorac Surg 32, 90–95. [DOI] [PubMed] [Google Scholar]
- Ruchat P, Dang L, Virag N, Schlaepfer J, von Segesser LK & Kappenberger L (2007. b). A biophysical model of atrial fibrillation to define the appropriate ablation pattern in modified maze. Eur J Cardiothorac Surg 31, 65–69. [DOI] [PubMed] [Google Scholar]
- Ruchat P, Virag N, Dang L, Schlaepfer J, Pruvot E & Kappenberger L (2007. c). A biophysical model of atrial fibrillation ablation: what can a surgeon learn from a computer model? Europace 9 (Suppl. 6), 71. [DOI] [PubMed] [Google Scholar]
- Sabouri S, Matene E, Vinet A, Richer LP, Cardinal R, Armour JA, Page P, Kus T & Jacquemet V (2014). Simultaneous epicardial and noncontact endocardial mapping of the canine right atrium: simulation and experiment. PLoS One 9, e91165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Bueno‐Orovio A, Wettwer E, Loose S, Simon J, Ravens U, Pueyo E & Rodriguez B (2014). Inter‐subject variability in human atrial action potential in sinus rhythm versus chronic atrial fibrillation. PLoS One 9, e105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann G, Höper C, Sachse FB, Dössel O, Holden AV & Zhang H (2006). Heterogeneous three‐dimensional anatomical and electrophysiological model of human atria. Philos Trans A Math Phys Eng Sci 364, 1465–1481. [DOI] [PubMed] [Google Scholar]
- Spector P (2013). Principles of cardiac electric propagation and their implications for re‐entrant arrhythmias. Circ Arrhythm Electrophysiol 6, 655–661. [DOI] [PubMed] [Google Scholar]
- Spector PS, Correa de Sa DD, Tischler ES, Thompson NC, Habel N, Stinnett‐Donnelly J, Benson BE, Bielau P & Bates JH (2012). Ablation of multi‐wavelet re‐entry: general principles and in silico analyses. Europace 14 (Suppl. 5), v106–v111. [DOI] [PubMed] [Google Scholar]
- Stiles MK, John B, Wong CX, Kuklik P, Brooks AG, Lau DH, Dimitri H, Roberts‐Thomson KC, Wilson L, De Sciscio P, Young GD & Sanders P (2009). Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the ‘second factor’. J Am Coll Cardiol 53, 1182–1191. [DOI] [PubMed] [Google Scholar]
- Swarup V, Baykaner T, Rostamian A, Daubert JP, Hummel J, Krummen DE, Trikha R, Miller JM, Tomassoni GF & Narayan SM (2014). Stability of rotors and focal sources for human atrial fibrillation: focal impulse and rotor mapping (FIRM) of AF sources and fibrillatory conduction. J Cardiovasc Electrophysiol 25, 1284–1292. [DOI] [PubMed] [Google Scholar]
- Trayanova NA (2014). Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management. Circ Res 114, 1516–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheule S, Eckstein J, Linz D, Maesen B, Bidar E, Gharaviri A & Schotten U (2014). Role of endo‐epicardial dissociation of electrical activity and transmural conduction in the development of persistent atrial fibrillation. Prog Biophys Mol Biol 115, 173–185. [DOI] [PubMed] [Google Scholar]
- Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P & Sanders P; STAR AF II Investigators (2015). Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372, 1812–1822. [DOI] [PubMed] [Google Scholar]
- Vigmond EJ, Tsoi V, Kuo S, Arevalo H, Kneller J, Nattel S & Trayanova N (2004). The effect of vagally induced dispersion of action potential duration on atrial arrhythmogenesis. Heart Rhythm 1, 334–344. [DOI] [PubMed] [Google Scholar]
- Vincenti A, Brambilla R, Fumagalli MG, Merola R & Pedretti S (2006). Onset mechanism of paroxysmal atrial fibrillation detected by ambulatory Holter monitoring. Europace 8, 204–210. [DOI] [PubMed] [Google Scholar]
- Virag N, Blanc O, Eick O & Kappenberger L (2001). A computer model to test therapeutic interventions for atrial fibrillation In Computer Simulation and Experimental Assessment of Cardiac Electrophysiology, ed. Virag N, Blanc O. & Kappenberger L, pp. 139–144. Futura Publishing, Armonk, NY. [Google Scholar]
- Virag N, Jacquemet V, Henriquez CS, Zozor S, Blanc O, Vesin JM, Pruvot E & Kappenberger L (2002). Study of atrial arrhythmias in a computer model based on magnetic resonance images of human atria. Chaos 12, 754–763. [DOI] [PubMed] [Google Scholar]
- Virag N, Jacquemet V & Kappenberger L (2012). Modeling of atrial fibrillation In Cardiac Mapping, ed. Shenasa M, Hindricks G, Borggrefe M, Breithardt G. & Josephson ME, pp. 131–138. Wiley‐Blackwell, Oxford, UK. [Google Scholar]
- Wilhelms M, Hettmann H, Maleckar MM, Koivumaki JT, Dossel O & Seemann G (2012). Benchmarking electrophysiological models of human atrial myocytes. Front Physiol 3, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow RL, Trayanova N, Geman D & Miller MI (2012). Computational medicine: translating models to clinical care. Science Transl Med 4, 158rv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Gutbrod SR, Bonifas AP, Su Y, Sulkin MS, Lu N, Chung HJ, Jang KI, Liu Z, Ying M, Lu C, Webb RC, Kim JS, Laughner JI, Cheng H, Liu Y, Ameen A, Jeong JW, Kim GT, Huang Y, Efimov IR & Rogers JA (2014). 3D multifunctional integumentary membranes for spatiotemporal cardiac measurements and stimulation across the entire epicardium. Nat Commun 5, 3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RJ & Panfilov AV (2010). Anisotropy of wave propagation in the heart can be modeled by a Riemannian electrophysiological metric. Proc Natl Acad Sci USA 107, 15063–15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman JA & Peters NS (2014). The rotor revolution: conduction at the eye of the storm in atrial fibrillation. Circ Arrhythm Electrophysiol 7, 1230–1236. [DOI] [PubMed] [Google Scholar]
- Zhao J, Butters TD, Zhang H, Pullan AJ, LeGrice IJ, Sands GB & Smaill BH (2012). An image‐based model of atrial muscular architecture: effects of structural anisotropy on electrical activation. Circ Arrhythm Electrophysiol 5, 361–370. [DOI] [PubMed] [Google Scholar]
- Zhao J, Kharche SR, Hansen BJ, Csepe TA, Wang Y, Stiles MK & Fedorov VV (2015). Optimization of catheter ablation of atrial fibrillation: insights gained from clinically‐derived computer models. Int J Mol Sci 16, 10834–10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Hou Y & Yang S (2011). A meta‐analysis of the comparative efficacy of ablation for atrial fibrillation with and without ablation of the ganglionated plexi. Pacing Clin Electrophysiol 34, 1687–1694. [DOI] [PubMed] [Google Scholar]


