Abstract
The unpredictable nature and potentially catastrophic consequences of ventricular arrhythmias (VAs) have obligated physicians to search for therapies to prevent sudden cardiac death (SCD). At present, a low left ventricular ejection fraction (LVEF) has been used as a risk factor to predict SCD in patients with structural heart disease and has been consistently adopted as the predominant, and sometimes sole, indication for implantable cardioverter defibrillator (ICD) therapy. Although the ICD remains the mainstay life‐saving therapy for SCD, it does not modify the underlying arrhythmic substrate and may be associated with adverse effects from perioperative and long‐term complications. Preventative pharmacological therapy has been associated with limited benefits, but anti‐arrhythmic medications have significant side effects profiles. Catheter ablation of VAs has greatly evolved over the last few decades. Substrate mapping in sinus rhythm has allowed haemodynamically unstable VAs to be successfully treated. Both LVEF as an indication for ICD therapy and electro‐anatomical mapping for substrate modification identify static components of underlying myocardial arrhythmogenicity. They do not take into account dynamic factors, such as the mechanisms of arrhythmia initiation and development of new anatomical or functional lines of block, leading to the initiation and maintenance of VAs. Dynamic factors are difficult to evaluate and consequently are not routinely used in clinical practice to guide treatment. However, progress in the treatment of VAs should consider and integrate dynamic factors with static components to fully characterize the myocardial arrhythmic substrate.
Abbreviations
- APD
action potential duration
- EPS
electrophysiological study
- ICD
implantable cardioverter defibrillator
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- SCD
sudden cardiac death
- SHD
structural heart disease
- VA
ventricular arrhythmia
- VT
ventricular tachycardia
Introduction
The study of cardiac electrophysiology in the treatment of ventricular arrhythmias (VAs) presents interesting pathological, clinical and technical aspects. In this review, the various aspects of VAs will be discussed as a whole, as the complexities of the pathophysiological mechanisms underlying VAs relate directly to the clinical and technical aspects of their therapy.
From a clinical point of view, it is well known that VAs may represent life‐threatening events that can develop into fatal outcomes within a short period of time. Moreover, the occurrence of an event is unpredictable, sudden and often catastrophic. These characteristics make the prevention of sudden cardiac death (SCD), which according to recent data can be attributable to a malignant VA in up to 30–40% of cases (Cobb et al. 2002; Goldberger et al. 2014; Tomaselli, 2015), one of the most important issues in public health today. In recent years, the identification of ion channel mutations that may trigger fatal arrhythmias in the absence of structural heart disease (SHD) has been an important new area of research (Napolitano et al. 2012). Notably, the percentage of ion channel defects as detected in victims of SCD is significant, ranging between 4 and 30% in various studies (Napolitano et al. 2012). The definition of myocardial disease has been altered to include not only the mechanical impairment of the heart but also an electrical abnormality that is capable of impacting heart function (Maron et al. 2006; Thiene et al. 2008). As demonstrated by Myerburg & Kessler (1993), the number of SCD events per year is higher in the general population than in patients with identified risk factors, such as SHD.
Proposed mechanisms of arrhythmias in SHD
The presence of SHD, whether from an ischaemic or other aetiology, has been associated with a heightened risk of developing VAs and SCD. However, this correlation is not absolute; clinical experience has demonstrated that the mere presence of scar tissue inside healthy myocardium does not necessarily lead to arrhythmia. There may be unique electrophysiological properties that differentiate a myocardial scar that has a high risk of causing VAs. A thorough exploration of the arrhythmic mechanism may better guide clinical therapies. Ischaemic and non‐ischaemic cardiomyopathies present different anatomical and electrophysiological characteristics. Scar in non‐ischaemic cardiomyopathies is typically localized to the base of the heart, is more scattered and often involves an epicardial site. The scar in ischaemic cardiomyopathies is typically denser and more often accessible from the endocardium. The fibrosis of the myocardial scar may cause arrhythmias through different mechanisms. Fibrosis can unbalance the sink–source mechanism that is responsible for eliminating early after‐depolarizations induced by oxidative stress, allowing for a greater susceptibility to early after‐depolarizations and resulting triggered activity (Morita et al. 2014).
However, re‐entry remains the main arrhythmic mechanism described in patients with SHD. The mix of different degrees of functional cells within a scar interspersed with areas of fibrosis has led to the realization of circus movements of a cardiac impulse similar to that first described by Mines (1913) in a frog heart preparation.
The site of origin of VA is typically located at the border of a scar in 68% of cases and in a small percentage of cases (4%) located within the healthy myocardium (Verma et al. 2005). Notably, the onset of VAs can be linked not only to a structural alteration determined by the scar but also to functional heterogeneity in the surrounding healthy myocardium (Dan, 2010). This heterogeneity can influence the normal behaviour of the myocyte action potential duration (APD) restitution and may create further calcium overload. Spatial differences in APD and conduction velocity alternans may favour the occurrence of a re‐entry mechanism (Dan, 2010).
The presence of circuits involving deeper layers should confirm the over‐simplistic nature of the bi‐dimensional image of a re‐entry circuit (Comtois et al. 2005).
Conversely, the occurrence of rotating and spiral waves inside the wall of the myocardium are less familiar and more challenging for the electrophysiologist (Comtois et al. 2005). The correlation between re‐entry circuits and spiral waves needs to be fully elucidated (Pertsov et al. 1984).
Prior electrophysiological studies performed during cardiac surgery have revealed the occurrence of spiral and rotating waves within the myocardial wall during ventricular tachycardia (VT) (Pogwizd et al. 1992; Downar et al. 1995). A theoretical model of circular impulse propagation in a two‐dimensional homogeneous tissue had initially been proposed (Belakhovski, 1965) with subsequent demonstration of spiral waves in a non‐homogeneous medium (Krinskii, 1966). Finally, the development of spiral waves in an isolated preparation of cardiac tissue was established (Davidenko, 1993).
In canine hearts, the progression in the degree of delayed refractoriness from the periphery to the centre of the myocardial scar was shown to be required for functional re‐entry to develop and persist (Gough et al. 1985). Animal models have shown that the rotating waves of a spiral anchored to a fixed obstacle can resemble the wave propagation in a re‐entrant circuit (Isomura et al. 2008). Additionally, the drift or shift in the centre of the spiral wave has been correlated to a change in VT morphology (Boersma et al. 2002).
Furthermore, it seems necessary to consider the outbreak of spiral waves to explain the three‐dimensional propagation of ventricular arrhythmias. Wiener & Rosenblueth (1946) described a re‐entrant circuit occurring in two dimensions that assumes the shape of a spiral wave. Winfree (1989) proposed the term ‘scroll wave’ to describe the form of the re‐entry circuit propagating in three dimensions. Finally, stable rotors and multiple wavelets have been recently mapped during ventricular fibrillation in patients with and without SHD (Nash et al. 2006; Krummen et al. 2014).
ICD therapy in patients with SHD
The implantable cardioverter defibrillator (ICD) was developed in the 1970s by the physician Micheal Mirowski in response to the SCD of his friend (Mirowski et al. 1972). ICDs have become the mainstay therapy in patients with SCD and underlying SHD with almost 225,000 worldwide implants per year (Mond & Proclemer, 2011). As a result of clinical trials demonstrating a mortality benefit, the ICD is the cornerstone therapy for both secondary prevention (patients with prior sustained VAs or cardiac arrest) and primary prevention (patients without documented sustained VA or cardiac arrest, but at high risk based on clinical factors) of SCD (Tracy et al. 2013).
Clinical risk stratification
Until now, the principal clinical tools that have been shown to assist in risk stratification in susceptible patients to guide ICD therapy include an invasive electrophysiological study (EPS) and the measurement of left ventricular ejection function (LVEF).
The EPS uses ventricular pacing with programmed ventricular extra‐stimuli at critically timed intervals to assess VA inducibility (Thomas & Josephson, 2008). In patients with prior myocardial infarction (MI) and non‐sustained VT, EPS‐guided ICD therapy has been shown to reduce mortality in two clinical trials (Moss et al. 1996; Buxton et al. 1999, 2000). On the basis of these trials, VT inducibility at EPS is included in current guidelines as an indication for ICD therapy in patients with non‐sustained VT, prior MI and borderline LVEF (36–40%) (Tracy et al. 2013). In patients with syncope of undetermined origin, guidelines recommend ICD therapy in patients with clinically relevant, haemodynamically significant sustained VT or ventricular fibrillation induced at EPS (Tracy et al. 2013).
While the EPS continues to be used as a risk stratification tool in select cases, its value remains limited, particularly in patients without prior MI. Studies of EPS in patients with non‐ischaemic cardiomyopathy, and with hypertrophic cardiomyopathy have found it to have poor specificity and sensitivity for SCD prediction (Poll et al. 1986; Fananapazir et al. 1992). As such, an EPS is not indicated for guiding ICD therapy in patients with non‐ischaemic cardiomyopathy with no prior evidence of sustained VA (Tracy et al. 2013).
An EPS may be also performed non‐invasively through the ICD, the results of which have been shown to be predictive of further therapy requirements in subgroups of patients with SHD (Daubert et al. 2009). The non‐invasive EPS may also assess outcomes after VT ablation (Santangeli et al. 2014).
Among the clinical markers of SCD, the LVEF alone in patients with SHD has correlated well with an increased risk of SCD, as demonstrated in several randomized clinical trials (Moss et al. 2002; Bardy et al. 2005). A relatively arbitrary cut‐off value of an LVEF ≤30% or ≤35% was used as an inclusion criterion in ICD trials that demonstrated a reduction in mortality with prophylactic ICD implantation (Greenberg et al. 2004; Bardy et al. 2005). Guidelines have since recommended consideration of prophylactic ICD implantation in patients with an LVEF ≤35% (Tracy et al. 2013).
An imbalance of the autonomic nervous system has been linked to an increased risk of SCD; specifically, a decrease in vagal tone and an increase in sympathetic tone is pro‐arrhythmic (Wellens et al. 2014). However, the clinical value of such impairment has been limited by several factors including the presence of beta‐blockers, which may interfere with surrogate markers of autonomic function including basal heart rate and heart rate variability (Wellens et al. 2014). Moreover the assessment of autonomic nervous system impairment as a marker of SCD is more accurate if performed by provocative testing rather than at baseline.
Recent evidence has shown that heart rate turbulence and deceleration capacity detected by electrocardiographic monitoring are able to identify an increased risk of SCD in patients with ischaemic cardiomyopathy and preserved ejection fraction (Mäkikallio et al. 2005; Bauer et al. 2009).
In patients with ischaemic cardiomyopathy and depressed ejection fraction, QRS duration, particularly in the presence of a left bundle branch block, baroreflex sensitivity and T wave alternans are markers of increased risk for SCD (Wellens et al. 2014).
Microvolt T wave alternans, which express a dispersion of the repolarization process of the myocardium, have also been proposed as a marker of SCD (Bloomfield et al. 2006; Narayan, 2006). The published REFINE study (Exner et al. 2007) found that the combination of abnormal T wave alternans, impaired heart rate turbulence and LVEF <50% was predictive of cardiac death or resuscitated cardiac arrest. The ongoing REFINE‐ICD trial (ClinicalTrials.gov Identifier: NCT00673842) will assess the applicability of T wave alternans and heart rate turbulence (via 24 h Holter monitor) as risk markers in patients with prior MI who reside outside an ICD indication with an LVEF between 35 and 50%. Patients deemed at high risk by the Holter monitor will be randomized to ICD to determine if prophylactic device therapy can reduce mortality in MI survivors with better preserved LV function. Among biochemical markers, the presence of pro‐hormone brain natriuretic peptide has been associated with an increased risk of SCD in patients with ischaemic cardiomyopathy (Scott et al. 2009) and increased incidence of shocks in patients with ICDs (Verma et al. 2006).
Recently, an attempt at using imaging modalities to characterize the arrhythmogenic substrate has been made, specifically with the use of cardiac magnetic resonance imaging. Late gadolinium enhancement on cardiac magnetic resonance imaging has been correlated with the occurrence of VAs in patients with low ejection fraction, although its application as an arrhythmic stratification tool in clinical practice is difficult to implement (Scott et al. 2013).
Nuclear imaging techniques are able to detect alterations in autonomic innervation and have been shown to be markers of increased risk for SCD. Both single photon emission computed tomography (Jacobson et al. 2010) and positron emission tomography (Fallavollita et al. 2014) have been used to characterize arrhythmic risk in patients with ischaemic cardiomyopathy.
Drawbacks of ICD therapy
It is important to note the strain that ICDs have placed on the healthcare system. Despite their clear benefits, significant costs may be incurred at time of device implant and during follow‐up (Essebag & Eisenberg, 2003; Camm et al. 2007; Hlatky & Mark, 2007). Current data ascribe a 3.6–5% peri‐procedural and 2.5% long term complication rate for an ICD implant (Anderson, 2009; Peterson et al. 2009) with further challenges resulting from inappropriate and appropriate ICD shocks including increased anxiety, depression and an overall decreased quality of life (Germano et al. 2006; Manzoni et al. 2015). Moreover, malfunctions, advisories and recalls of ICDs are consistently reported with a detrimental impact on the quality of life in patients implanted with ICDs and lead to additional costs for the healthcare system (Maisel et al. 2001).
The introduction of algorithms for anti‐tachycardia pacing (Schaumann et al. 1998) and of less stringent programming for ICD therapy delivery (Moss et al. 2012) have greatly reduced the burden of unnecessary ICD shocks delivered. As will be discussed later, anti‐arrhythmic medication and catheter ablation can also reduce ICD shocks.
It has been found that ICD therapies are associated with adverse outcomes (Poole et al. 2008). A recent meta‐analysis performed by our group (Proietti et al. 2015) has demonstrated that following the delivery of a first appropriate ICD shock, the odds ratio (OR) for mortality was increased to 2.9 as compared to 1.6 following an inappropriate shock. The difference detected in the OR for mortality between appropriate and inappropriate shock suggests that the underlying arrhythmogenic substrate plays a pivotal role in determining the prognosis, while the direct damage caused by the shock per se appears less relevant. Notably, clinical variables such as ejection fraction and New York Heart Association (NYHA) class do not influence the association between ICD shocks and mortality. A similar conclusion can be drawn from a recent study that shows an increase in mortality in patients receiving ATP therapy (Kleemann et al. 2015).
Current data indicate that 14–18% of primary prevention patients should expect to receive an appropriate shock after 2 years of follow up, whereas 12–21% of these patients will receive an inappropriate ICD shock (Mishkin et al. 2009). At 1 year, 39 and 20% of secondary prevention patients will expect to receive an appropriate or inappropriate shock, respectively (Mishkin et al. 2009). It is important to determine the key differences between patients at higher risk as compared to those who will never require ICD therapy. Patients who are candidates for ICD implantation may be divided into two groups: those who will not have any ICD therapy, and those who will benefit from ICD therapy but may also benefit from a more aggressive approach in reducing the arrhythmogenic potential. The problem lies in our limited ability to determine in advance whether a given patient with current ICD indication will or will not ever require ICD therapy.
The limits of the ICD therapy are implicit in its nature. Although constituting a life‐saving therapy, ICDs do not have any effect on the underlying arrhythmic substrate. The ICD remains a critical part of current management, but must be considered a rescue therapy to terminate VAs and prevent SCD. However, adjunctive therapy aimed at modifying the underlying arrhythmogenic substrate is required to prevent the occurrence of VAs, using pharmacological therapy and/or interventional therapy such as ablation (Abstract Figure).
Pharmacological therapy
The use of pharmacological therapy in patients with SHD has been consistently demonstrated to be inferior to ICDs for the prevention of SCD (Wyse et al. 1997; Connolly et al. 2000; Greenberg et al. 2004; Bardy et al. 2005). Moreover, any potential benefits from anti‐arrhythmic medications may be outweighed by their associated adverse side effects. The CAST (Echt et al. 1991) and CAST II (Anon, 1992) trials assessing the efficacy of class IC drugs as compared to standard of care in patients with premature ventricular contractions and SHD were both terminated prematurely due to an increased mortality in the medical therapy arm. Similarly, the SWORD (Waldo et al. 1996) trial was terminated early because sotalol increased mortality in patients with left ventricular dysfunction and prior MI. Studies with amiodarone have also failed to reduce all‐cause mortality in patient subgroups at increased risk of SCD (Cairns et al. 1997; Julian et al. 1997; Bardy et al. 2005). Amiodarone is of limited efficacy in the long term because most patients eventually develop side effects, experience recurrence of VAs or die (Bokhari et al. 2004).
The OPTIC trial (Connolly et al. 2006) compared the ability of beta‐blockers alone or in combination with amiodarone, versus sotalol alone to reduce the occurrence of ICD shocks in patients with SHD. Although the combination of amiodarone with beta‐blockers was more effective, these patients experienced a significant increase of pharmacologically related adverse events. The OPTIC trial suggests that anti‐arrhythmic medication may be useful as an adjunct to ICD (to reduce arrhythmic events), but not effective as a substitute for ICD, as previously discussed.
Catheter ablation of ventricular arrhythmias
Catheter ablation has also been evaluated as adjunctive therapy in patients with ICD and documented VT. Two randomized trials, SMASH‐VT (Reddy et al. 2007) and VTach (Kuck et al. 2010), demonstrated that VT ablation reduces the burden of appropriate ICD therapy. Other studies have also found that ablation of VT reduces ICD shocks in patients with SHD (Calkins et al. 2000; Mallidi et al. 2011), and guidelines (Pedersen et al. 2014) recommend consideration of anti‐arrhythmic medication or ablation as adjunctive therapy for VT in patients with ICD. However, despite evidence of reduced VT recurrences with adjunctive ablation therapy, no impact on mortality has been shown. Furthermore, superiority of ablation over anti‐arrhythmic therapy has yet to be demonstrated in a randomized trial. The ongoing Ventricular tachycardia Ablation vs. eNhanced drug therapy In Structural Heart disease (VANISH) trial (ClinicalTrials.gov Identifier: NCT00905853) has been designed to compare increasing anti‐arrhythmic medication versus catheter ablation following failure of anti‐arrhythmic therapy. With a larger sample size and longer follow‐up time, the study will provide information on mortality as a secondary outcome.
Radiofrequency ablation of VAs is based on the understanding of the arrhythmic substrate to selectively target areas of myocardium in which these circuits originate.
For haemodynamically stable VT, mapping and ablation may be performed during VT, so‐called ‘entrainment mapping’ (Fig. 1). Alternatively, substrate mapping and ablation can be employed for those VTs that are haemodynamically unstable.
Figure 1. Entrainment with concealed fusion .
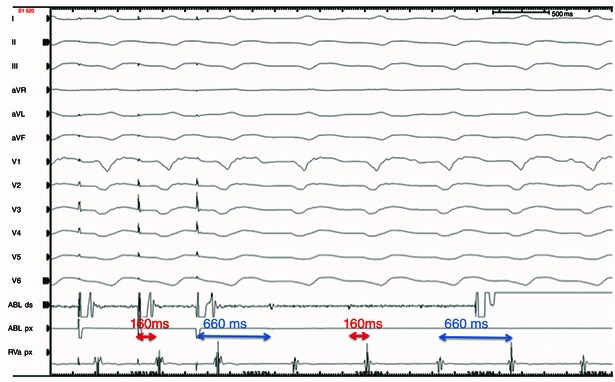
Overdrive pacing from the ablation catheter during arrhythmia shows entrainment with concealed fusion, which is consistent with a re‐entry mechanism of the VA. The same morphology of the paced QRS and spontaneous VA QRS indicates that the pacing catheter is positioned inside the VA circuit. The post pacing interval (distance from the pacing stimulus to the ventricular EGM in the pacing channel) is similar to VA cycle length of 660 ms, and the distance from the pacing stimulus to the onset of the QRS is the same as the distance from the spontaneous electrogram (EGM) to QRS. This indicates that the pacing ablation catheter is positioned within the central isthmus of the VA circuit. The above criteria together indicate that this is an optimal site for ablation and VA interruption.
The first strategy aims to map VT circuits within and adjacent to areas of scar through the use of overdrive ventricular pacing resulting in entrainment and fusion during VT (Almendral et al. 1986; Stevenson et al. 1993, 1997; Josephson et al. 2014). With modern three‐dimensional electro‐anatomical mapping systems, an activation map can be created to define the VT circuit and complement the entrainment mapping technique while the patient is actually in VT (Potse & Essebag, 2009). The entrainment mapping approach invokes re‐entry as the principal mechanism of VT and requires the VT to be haemodynamically tolerated.
The more common scenario is one in which the patient becomes rapidly hypotensive with decreased cerebral perfusion during VT or when multiple VT morphologies are present. Recently, a score based on clinical parameters has been proposed to identify patients at high risk of haemodynamic deterioration (Santangeli et al. 2015).
To improve the feasibility and safety of the procedure, substrate mapping and ablation may be performed during sinus rhythm. This approach uses a non‐fluoroscopic three‐dimensional mapping system that produces an electro‐anatomical reconstruction of the heart (Fig. 2). Based on voltage signals, areas of scar (low voltage) can be identified. Areas of delayed electrical activation (late potentials) can also be noted on the three‐dimensional electro‐anatomical map. Channels of viable cells within scar represented by low voltage fractionated and late potentials are considered markers of the VT circuit and are targeted with either scar homogenization in an effort to directly eliminate all fragmented activity, or with the use of ablation lines focused on electrically isolating scar from healthy myocardium (Marchlinski et al. 2000; Di Biase et al. 2012)
Figure 2. High‐resolution substrate map of the left ventricle performed in sinus rhythm, with a PentaRay® NAV (Biosense Webster) catheter that collects electro‐anatomical data simultaneously from 10 electrode pairs .
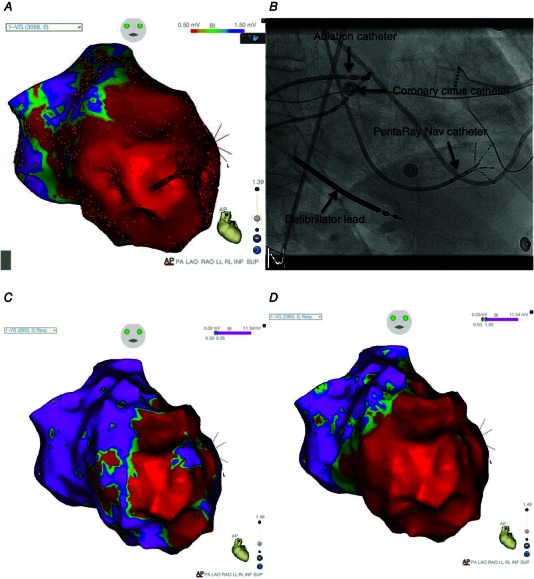
A, bipolar voltage map (0.5–1.5 mV) with the PentaRay NAV inside and the dots showing the points taken (more than 3000 points). The colour scale (from red to purple) depicts a large area of scar (voltage below 0.5 mV) in red, and areas of normal tissue (voltage above 1.5 mV) in purple. B, fluoroscopy image of the star‐shaped PentaRay NAV catheter mapping the lateral wall of the left ventricle. The five branches of the PentaRay NAV catheter are flexible and allow manipulation of the catheter inside the heart chamber. C, bipolar voltage map in same patient with settings of the voltage map adjusted (0.30–0.35 mV) to allow the identification of potential conducting channels inside the myocardial scar. Note these ‘channels’ in purple (voltage above 0.35 mV) now appearing within areas in A shown as scar (where setting displayed voltage <0.5 mV in red). D, this map in the same patient now uses the colour scale to display electrical activation (rather than voltage) of the same points collected in sinus rhythm. The area in red represents electrogram activation that occurs before the end of the QRS. The area in purple represents ‘late potentials’ occurring >10 ms after the end of the QRS. Note that some purple areas of ‘late potentials’ occur within purple voltage ‘channels’ (shown in C) and may be considered potential targets for substrate ablation.
Our group has demonstrated that a substrate ablation strategy alone for either haemodynamically tolerated or non‐tolerated VT can be successful (Proietti et al. 2014). In our experience this approach has a success rate of 66% arrhythmia‐free survival at 18 months follow‐up (Proietti et al. 2014). Our results are consistent with the findings reported by other centres and are more favourable in ischaemic than in non‐ischaemic VT.
The main limitation of substrate ablation is that it targets surrogate markers of the arrhythmic circuit as identified by channels of viable cells represented as late or fragmented potentials within or adjacent to a scar. However, the correlation between these surrogate markers detected in sinus rhythm and tachycardia circuits do not always correspond, and should be evaluated carefully. With current technology, substrate ablation may require ablation of myocardial tissue that would not otherwise be part of potential VT circuits in order to achieve the published procedural success rates. It is unclear whether such additional ablation can alter areas of myocardial fibrosis and cellular disarray in a way that may lead to areas of increased arrhythmogenesis.
A further limitation of a purely substrate‐based ablation approach is the limited ability to evaluate the potential dynamic or functional components linked to the behaviour of viable myocardial cells at the border or inside the scar that can play a role in the mechanism of VT initiation. Substrate ablation does not take into account this vital component and may not target myocardium that is relevant for arrhythmogenicity.
Future directions
As mentioned, one of the major challenges of catheter ablation for VT concerns the correct evaluation of surrogate markers. Attempts to re‐define the presence of fractionated electrical activity have been made whereby the Bordeaux group have described fragmented activities inside or delayed within the QRS (Jaïs et al. 2012).
Another approach is to perform an off‐line analysis capable of distinguishing signals relevant for the critical part of the VT circuit during sinus rhythm. Several authors have pursued further research into this strategy (Mountantonakis et al. 2013; Jamil‐Copley et al. 2014; Campos et al. 2015), which appears to be a promising direction that merits further exploration. Further analysis should clarify the role of dynamic components at time of onset and during maintenance of arrhythmias. Recently a ‘re‐entry vulnerability index’ has been proposed that can take into account these dynamic components including the heterogeneity during the repolarization and depolarization process of contiguous areas of the myocardium (Child et al. 2015).
A better understanding and definition of the involvement of occurrence of rotating activity involving the mid‐layer of the myocardial wall may improve the outcomes of VT ablation. Targeting this mid layer of the myocardial wall by catheter ablation is technically difficult. A needle able to penetrate into the myocardial wall is being developed that is able to penetrate arrhythmia circuits originating from the mid‐myocardium (Sapp et al. 2013).
Moreover, the need for epicardial ablation either alone or in conjunction with endocardial ablation is often described not only in non‐ischaemic VTs, but in ischaemic VTs as well. An epicardial approach requires particular expertise and can be associated with significant complications (Della Bella et al. 2011).
Navigation of the ablation catheter in the left ventricle is challenging due to the presence of complex intra‐cavitary structures such as papillary muscles. The use of intra‐cardiac echocardiography is useful in VT ablation. It improves the navigation of the catheter inside the ventricle but can also identify intra‐cavitary structures (Sadek et al. 2015) and epicardial region (Bala et al. 2011) that are sites of VT origin.
Recently, a star‐shaped catheter, PentaRay® NAV (Biosense Webster, South Diamond Bar, CA, USA), has been developed. The catheter, equipped with 20 bipolar electrodes located along five flexible branches, allows the relatively rapid creation of a high‐density map by navigation of the catheter inside the ventricle (Fig. 2). Moreover, the ability to pace from any electrodes of the catheter may provide information regarding the conduction velocity of the myocardium. Conduction velocity can potentially represent an additional parameter to include in the characterization of the underlying arrhythmogenic substrate and improve substrate ablation strategies.
Finally, even in cases where the target area has been identified, it can be difficult to reach and maintain stable contact between the tip of the catheter and the surface of the myocardium in order to deliver an effective lesion through the thickness of the left ventricular endocardium. For this reason, new technologies, including the ability to measure contact force (Kuck et al. 2012; Mizuno et al. 2013), are continuously being developed to address these challenges.
Conclusions
The unpredictable nature and potentially catastrophic consequences of VAs related to SHD have directed physicians to identify clinical tools to predict and prevent SCD. Low LVEF is the most reliable marker of SCD in patients with SHD and is used as an indication for ICD therapy. Although the ICD remains the mainstay therapy to prevent SCD, it can be associated with adverse events, and patients receiving appropriate ICD shocks have an increased risk of cardiac mortality.
The ICD is a lifesaving therapy but it does not modify the underlying arrhythmic substrate and propensity for VA onset. Preventative pharmacological anti‐arrhythmic therapy can be used, but may also cause serious adverse effects. Catheter ablation of VAs has greatly evolved to treat not only monomorphic, haemodynamically tolerated VT, but also polymorphic and haemodynamically unstable VT through substrate ablation guided by three‐dimensional electro‐anatomical mapping.
Both LVEF as an indication for ICD therapy and the electro‐anatomical map for substrate ablation identify static components of the underlying myocardial arrhythmogenicity and do not take into account the dynamic factors involved in the onset and maintenance of the VAs. These factors are difficult to assess and fully recognize. However, progress in the treatment of VAs should take into account these factors and integrate them with the static components in order to fully characterize the myocardial arrhythmic substrate.
Additional information
Competing interests
V.E. has received honoraria from Biosense‐Webster, Boston Scientific, Medtronic and St. Jude Medical. The remaining authors have no conflicts of interest.
Funding
V.E. is the recipient of a Clinician Scientist award from the Canadian Institutes of Health Research (CIHR).
Biographies
Riccardo Proietti, MD, PhD, graduated from the Catholic University of Rome in1997 and then specialized in cardiology at the University of Perugia. He achieved a PhD in Cardiovascular Science at the University of Padua and accomplished a fellowship in clinical electrophysiology at McGill University, Montreal. Since 2010, he has been a consultant cardiologist at Luigi Sacco Hospital of Milan, primarily focusing on cardiac pacing and electrophysiology. His research interests include mechanisms and therapy of cardiac arrhythmias.

Jacqueline Joza completed her cardiology training at McGill University and is in her final year of fellowship in cardiac electrophysiology at New York University Langone Medical Centre. She will be joining the division of cardiac electrophysiology at McGill University as an attending staff member in 2016. Her research focus is on atrial fibrillation and in the field of cardio‐genetics and understanding sudden cardiac death mechanisms in patients with inherited arrhythmias.
Dr Vidal Essebag completed his MD, cardiology training, MSc and PhD in epidemiology and biostatistics at McGill University. He subsequently completed a clinical and research fellowship in cardiac electrophysiology at Harvard University. He is currently Director of Cardiac Electrophysiology at the McGill University Health Centre, Associate Professor of Medicine at McGill University and a Clinician Scientist supported by the Canadian Institutes of Health Research (CIHR). His clinical research interests include ablation of atrial fibrillation and ventricular tachycardia, defibrillator and cardiac resynchronization therapy and peri‐operative anticoagulation management.
This review was presented at the symposium “Cardiac Arrhythmias: Challenges for Diagnosis and Treatment. A symposium in honour of George Ralph Mines (1886–1914)”, which took place at McGill University, Montreal, QC, Canada, between 6–7 November 2014.
References
- Almendral JM, Rosenthal ME, Stamato NJ, Marchlinski FE, Buxton AE, Frame LH, Miller JM & Josephson ME (1986). Analysis of the resetting phenomenon in sustained uniform ventricular tachycardia: incidence and relation to termination. J Am Coll Cardiol 8, 294–300. [DOI] [PubMed] [Google Scholar]
- Anderson KP (2009). Estimates of implantable cardioverter‐defibrillator complications: caveat emptor. Circulation 119, 1069–1071. [DOI] [PubMed] [Google Scholar]
- Anon (1992). Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. The Cardiac Arrhythmia Suppression Trial II Investigators. N Engl J Med 327, 227–233. [DOI] [PubMed] [Google Scholar]
- Bala R, Ren J‐F, Hutchinson MD, Desjardins B, Tschabrunn C, Gerstenfeld EP, Deo R, Dixit S, Garcia FC, Cooper J, Cooper J, Lin D, Riley MP, Tzou WS, Verdino R, Epstein AE, Callans DJ, Marchlinski FE (2011). Assessing epicardial substrate using intracardiac echocardiography during VT ablation. Circ Arrhythmia Electrophysiol 4, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM & Ip JH (2005). Amiodarone or an implantable cardioverter–defibrillator for congestive heart failure. N Engl J Med 352, 225–237. [DOI] [PubMed] [Google Scholar]
- Bauer A, Barthel P, Schneider R, Ulm K, Muller A, Joeinig A, Stich R, Kiviniemi A, Hnatkova K, Huikuri H, Schomig A, Malik M, Schmidt G, Müller A & Schömig A (2009). Improved stratification of autonomic regulation for risk prediction in post‐infarction patients with preserved left ventricular function (ISAR‐Risk). Eur Heart J 30, 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belakhovski IS (1965). Several modes of excitation movement in ideal excitable tissue. Biofizika 10, 1063–1067. [PubMed] [Google Scholar]
- Della Bella P, Brugada J, Zeppenfeld K, Merino J, Neuzil P, Maury P, Maccabelli G, Vergara P, F Baratto, Berruezo A, Wijnmaalen AP (2011). Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol 4, 653–659. [DOI] [PubMed] [Google Scholar]
- Di Biase L, Santangeli P, Burkhardt DJ, Bai R, Mohanty P, Carbucicchio C, Dello Russo A, Casella M, Mohanty S, Pump A, Hongo R, Beheiry S, Pelargonio G, Santarelli P, Zucchetti M, Horton R, Sanchez JE, Elayi CS, Lakkireddy D, Tondo C, Natale A (2012). Endo‐epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol 60, 132–141. [DOI] [PubMed] [Google Scholar]
- Bloomfield DM, Bigger JT, Steinman RC, Namerow PB, Parides MK, Curtis AB, Kaufman ES, Davidenko JM, Shinn TS & Fontaine JM (2006). Microvolt T‐wave alternans and the risk of death or sustained ventricular arrhythmias in patients with left ventricular dysfunction. J Am Coll Cardiol 47, 456–463. [DOI] [PubMed] [Google Scholar]
- Boersma L, Zetelaki Z, Brugada J & Allessie M (2002). Polymorphic reentrant ventricular tachycardia in the isolated rabbit heart studied by high‐density mapping. Circulation 105, 3053–3061. [DOI] [PubMed] [Google Scholar]
- Bokhari F, Newman D, Greene M, Korley V, Mangat I & Dorian P (2004). Long‐term comparison of the implantable cardioverter defibrillator versus amiodarone: eleven‐year follow‐up of a subset of patients in the Canadian Implantable Defibrillator Study (CIDS). Circulation 110, 112–116. [DOI] [PubMed] [Google Scholar]
- Buxton AE, Lee KL, DiCarlo L, Gold MR, Greer GS, Prystowsky EN, O'Toole MF, Tang A, Fisher JD, Coromilas J, Talajic M, Hafley G (2000). Electrophysiologic testing to identify patients with coronary artery disease who are at risk for sudden death. N Engl J Med 342, 1937–1945. [DOI] [PubMed] [Google Scholar]
- Buxton AE, Lee KL, Fisher JD, Josephson ME, Prystowsky EN & Hafley G (1999). A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med 341, 1882–1890. [DOI] [PubMed] [Google Scholar]
- Cairns JA, Connolly SJ, Roberts R & Gent M (1997). Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian Amiodarone Myocardial Infarction Arrhythmia Trial Investigators [published erratum appears in Lancet 19]. Lancet 349, 675–682. [DOI] [PubMed] [Google Scholar]
- Calkins H, Epstein A, Packer D, Arria AM, Hummel J, Gilligan DM, Trusso J, Carlson M, Luceri R, Kopelman H, Wilber D, Wharton JM, Stevenson W (2000). Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. J Am Coll Cardiol 35, 1905–1914. [DOI] [PubMed] [Google Scholar]
- Camm J, Klein H & Nisam S (2007). The cost of implantable defibrillators: Perceptions and reality. Eur Heart J 28, 392–397. [DOI] [PubMed] [Google Scholar]
- Campos B, Jauregui ME, Marchlinski FE, Dixit S & Gerstenfeld EP (2015). Use of a novel fragmentation map to identify the substrate for ventricular tachycardia in postinfarction cardiomyopathy. Heart Rhythm 12, 95–103. [DOI] [PubMed] [Google Scholar]
- Child N, Bishop MJ, Hanson B, Coronel R, Opthof T, Boukens BJ, Walton RD, Efimov IR, Bostock J, Hill Y, CA Rinaldi, Razavi R, Gill J & Taggart P (2015). An activation‐repolarization time metric to predict localized regions of high susceptibility to re‐entry. Heart Rhythm 12, 1644–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb LA, Fahrenbruch CE, Olsufka M & Copass MK (2002). Changing incidence of out‐of‐hospital ventricular fibrillation, 1980–2000. JAMA 288, 3008–3013. [DOI] [PubMed] [Google Scholar]
- Comtois P, Kneller J & Nattel S (2005). Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace 7, 10–20. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES, Thorpe K, Champagne J, Talajic M, Coutu B, Gronefeld GC & Hohnloser SH (2006). Comparison of beta‐blockers, amiodarone plus beta‐blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA 295, 165–171. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, Sheldon RS, Mitchell LB, Green MS, Klein GJ, O'Brien B (2000). Canadian Implantable Defibrillator Study (CIDS) a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 101, 1297–1302. [DOI] [PubMed] [Google Scholar]
- Dan GA (2010). The dynamic nature of ventricular tachycardia initiation. Drugs Today (Barc) 46, 777–783. [DOI] [PubMed] [Google Scholar]
- Daubert JP, Winters SL, Subačius H, Berger RD, Ellenbogen KA, Taylor SG, Schaechter A, Howard A & Kadish A (2009). Ventricular arrhythmia inducibility predicts subsequent ICD activation in nonischemic cardiomyopathy patients. Pacing Clin Electrophysiol 32, 755–761. [DOI] [PubMed] [Google Scholar]
- Davidenko JM (1993). Spiral wave activity: a possible common mechanism for polymorphic and monomorphic ventricular tachycardias. J Cardiovasc Electrophysiol 4, 730–746. [DOI] [PubMed] [Google Scholar]
- Downar E, Saito J, Doig JC, Chen TC, Sevaptsidis E, Masse S, Kimber S, Mickleborough L & Harris L (1995). Endocardial mapping of ventricular tachycardia in the intact human ventricle. III. Evidence of multiuse reentry with spontaneous and induced block in portions of reentrant path complex. J Am Coll Cardiol 25, 1591–1600. [DOI] [PubMed] [Google Scholar]
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias‐Manno D, Barker AH, Arensberg D, Baker A, Friedman L & Greene HL (1991). Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324, 781–788. [DOI] [PubMed] [Google Scholar]
- Essebag V & Eisenberg MJ (2003). Expanding indications for defibrillators after myocardial infarction: risk stratification and cost effectiveness. Card Electrophysiol Rev 7, 43–48. [DOI] [PubMed] [Google Scholar]
- Exner D V, Kavanagh KM, Slawnych MP, Mitchell LB, Ramadan D, Aggarwal SG, Noullett C, Van Schaik A, Mitchell RT, Shibata MA, Gulamhussein S, McMeekin J, Tymchak W, Schnell G, Gillis AM, Sheldon RS, Fick GH, Duff HJ; REFINE Investigators (2007). Noninvasive risk assessment early after a myocardial infarction: the REFINE study. J Am Coll Cardiol 50, 2275–2284. [DOI] [PubMed] [Google Scholar]
- Fallavollita JA, Heavey BM, Luisi AJ, Michalek SM, Baldwa S, Mashtare TL, Hutson AD, Haka MS, Sajjad M, Cimato TR, Curtis AB, Cain ME, Canty JM (2014). Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol 63, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fananapazir L, Chang AC, Epstein SE & McAreavey D (1992). Prognostic determinants in hypertrophic cardiomyopathy. Prospective evaluation of a therapeutic strategy based on clinical, Holter, hemodynamic, and electrophysiological findings. Circulation 86, 730–740. [DOI] [PubMed] [Google Scholar]
- Germano JJ, Reynolds M, Essebag V & Josephson ME (2006). Frequency and causes of implantable cardioverter‐defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol 97, 1255–1261. [DOI] [PubMed] [Google Scholar]
- Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM Jr, Chen PS, Chugh SS, Costantini O, Exner DV, Kadish AH, Lee B, Lloyd‐Jones D, Moss AJ, Myerburg RJ, Olgin JE, Passman R, Stevenson WG, Tomaselli GF, Zareba W, Zipes DP, Zoloth L (2014). Risk stratification for sudden cardiac death: a plan for the future. Circulation 129, 516–526. [DOI] [PubMed] [Google Scholar]
- Gough WB, Mehra R, Restivo M, Zeiler RH & el‐Sherif N (1985). Reentrant ventricular arrhythmias in the late myocardial infarction period in the dog. 13. Correlation of activation and refractory maps. Circ Res 57, 432–442. [DOI] [PubMed] [Google Scholar]
- Greenberg H, Case RB, Moss AJ, Brown MW, Carroll ER & Andrews ML (2004). Analysis of mortality events in the multicenter automatic defibrillator implantation trial (MADIT‐II). J Am Coll Cardiol 43, 1459–1465. [DOI] [PubMed] [Google Scholar]
- Hlatky MA & Mark DB (2007). The high cost of implantable defibrillators. Eur Heart J 28, 388–391. [DOI] [PubMed] [Google Scholar]
- Isomura A, Hörning M, Agladze K & Yoshikawa K (2008). Eliminating spiral waves pinned to an anatomical obstacle in cardiac myocytes by high‐frequency stimuli. Phys Rev E Stat Nonlin Soft Matter Phys 78, 1–6. [DOI] [PubMed] [Google Scholar]
- Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H & Narula J (2010). Myocardial iodine‐123 meta‐iodobenzylguanidine imaging and cardiac events in heart failure: results of the prospective ADMIRE‐HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 55, 2212–2221. [DOI] [PubMed] [Google Scholar]
- Jaïs P, Maury P, Khairy P, Sacher F, Nault I, Komatsu Y, Hocini M, Forclaz A, Jadidi AS, Weerasooryia R, Shah A, Derval N, Cochet H, Knecht S, Miyazaki S, Linton N, Rivard L, Wright M, Wilton SB, Scherr D, Pascale P, Roten L, Pederson M, Bordachar P, Laurent F, Kim SJ, Ritter P, Clementy J, Haïssaguerre M (2012). Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar‐related ventricular tachycardia. Circulation 125, 2184–2196. [DOI] [PubMed] [Google Scholar]
- Jamil‐Copley S, Vergara P, Carbucicchio C, Linton N, Koa‐Wing M, Luther V, Francis DP, Peters NS, Davies DW, Tondo C, Bella PD & Kanagaratnam P (2014). Application of ripple mapping to visualize slow conduction channels within the infarct‐related left ventricular scar. Circ Arrhythmia Electrophysiol 8, 76–86. [DOI] [PubMed] [Google Scholar]
- Josephson ME, Almendral J & Callans DJ (2014). Resetting and entrainment of reentrant ventricular tachycardia associated with myocardial infarction. Heart Rhythm 11, 1239–1249. [DOI] [PubMed] [Google Scholar]
- Julian DG, Camm AJ, Frangin G, Janse MJ, Munoz A, Schwartz PJ & Simon P (1997). Randomised trial of effect of amiodarone on mortality in patients with left‐ventricular dysfunction after recent myocardial infarction: EMIAT. European Myocardial Infarct Amiodarone Trial Investigators. Lancet 349, 667–674. [DOI] [PubMed] [Google Scholar]
- Kleemann T, Strauss M, Kouraki K & Zahn R (2015). Clinical course and prognostic relevance of antitachycardia pacing‐terminated ventricular tachyarrhythmias in implantable cardioverter‐defibrillator patients. Europace 17, 1068–1075. [DOI] [PubMed] [Google Scholar]
- Krinskii VI (1966). Spread of excitation in an inhomogeneous medium (state similar to cardiac fibrillation). Biophys USSR 11, 776. [Google Scholar]
- Krummen DE, Hayase J, Morris DJ, Ho J, Smetak MR, Clopton P, Rappel W‐J & Narayan SM (2014). Rotor stability separates sustained ventricular fibrillation from self‐terminating episodes in humans. J Am Coll Cardiol 63, 2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuck KH, Reddy VY, Schmidt B, Natale A, Neuzil P, Saoudi N, Kautzner J, Herrera C, Hindricks G, Jaïs P, Nakagawa H, Lambert H, Shah DC (2012). A novel radiofrequency ablation catheter using contact force sensing: Toccata study. Heart Rhythm 9, 18–23. [DOI] [PubMed] [Google Scholar]
- Kuck K‐H, Schaumann A, Eckardt L, Willems S, Ventura R, Delacrétaz E, Pitschner H‐F, Kautzner J, Schumacher B & Hansen PS (2010). Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 375, 31–40. [DOI] [PubMed] [Google Scholar]
- Maisel WH, Sweeney MO, Stevenson WG, Ellison KE & Epstein LM (2001). Recalls and safety alerts involving pacemakers and implantable cardioverter‐defibrillator generators. JAMA 286, 793–799. [DOI] [PubMed] [Google Scholar]
- Mäkikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Schmidt G & Huikuri HV (2005). Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J 26, 762–769. [DOI] [PubMed] [Google Scholar]
- Mallidi J, Nadkarni GN, Berger RD, Calkins H & Nazarian S (2011). Meta‐analysis of catheter ablation as an adjunct to medical therapy for treatment of ventricular tachycardia in patients with structural heart disease. Heart Rhythm 8, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni GM, Castelnuovo G, Compare A, Pagnini F, Essebag V & Proietti R (2015). Psychological effects of implantable cardioverter defibrillator shocks. A review of study methods. Front Psychol 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlinski FE, Callans DJ, Gottlieb CD & Zado E (2000). Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 101, 1288–1296. [DOI] [PubMed] [Google Scholar]
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE & Young JB (2006). Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Function. Circulation 113, 1807–1816. [DOI] [PubMed] [Google Scholar]
- Mines GR (1913). On dynamic equilibrium in the heart. J Physiol 46, 349–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowski M, Mower MM, Staewen WS, Denniston RH & Mendeloff AI (1972). The development of the transvenous automatic defibrillator. Arch Intern Med 129, 773–779. [PubMed] [Google Scholar]
- Mishkin JD, Saxonhouse SJ, Woo GW, Burkart TA, Miles WM, Conti JB, Schofield RS, Sears SF & Aranda JM (2009). Appropriate evaluation and treatment of heart failure patients after implantable cardioverter‐defibrillator discharge. Time to go beyond the initial shock. J Am Coll Cardiol 54, 1993–2000. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Vergara P, Maccabelli G, Trevisi N, ENG SC, Brombin C, Mazzone P & Della Bella P (2013). Contact force monitoring for cardiac mapping in patients with ventricular tachycardia. J Cardiovasc Electrophysiol 24, 519–524. [DOI] [PubMed] [Google Scholar]
- Mond HG & Proclemer A (2011). The 11th world survey of cardiac pacing and implantable cardioverter‐defibrillators: Calendar year 2009 ‐ A world society of Arrhythmia's project. Pacing Clin Electrophysiol 34, 1013–1027. [DOI] [PubMed] [Google Scholar]
- Morita N, Mandel WJ, Kobayashi Y & Karagueuzian HS (2014). Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J Arrrhythm 30, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, Levine JH, Saksena S, Waldo AL, Wilber D, Brown MW, Heo M (1996). Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 335, 1933–1940. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NAM, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D & Zareba W (2012). Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 367, 2275–2283. [DOI] [PubMed] [Google Scholar]
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW & Andrews ML (2002). Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346, 877–883. [DOI] [PubMed] [Google Scholar]
- Mountantonakis SE, Park RE, Frankel DS, Hutchinson MD, Dixit S, Cooper J, Callans D, Marchlinski FE & Gerstenfeld EP (2013). Relationship between voltage map “channels” and the location of critical isthmus sites in patients with post‐infarction cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol 61, 2088–2095. [DOI] [PubMed] [Google Scholar]
- Myerburg RJ & Kessler KMCA (1993). Sudden cardiac death: epidemiology, transient risk, and intervention assessment. Ann Intern Med 119, 1187–1197. [DOI] [PubMed] [Google Scholar]
- Napolitano C, Bloise R, Monteforte N & Priori SG (2012). Sudden cardiac death and genetic ion channelopathies: long QT, Brugada, short QT, catecholaminergic polymorphic ventricular tachycardia, and idiopathic ventricular fibrillation. Circulation 125, 2027–2034. [DOI] [PubMed] [Google Scholar]
- Narayan SM (2006). T‐wave alternans and the susceptibility to ventricular arrhythmias. J Am Coll Cardiol 47, 269–281. [DOI] [PubMed] [Google Scholar]
- Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, Hayward M, Paterson DJ & Taggart P (2006). Evidence for multiple mechanisms in human ventricular fibrillation. Circulation 114, 536–542. [DOI] [PubMed] [Google Scholar]
- Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della‐Bella P, Dickfeld T, Dorian P, Huikuri H, Kim YH, Knight B, Marchlinski F, Ross D, Sacher F, Sapp J, Shivkumar K, Soejima K, Tada H, Alexander ME, Triedman JK, Yamada T, Kirchhof P, Lip GY, Kuck KH, Mont L, Haines D, Indik J, Dimarco J, Exner D, Iesaka Y, Savelieva I; EP‐Europace, UK (2014). EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Heart Rhythm 11, e166–e196. [DOI] [PubMed] [Google Scholar]
- Pertsov AM, Ermakova EA & Panfilov AV (1984). Rotating spiral waves in a modified Fitz‐Hugh‐Nagumo model. Phys D Nonlinear Phenom 14, 117–124. [Google Scholar]
- Peterson PN, Daugherty SL, Wang Y, Vidaillet HJ, Heidenreich PA, Curtis JP & Masoudi FA (2009). Gender differences in procedure‐related adverse events in patients receiving implantable cardioverter‐defibrillator therapy. Circulation 119, 1078–1084. [DOI] [PubMed] [Google Scholar]
- Pogwizd SM, Hoyt RH, Saffitz JE, Corr PB, Cox JL & Cain ME (1992). Reentrant and focal mechanisms underlying ventricular tachycardia in the human heart. Circulation 86, 1872–1887. [DOI] [PubMed] [Google Scholar]
- Poll DS, Marchlinski FE, Buxton AE & Josephson ME (1986). Usefulness of programmed stimulation in idiopathic dilated cardiomyopathy. Am J Cardiol 58, 992–997. [DOI] [PubMed] [Google Scholar]
- Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, Reddy RK, Marchlinski FE, Yee R, Guarnieri T, Talajic M, Wilber DJ, Fishbein DP, Packer DL, Mark DB, Lee KL, Bardy GH (2008). Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 359, 1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potse M & Essebag V (2009). Guidance for catheter ablation of ventricular arrhythmia. Med Biol Eng Comput 47, 241–243. [DOI] [PubMed] [Google Scholar]
- Proietti R, Essebag V, Beardsall J, Hache P, Pantano A, Wulffhart Z, Juta R, Tsang B, Joza J, Nascimento T, Pegoraro V, Khaykin Y & Verma A (2014). Substrate‐guided ablation of haemodynamically tolerated and untolerated ventricular tachycardia in patients with structural heart disease: effect of cardiomyopathy type and acute success on long‐term outcome. Europace 3, 461–467. [DOI] [PubMed] [Google Scholar]
- Proietti R, Labos C, Davis M, Thanassoulis G, Santangeli P, Russo V, Di Biase L, Roux J‐F, Verma A, Natale A & Essebag V (2015). A systematic review and meta‐analysis of the association between implantable cardioverter‐defibrillator shocks and long‐term mortality. Can J Cardiol 31, 270–277. [DOI] [PubMed] [Google Scholar]
- Reddy VY, Reynolds MR, Neuzil P, Richardson AW, Taborsky M, Jongnarangsin K, Kralovec S, Sediva L, Ruskin JN & Josephson ME (2007). Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med 357, 2657–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek MM, Benhayon D, Sureddi R, Chik W, Santangeli P, Supple GE, Hutchinson MD, Bala R, Carballeira L, Zado ES, Patel VV, Callans DJ, Marchlinski FE, Garcia FC (2015). Idiopathic ventricular arrhythmias originating from the moderator band: electrocardiographic characteristics and treatment by catheter ablation. Heart Rhythm 12, 67–75. [DOI] [PubMed] [Google Scholar]
- Santangeli P, Frankel DS & Marchlinski FE (2014). End points for ablation of scar‐related ventricular tachycardia. Circ Arrhythm Electrophysiol 7, 949–960. [DOI] [PubMed] [Google Scholar]
- Santangeli P, Muser D, Zado ES, Magnani S, Khetpal S, Hutchinson MD, Supple G, Frankel DS, Garcia FC, Bala R, Riley MP, Lin D, Rame JE, Schaller R, Dixit S, Marchlinski FE & Callans DJ (2015). Acute hemodynamic decompensation during catheter ablation of scar‐related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ Arrhythm Electrophysiol 8, 68–75. [DOI] [PubMed] [Google Scholar]
- Sapp JL, Beeckler C, Pike R, Parkash R, Gray CJ, Zeppenfeld K, Kuriachan V & Stevenson WG (2013). Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation 128, 2289–2295. [DOI] [PubMed] [Google Scholar]
- Schaumann A, von zur Mühlen F, Herse B, Gonska B‐D & Kreuzer H (1998). Empirical versus tested antitachycardia pacing in implantable cardioverter defibrillators a prospective study including 200 patients. Circulation 97, 66–74. [DOI] [PubMed] [Google Scholar]
- Scott PA, Rosengarten JA, Curzen NP & Morgan JM (2013). Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta‐analysis. Eur J Heart Fail 15, 1019–1027. [DOI] [PubMed] [Google Scholar]
- Scott PA, Barry J, Roberts PR & Morgan JM (2009). Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta‐analysis. Eur J Heart Fail 11, 958–966. [DOI] [PubMed] [Google Scholar]
- Stevenson WG, Friedman PL, Sager PT, Saxon LA, Kocovic D, Harada T, Wiener I & Khan H (1997). Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol 29, 1180–1189. [DOI] [PubMed] [Google Scholar]
- Stevenson WG, Khan H, Sager P, Saxon LA, Middlekauff HR, Natterson PD & Wiener I (1993). Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation 88, 1647–1670. [DOI] [PubMed] [Google Scholar]
- Thiene G, Corrado D & Basso C (2008). Revisiting definition and classification of cardiomyopathies in the era of molecular medicine. Eur Heart J 29, 144–146. [DOI] [PubMed] [Google Scholar]
- Thomas KE & Josephson ME (2008). The role of electrophysiology study in risk stratification of sudden cardiac death. Prog Cardiovasc Dis 51, 97–105. [DOI] [PubMed] [Google Scholar]
- Tomaselli GF (2015). Introduction to a compendium on sudden cardiac death: epidemiology, mechanisms, and management. Circ Res 116, 1883–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NAM, Ferguson TB, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG & Varosy PD (2013). 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device‐based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guide. J Am Coll Cardiol 61, e6–e75. [DOI] [PubMed] [Google Scholar]
- Verma A, Kilicaslan F, Martin DO, Minor S, Starling R, Marrouche NF, Almahammed S, Wazni OM, Duggal S, Zuzek R, Yamaji H, Cummings J, Chung MK, Tchou PJ, Natale A (2006). Preimplantation B‐type natriuretic peptide concentration is an independent predictor of future appropriate implantable defibrillator therapies. Heart 92, 190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Marrouche NF, Schweikert RA, Saliba W, Wazni O, Cummings J, Abdul‐Karim A, Bhargava M, Burkhardt JD, Kilicaslan F, Martin DO & Natale A (2005). Relationship between successful ablation sites and the scar border zone defined by substrate mapping for ventricular tachycardia post‐myocardial infarction. J Cardiovasc Electrophysiol 16, 465–471. [DOI] [PubMed] [Google Scholar]
- Waldo AL, Camm AJ, Friedman PL, MacNeil DJ, Pauls JF, Pitt B, Pratt CM, Schwartz PJ, Veltri EP (1996). Effect of d‐sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. Lancet 348, 7–12. [DOI] [PubMed] [Google Scholar]
- Wellens HJJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, Huikuri HV, Kääb S, La Rovere MT, Malik M, Myerburg RJ, Simoons ML, Swedberg K, Tijssen J, Voors AA & Wilde AA (2014). Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 35, 1642–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener N & Rosenblueth A (1946). The mathematical formulation of the problem of conduction of impulses in a network of connected excitable elements, specifically in cardiac muscle. Arch Inst Cardiol Mex 16, 205–265. [PubMed] [Google Scholar]
- Winfree AT (1989). Electrical instability in cardiac muscle: phase singularities and rotors. J Theor Biol 138, 353–405. [DOI] [PubMed] [Google Scholar]
- Wyse DG, Friedman PL & Epstein AE (1997). A comparison of antiarrhythmic‐drug therapy with implantable defibrillators in patients resuscitated from near‐fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med 337, 1576–1583. [DOI] [PubMed] [Google Scholar]


