Abstract
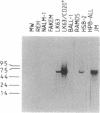
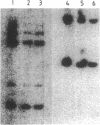
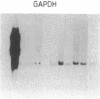
We describe the molecular cloning of a receptor tyrosine kinase from a cell line (LK63) derived from a case of human pre-B-cell leukemia. We have previously shown that a monoclonal antibody (IIIA4) raised against LK63 recognized a glycosylated, cell-surface 135-kDa molecule (HEK), which displayed tyrosine kinase activity in vitro. The HEK protein was purified by using a IIIA4 antibody column and both N-terminal and internal amino acid sequences were obtained. A 51-mer degenerate oligonucleotide based on the internal amino acid sequence was used to screen an LK63-derived lambda gt10 cDNA library under low-stringency hybridization conditions. One clone of 2.5 kilobases (kb) was isolated and characterized and used to rescreen the library under more-stringent hybridization conditions. A 4.5-kb clone containing the entire HEK coding region was isolated and its complete DNA sequence was determined. The 4.5-kb insert was subcloned into the expression vector CDM8 and transfected into COS cells. COS cells transfected with the sense HEK/CDM8 construct stained specifically with the IIIA4 antibody, thereby confirming that the antigen recognized by the IIIA4 antibody and the expressed protein product of the HEK cDNA clone were identical. DNA sequence analysis revealed that HEK is a newly discovered member of the EPH/ELK family of receptor tyrosine kinases. Northern blot analysis of a number of cell lines demonstrated the expression of 5.5- to 6.0-kb HEK transcripts in LK63 and the T-cell lines JM and HSB-2. Southern blot analysis of DNA from LK63 suggested that the HEK gene was neither amplified nor rearranged in the LK63 tumor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Bowtell D. D. Rapid isolation of eukaryotic DNA. Anal Biochem. 1987 May 1;162(2):463–465. doi: 10.1016/0003-2697(87)90421-0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Chan J., Watt V. M. eek and erk, new members of the eph subclass of receptor protein-tyrosine kinases. Oncogene. 1991 Jun;6(6):1057–1061. [PubMed] [Google Scholar]
- Draetta G., Piwnica-Worms H., Morrison D., Druker B., Roberts T., Beach D. Human cdc2 protein kinase is a major cell-cycle regulated tyrosine kinase substrate. Nature. 1988 Dec 22;336(6201):738–744. doi: 10.1038/336738a0. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., Gough N. M., King J. A., Hilton D. J., Nicola N. A., Simpson R. J., Nice E. C., Kelso A., Metcalf D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J. 1987 Dec 20;6(13):3995–4002. doi: 10.1002/j.1460-2075.1987.tb02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Hirai H., Maru Y., Hagiwara K., Nishida J., Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987 Dec 18;238(4834):1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- Lhoták V., Greer P., Letwin K., Pawson T. Characterization of elk, a brain-specific receptor tyrosine kinase. Mol Cell Biol. 1991 May;11(5):2496–2502. doi: 10.1128/mcb.11.5.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg R. A., Hunter T. cDNA cloning and characterization of eck, an epithelial cell receptor protein-tyrosine kinase in the eph/elk family of protein kinases. Mol Cell Biol. 1990 Dec;10(12):6316–6324. doi: 10.1128/mcb.10.12.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Castro M. M., Aboul-ela F., Tinoco I., Jr Base pairing involving deoxyinosine: implications for probe design. Nucleic Acids Res. 1985 Dec 20;13(24):8927–8938. doi: 10.1093/nar/13.24.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maru Y., Hirai H., Takaku F. Overexpression confers an oncogenic potential upon the eph gene. Oncogene. 1990 Mar;5(3):445–447. [PubMed] [Google Scholar]
- Maru Y., Hirai H., Yoshida M. C., Takaku F. Evolution, expression, and chromosomal location of a novel receptor tyrosine kinase gene, eph. Mol Cell Biol. 1988 Sep;8(9):3770–3776. doi: 10.1128/mcb.8.9.3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews W., Jordan C. T., Wiegand G. W., Pardoll D., Lemischka I. R. A receptor tyrosine kinase specific to hematopoietic stem and progenitor cell-enriched populations. Cell. 1991 Jun 28;65(7):1143–1152. doi: 10.1016/0092-8674(91)90010-v. [DOI] [PubMed] [Google Scholar]
- Pasquale E. B. Identification of chicken embryo kinase 5, a developmentally regulated receptor-type tyrosine kinase of the Eph family. Cell Regul. 1991 Jul;2(7):523–534. doi: 10.1091/mbc.2.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J. The structure and insertion of integral proteins in membranes. Annu Rev Cell Biol. 1990;6:247–296. doi: 10.1146/annurev.cb.06.110190.001335. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wilks A. F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1603–1607. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Y. G., Jubinsky P. T., Sengupta A., Yeung D. C., Stanley E. R. Purification of the colony-stimulating factor 1 receptor and demonstration of its tyrosine kinase activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1268–1271. doi: 10.1073/pnas.84.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel I. R., Wilks A. F., Pietersz G. A., Goding J. W. Murine plasma cell membrane antigen PC-1: molecular cloning of cDNA and analysis of expression. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8619–8623. doi: 10.1073/pnas.82.24.8619. [DOI] [PMC free article] [PubMed] [Google Scholar]