Abstract
Despite advances in other realms of cardiac care, the mortality attributable to ischaemic cardiomyopathy has only marginally decreased over the last 10 years. These findings highlight the growing realization that current pharmacological and device therapies rarely reverse disease progression and rationalize a focus on novel means to reverse, repair and re‐vascularize damaged hearts. As such, multiple candidate cell types have been used to regenerate damaged hearts either directly (through differentiation to form new tissue) or indirectly (via paracrine effects). Emerging literature suggests that robust engraftment of electrophysiolgically heterogeneous tissue from transplanted cells comes at the cost of a high incidence of ventricular arrhythmias. Similar electrophysiological studies of haematological stem cells raised early concerns that transplant of depolarized, inexcitable cells that also induce paracrine‐mediated electrophysiological remodelling may be pro‐arrhythmic. However, meta‐analyses suggest that patients receiving haematological stem cells paradoxically may experience a decrease in ventricular arrhythmias, an observation potentially related to the extremely poor long‐term survival of injected cells. Finally, early clinical and preclinical data from technologies capable of differentiating to a mature cardiomyocyte phenotype (such as cardiac‐derived stem cells) suggests that these cells are not pro‐arrhythmic although they too lack robust long‐term engraftment. These results highlight the growing understanding that as next generation cell therapies are developed, emphasis should also be placed on understanding possible anti‐arrhythmic contributions of transplanted cells while vigilance is needed to predict and treat the inadvertent effects of regenerative cell therapies on the electrophysiological stability of the ischaemic cardiomyopathic heart.
Abbreviations
- BMC
unselected bone marrow cell
- CSC
cardiac‐derived stem cell
- Cx43
connexin43
- EDC
explant‐derived cell
- EPC
endothelial progenitor cell
- ESC
embryonic stem cell
- ESC‐CM
embryonic stem cell‐derived cardiomyocyte
- ICM
ischaemic cardiomyopathy
- iPSC
induced pluripotent stem cell
- iPSC‐CM
induced pluripotent stem cell‐derived cardiomyocyte
- LAD
left anterior descending
- MSC
mesenchymal stem cell
- SCID
severe combined immunodeficiency
- SkM
skeletal myoblast
Ischaemic cardiomyopathy (ICM), the most common form of heart failure, reflects underlying coronary artery disease whereby one or more myocardial infarcts convert working heart tissue to scar and progressively weaken pump function. In many ways, ischaemic cardiomyopathy (ICM) mirrors an advanced malignant disease with 27% of patients dying within 1 year of a heart failure diagnosis (Alter et al. 2012). Despite advances in other realms of cardiac care, the mortality attributable to ICM has only marginally decreased from 27 to 25% amongst all patients (P = 0.03) over the last 10 years (Yeung et al. 2012). These findings highlight the growing realization that current pharmacological and device therapies rarely reverse disease progression, thus rationalizing a new focus on novel means to reverse, repair, and vascularize damaged ICM hearts. To accomplish this, cell therapy has emerged as a solution to promote cardiac repair. Ideally, transplanted stem cells should display extensive engraftment and differentiation while electromechanically coupling with surrounding host myocardium to improve cardiac function. To date, a number of cell types have been explored with varying degrees of success. Evidence regarding the effects of cell transplantation on cardiac rhythm is only now beginning to emerge and is providing fascinating insights into how cardiac and non‐cardiac cell sources alter the electrophysiology of the damaged/remodelled ICM heart.
Patient with ischaemic cardiomyopathy: a susceptible host to cardiac dysrhythmias
In ICM patients, ventricular arrhythmias account for significant morbidity and mortality, with premature ventricular depolarizations occurring in 70–95% of ICM patients and 50–60% of deaths attributed to an arrhythmic cause (Brodsky et al. 1986; Kannel et al. 1988). In the failing heart areas of myocardial fibrosis intermix with living tissue, creating functional and structural inhomogeneities that favour arrhythmogenesis. In most cases, the underlying mechanism is either re‐entry or triggered activity.
In the case of re‐entry, fibrous tissue impairs the uniform propagation of electrical impulses within the ventricle by forming complex channels in dense electrical scars. Electrical remodelling in response to abnormal intraventricular stress and strain distribution results in decreased conduction velocity through downregulation of connexin43 (Cx43) (Kitamura et al. 2002). As such, re‐entrant circuits account for the majority of ventricular tachyarrhythmias in ICM patients.
Tissue inhomogeneities and remodelling also increase the risk of triggered activity. Abnormal depolarization may result from altered calcium handling due to increased sodium–calcium exchanger activity, leading to premature and extrasystolic beats. These effects are further compounded as greater mechanical stress is placed on non‐ischaemic tissue, altering calcium handling and creating areas predisposed to increased automaticity.
As new candidate cell therapies are explored, understanding their pro‐ and anti‐arrhythmic contributions is critical to developing safe and effective therapies for heart failure that reduce arrhythmic risk rather than create or exacerbate existing electrical instabilities.
Clinical cell candidates for cardiac repair
Over the past two decades, a number of cell products have undergone clinical consideration based on their presumed capacity to directly replace injured tissue (Table 1). Among these are skeletal myoblasts (SkMs), haematological cell products (unselected bone marrow cells (BMCs), mesenchymal stem cells (MSCs) and endothelial progenitor cells (EPCs)), pluripotent stem cells and cardiac‐derived stem cells (CSCs). In addition to the effects of direct differentiation and integration with host myocardium, there is strong evidence that transplanted cells often act indirectly, exhibiting modulatory effects on the ischaemic milieu. Already, a number of clinical trials using autologous adult progenitor cells have been completed, yielding positive and promising results (Chen et al. 2004; Schachinger et al. 2004, 2009; Makkar et al. 2012).
Table 1.
Pre‐clinical effects of cell therapy on cardiac function and rhythm
| Cell timing + delivery | ||||
|---|---|---|---|---|
| Cell type | Animal model | method | Cell transplant results | Reference |
| SkMs | Rats + LAD coronary artery ligation | 7 days post‐surgery + IC injection | 13/20 SkM‐treated rats had sustained ventricular tachycardia at electrophysiological testing. | Fernandes et al. (2006) |
| SkMs | Mice + cryolesion | At surgery + intra‐cardiac injection | 15/16 SkM‐treated mice had sustained ventricular tachycardia at electrophysiological testing. | Roell et al. (2007) |
| Over‐expression of Cx43 decreased SkM induced ventricular tachycardia by 62.5% at electrophysiological testing. | ||||
| MSCs | Pigs + catheter infarction | 1 month post‐infarct + catheter injection | Increased cardiac nerve sprouting no evidence for pro‐arrhythmia but not formally assessed. | Pak et al. (2003) |
| BMCs | Rats + LAD coronary artery ligation | 7 days post‐surgery + intra‐cardiac injection | No increase in sustained ventricular tachycardia at electrophysiological testing. | Fernandes et al. (2006) |
| MSCs | Pigs + catheter infarction | 30 min post infarct + intravenous infusion | Improved cardiac function but decreased refractoriness 3 months post‐cell transplant. | Price et al. (2006) |
| Human ESC‐CMs | Nude rats + LAD coronary artery ligation | 4 day post‐surgery + intra‐cardiac injection | Improved cardiac function with no evidence for pro‐arrhythmia but not formally assessed. | Laflamme et al. (2007) |
| ESC‐CMs | Mice + LAD coronary artery ligation | At surgery+ intra‐cardiac injection | Improved cardiac function with no evidence for pro‐arrhythmia but not formally assessed. | Ebert et al. (2007) |
| ESC‐CMs | Mice + cryolesion | At surgery + intra‐cardiac injection | Decrease sustained ventricular tachycardia at electrophysiological testing. | Roell et al. (2007) |
| Human ESC‐CMs | Immuno‐suppressed guinea‐pigs + cryolesion | 10 days post‐surgery + intra‐cardiac injection | Improved cardiac function while reducing spontaneous and inducible ventricular tachycardia | Shiba et al. (2012) |
| Human ESC‐CMs | Immuno‐suppressed macaques + catheter infarction | 14 days post infarct + catheter injection | 4/4 ESC‐CM treated macaques demonstrated premature ventricular contractions and spontaneous ventricular tachycardia. | Chong et al. (2014) |
| iPSCs | Mice + LAD coronary artery ligation | At surgery + intra‐cardiac injection | Improved cardiac function with no evidence for pro‐arrhythmia but not formally assessed. | Singla et al. (2011) |
| Human iPSC‐CM | Nude rats + LAD coronary artery ligation | At surgery | Trend to improved cardiac function with no evidence for pro‐arrhythmia but not formally assessed. | Carpenter et al. (2012) |
| Human iPSC‐CM | Immuno‐suppressed pigs + LAD constriction | 4 weeks post‐surgery | Improved cardiac function with no evidence for pro‐arrhythmia on 24 telemetry prior to sacrifice 8 weeks after cell delivery. | Kawamura et al. (2012) |
| iPSCs | Pigs + catheter infarction | 7 days post‐infarct | Improved cardiac function and perfusion with no evidence for pro‐arrhythmia but not formally assessed. | Li et al. (2013) |
| EDCs + CDCs | Rats + LAD coronary artery ligation | At surgery + intra‐cardiac injection | Both EDCs and CDCs improved cardiac function to a similar degree with no evidence for pro‐arrhythmia but not formally assessed. | Davis et al. (2010 a) |
| Human EDCs | SCID Mice + LAD coronary artery ligation | 7 days post‐infarct + intra‐cardiac injection | Improved cardiac function with enhanced EDC engraftment and no evidence for pro‐arrhythmia but not formally assessed. | Mayfield et al. (2014 b) |
| Human EDCs | SCID Mice + LAD coronary artery ligation | 7 days post‐infarct + intra‐cardiac injection | Diabetes impairs the EDC‐mediated improvements in cardiac function with no evidence for pro‐arrhythmia but not formally assessed. | Molgat et al. (2014) |
| Human EDCs + EPCs | SCID Mice + LAD coronary artery ligation | 7 days post‐infarct + intra‐cardiac injection | Both EDCs and EPCs improved cardiac function to a similar degree with no evidence for pro‐arrhythmia but not formally assessed. | Latham et al. (2013) |
| Human c‐Kit+ cells | SCID Mice and rats + LAD coronary artery ligation | At surgery + intra‐cardiac injection | Improved cardiac function with no evidence for pro‐arrhythmia but not formally assessed. | Bearzi et al. (2007) |
| Cardiospheres | Mice + LAD coronary artery ligation | At surgery + intra‐cardiac injection | Improved cardiac function with no evidence for pro‐arrhythmia but not formally assessed. | Messina et al. (2004) |
| Human CDCs | SCID Mice + LAD ligation | At surgery + intra‐cardiac injection | CDCs improved cardiac function with no evidence for pro‐arrhythmia but not formally assessed. | Smith et al. (2007) |
| Human cardiospheres + CDCs | SCID Mice + LAD ligation | At surgery + intra‐cardiac injection | Cardiospheres improved cardiac function more than CDCs. No evidence for pro‐arrhythmia but not formally assessed. | Li et al. (2010) |
| CDCs | Pig + catheter infarction | 4 weeks post‐infarct + catheter injection | CDCs improved cardiac function while not increasing inducible ventricular tachycardia on electrophysiological testing. | Johnston et al. (2009) |
| CDCs or cardiospheres | Pig + catheter infarction | 4–5 weeks post‐infarct + catheter injection | CDCs and cardiospheres improved cardiac function to a similar extent while not resulting in deaths (sudden or otherwise) in either group. | Lee et al. (2011) |
Skeletal myoblasts
SkMs represent an abundant source of skeletal progenitors (i.e. 5% of all cells located between the basal lamina and the sarcolemma of adult skeletal muscle) that were first delivered clinically to damaged hearts in 2001 (Menasche et al. 2001). Bolstered by their ability to be easily harvested, natural resistance to hypoxia, and formation of contractile myotubes after transplantation, SkMs served as a promising candidate to replace damaged myocardium and demonstrated long‐term persistence with functional benefits in ICM patients (Hagege et al. 2003; Pagani et al. 2003). In addition to providing hints of cardiac repair, these studies also indicated that SkM grafts remained functionally and electrically isolated from surrounding host myocardium, forming areas of slow conduction which favoured re‐entry (Fernandes et al. 2006) and resulted in a high incidence of clinical life‐threatening ventricular arrhythmias (Menasche et al. 2003; Smits et al. 2003; Povsic et al. 2011). This pro‐arrhythmic effect was owed largely to a lack of gap‐junction protein Cx43 expression, the principal cardiac connexin found in ventricular myocytes (Abraham et al. 2005). Downregulation of Cx43 has been demonstrated both in experimental models and in ICM patients (Ai & Pogwizd, 2005) with studies in heterozygous Cx43 knockout mice supporting the role of decreased Cx43 expression in arrhythmogenesis (Guerrero et al. 1997; Lerner et al. 2000). Efforts to overcome the electrical isolation of SkMs have shown promise, as electrical coupling and decreased arrhythmogenicity were achieved in co‐cultures (Abraham et al. 2005) and in vivo (Fernandes et al. 2006; Roell et al. 2007) by inducing Cx43 expression. However, equivocal clinical benefits (i.e. no enhancement in left ventricular ejection fraction 6 months after SkM injection; Menasche et al. 2008) combined with ongoing concerns regarding pro‐arrhythmias (i.e. the abruptly terminated MARVEL study demonstrated ventricular tachycardia in the first 4 of 7 patients receiving high dose SkMs; Povsic et al. 2011) combined to stall investigations regarding SkM‐mediated cardiac repair. Ultimately these findings motivated the field to explore parallel investigations using autologous cell products to maximize therapeutic benefits while carefully monitoring for proarrhythmic potential or complications.
Haematological stem cell products (BMCs, MSCs or EPCs)
With the demonstration of a self‐renewing population of cells in the bone marrow that possessed the ability of adopt a cardiomyogenic fate after exposure to inductive culture conditions, early interest focused on investigating the ability of haematological stem cell products to promote cardiac repair. Preclinical studies of unselected BMCs demonstrated remarkable improvements in cardiac structure and function that were initially attributed to BMC transdifferentiation into working myocytes (Jackson et al. 2001). Subsequent lineage tracing studies were unable to demonstrate significant transdifferentiation into cardiomyocytes despite validating equivalent benefits in cardiac function (Murry et al. 2004). These studies established the ‘paracrine’ hypothesis that pro‐cardiogenic cytokines released by transplanted BMCs promoted post‐infarct cardiac repair by stimulating endogenous repair and myocardial salvage (Kocher et al. 2001; Narmoneva et al. 2004; Fazel et al. 2006). While unselected BMCs provide modest cardiac repair in clinical trials (Jeevanantham et al. 2012), several strategies have been developed to improve the haematological cell products though candidate cell selection or enhanced cell culture techniques to provide EPCs or MSCs for cardiac transplantation (Davis & Stewart, 2014).
In contrast to application of SkMs to ICM hearts, the pro‐arrhythmic experience with blood‐ and bone marrow‐derived cells has been more contradictory. After transplantation, EPCs and MSCs maintain a depolarized membrane potential (−20 to −40 mV) characteristic of inexcitable cells (Heubach et al. 2004; Li et al. 2005). Unlike SkMs, they express Cx43 thus retaining the potential to electrically couple with surrounding cardiomyocytes to form inexcitable current sinks and promote electrical heterogeneity. In co‐cultures with neonatal cardiomyocytes, electrically coupled MSCs remain undifferentiated and functionally isolated, thus reducing the overall conduction velocities within culture and promoting the generation of re‐entrant arrhythmias (Chang et al. 2006). Although administration of MSCs after myocardial infarction enhances left ventricular function, intra‐myocardial injection has also been shown to reduce the effective refractory period (i.e. left ventricular free wall, left ventricular peri‐infarct and right ventricular free wall) and increase the spatiotemporal heterogeneity of restitution 3 months after MSC injection (Price et al. 2006). These findings heighten pro‐arrhythmic concerns as reduced refractoriness facilitates the induction of ventricular tachycardia (Kuo et al. 1983; Bode et al. 2002) while dispersion of cardiac restitution may contribute to electrical alternans, a harbinger of dynamic instability and the initiation of malignant ventricular arrhythmias (Weiss et al. 2002). In addition to introducing heterogeneity, MSCs have been shown to increase sympathetic innervation in both the atria and the ventricles 2 months after injection, potentially amplifying the effects of electrical instability and inhomogeneity (Pak et al. 2003). The widespread distribution of these electrophysiological and neurological changes suggests that the mechanism behind these effects is largely paracrine, linked to the early delivery of cytokines or exosomes that promote both cardiac repair and fundamental electrophysiological changes within the ICM heart.
Despite these concerning findings, haematological stem cell products have not been shown to promote arrhythmias in the 1200+ patients treated soon after myocardial infarction: rather meta‐analyses suggest that patients receiving blood‐ or bone marrow‐derived stem cells experience a decreased incidence of ventricular arrhythmias (Zhang et al. 2009 b). This paradoxical observation may be linked to differential expression of cytokines/exosomes by haematological stem cells sourced from patients (as compared to non‐infarcted animal models) and/or the minimal long‐term engraftment seen after intra‐coronary injection of haematological stem cells (i.e. long‐term retention reported at < 3%) (Kang et al. 2006; Lunde et al. 2006; Meyer et al. 2006). These findings also suggest that measures taken to improve acute engraftment may be detrimental to the observed salutary benefits derived from transplanted cells, and stem cell recipients need to be closely monitored for hints of pro‐arrhythmic changes. Nonetheless, this clinical experience has motivated further investigations, including the phase III BAMI trial (Barts & The London NHS Trust, 2014), using a single infusion of bone marrow‐derived mononuclear cells with the aim of reducing all‐cause and arrhythmic mortality following acute myocardial infarction.
Pluripotent stem cells
The capacity to form all three germ layers and freely differentiate into all lineages has made pluripotent stem cells an appealing target for research in regenerative medicine. Embryonic stem cells (ESCs) represent cell lines derived from the inner cell mass of the blastocyst, an early pre‐implantation embryo formed 4–5 days after fertilization (Thomson et al. 1998). ESCs can be induced to form functional cardiomyocytes in vitro, and electrophysiological studies reveal distinct atrial, ventricular and pacemaker phenotypes and excitation properties, even during early differentiation (He et al. 2003; Mummery et al. 2003; Satin et al. 2004). Even when cells are sorted for markers of ventricular lineage, significant electrophysiological heterogeneity exists including relatively depolarized resting membrane potential (−60 vs. −80 mV), reduced upstroke velocity (15–50 vs. 100–135 V s−1) and shortened action potential duration at 90% of repolarization (280–400 vs. 250–300 ms) (He et al. 2003; Zhang et al. 2009 a; Hartman et al. 2015). Small‐animal studies have shown that transplantation of ESC‐derived cardiomyocytes (ESC‐CMs) into the infarcted heart improves contractile function and electrical conduction, reducing susceptibility to arrhythmias (Kolossov et al. 2006; Ebert et al. 2007; Laflamme et al. 2007; Robey et al. 2008; Shiba et al. 2012). Initial hypotheses suggested that this effect may be mediated in part through integration and electrical coupling of transplanted ESCs as exogenously delivered ESC‐CMs electrically couple to the host heart in both guinea pig and primate models of myocardial infarction (Shiba et al. 2012; Chong et al. 2014). However, large‐animal studies revealed that despite uniform electrically coupling between human ESC‐CMs and recipient primate hearts, all animals that received ESCs experienced ventricular arrhythmias, including premature ventricular depolarizations and ventricular tachycardia (Chong et al. 2014). The reason for this conflicting result is unknown but it may reflect a combination of larger graft sizes and the relative immaturity of engrafted ESC‐CMs. The larger ESC‐derived graft sizes found in primates reflected a greater number of ESCs that could be delivered, engraft and differentiate (approximately 10‐fold larger than previous small‐animal studies). Given that the spread of activation within coupled immature cardiomyocytes is reduced (>50 ms for activation), this critical delay within a structurally abnormal heart may provide the ideal conditions for re‐entry and pro‐arrhythmia (Shiba et al. 2012). It is also possible that the injected cells were contaminated with ESC‐derived pacemaker cells (Laflamme et al. 2005; Zhu et al. 2009) or ESC‐derived cardiomyocytes with aberrant iK1 expression resulting in early and late afterdepolarizations (Lieu et al. 2013). Although further work is needed to establish what mechanisms account for the observed pro‐arrhythmia, these findings highlight the difficulties inherent in promoting engraftment of electrically heterogeneous non‐cardiac cells within an established scar.
Induced pluripotent stem cells (iPSCs) are autologous cell lines derived from cultured somatic cells after genetic reprogramming with transcription factors, microRNAs, synthetic self‐replicative RNAs or small molecules (Takahashi & Yamanaka, 2006). Akin to ESCs, iPSCs have the capacity to differentiate into cells of all three germ layers, while exhibiting the same characteristic morphology and surface antigens. In addition, iPSCs have the advantage of being autologous, mitigating ethical and limiting immune rejection concerns. Akin to ESC‐derived cardiomyocytes, electrophysiological profiling of iPSC‐derived cardiomyocytes (iPSC‐CMs) demonstrates significant heterogeneity reminiscent of immature atrial, nodal or ventricular cells (Moretti et al. 2010; Zhang et al. 2012). The capacity for infarct repair using iPSCs has been demonstrated in numerous preclinical animal models (Carpenter et al. 2012; Kawamura et al. 2012; Li et al. 2013). In one such study, iPSCs demonstrated cardiac transdifferentiation equivalent with that of embryonic stem cells, along with significant inhibition of apoptosis and fibrosis and improved ventricular function (Singla et al. 2011). Similar functional improvements were seen using undifferentiated iPSCs injected into a porcine model (Li et al. 2013). Although no animals were reported lost to sudden cardiac death, the incidence of ventricular arrhythmias was not assessed. In all studies following straightforward injection of iPSCs, cell tracking has revealed modest long‐term engraftment in a manner consistent with previous intra‐myocardial injection studies, suggesting that the therapeutic effect of iPSCs is predominantly mediated through a paracrine release of immunomodulatory, anti‐apoptotic and proangiogenic factors (Bobis‐Wozowicz et al. 2015).
Cardiac‐derived stem cells
Almost a decade ago, the adult heart was shown to contain reservoirs of cells that express stem cell markers, propagate in vitro and adopt the phenotypic features of heart cells after differentiation (Oh et al. 2003). Since then, several studies have demonstrated that adult hearts undergo lifelong replacement, with cardiomyocyte turnover estimates ranging between 0.5 and 2% per year (Soonpaa & Field, 1997; Bergmann et al. 2009; Walsh et al. 2010). While both pre‐existing cardiomyocytes and endogenous stem cells have been identified as important sources for ongoing cardiomyocyte replacement, controversy exists as to which is the dominant cell source (Senyo et al. 2013; Torella et al. 2015). However, based on these findings, several groups have developed independent methods for the culture and isolation of cardiac‐derived stem cells (CSCs). Among these are cardiac explant‐derived cells (EDCs), antigenically selected c‐Kit+ cells and culture guided cardiosphere‐derived cells (CDCs). The fundamental rationale for this approach is simple, with ex vivo amplification of resident CSCs followed by delivery to areas of injury, where they engraft and regenerate the heart.
Explant derived cells
Cardiac explant‐derived cells (EDCs) represent a heterogeneous mixture of cells cultured directly from minced and plated myocardial biopsies (Davis et al. 2010 a). EDCs contain complimentary subpopulations of cardiac (c‐Kit+), endothelial (CD31+, CD34+), and mesenchymal (CD90+) progenitor cells that act synergistically to provide myocardial repair (Latham et al. 2013; Molgat et al. 2014; Mayfield et al. 2014 a,b). Unfortunately, direct application of this initial cell product to the clinical setting is limited by a constant return to the proportion of explant tissue plated, thus necessitating either antigenic selection followed by ex vivo proliferation (i.e. c‐Kit+ cells) or direct culture guided expansion (i.e. CDCs) of the initial cell product.
c‐Kit+ cardiac stem cells
First identified in 2003, cardiac stem cells positive for the receptor tyrosine kinase c‐Kit represent a small resident population within the adult mammalian heart capable of cardiomyocte, vascular smooth muscle and endothelial differentiation (Beltrami et al. 2003). Antigenically sorted c‐Kit+ stem cells have been shown to differentiate and confer functional improvements after transplantation into ischaemic myocardium in both preclinical and clinical studies, despite lacking evidence that these cells express currents typical of mature cardiomyocytes (Bearzi et al. 2007). In the randomized open‐label SCIPIO trial, the safety and efficacy of intracoronary delivery of c‐Kit+ cardiac stem cells was established, revealing a 12.3% improvement in left ventricular ejection fraction in treated patients at 2 year follow‐up, with no mortality or major adverse cardiac events (Bolli et al. 2011; Chugh et al. 2012). Since publication, concerns have been raised about the SCIPIO trial regarding the integrity of data demonstrating c‐Kit+ cell characterization (The Lancet Editors, 2014). While troubling and the subject of ongoing investigation, the trial was designed such that cultured cells were shipped to a separate hospital for administration and patient follow‐up. Although this ‘division of labour’ has been suggested to preserve the integrity of patient outcome data, there are no immediate plans to start a Phase 2 trial involving c‐Kit+ cells (Grens, 2015).
Interestingly, emerging lineage tracking evidence also suggests that percentage of cardiomyocytes emerging from resident adult c‐Kit+ cells during development and response to cardiac injury are vanishingly small and highly unlikely to influence cardiac function (Jesty et al. 2012; van Berlo et al. 2014). While prolonged culture of c‐Kit+ in inductive media may influence the adoption of a cardiac phenotype, more preclinical work is needed to define the operative mechanisms and cardiogenic potential of ex vivo proliferated c‐Kit+ cells.
Cardiosphere‐derived cells
Three‐dimensional cardiospheres spontaneously assemble during brief exposure of EDCs to non‐adherent culture conditions (Messina et al. 2004). Although sphering EDCs does not amplify cell numbers, these conditions promote the expression of stem cell‐related antigens (c‐Kit+) and transcripts (Li et al. 2010; Davis et al. 2010 b). Cardiospheres can then be expanded within adherent culture conditions to yield cardiosphere‐derived cells (CDCs). Akin to EDCs, CDCs represent a heterogeneous mixture of cells, with subpopulations expressing stromal, mesenchymal, and progenitor related surface antigens (Smith et al. 2007; Davis et al. 2009). Importantly, CDCs express Cx43, and maintain a capacity for differentiation into electrophysiologically functionally mature myocytes (Davis et al. 2010 b). When cocultured with neonatal rat ventricular myocytes, human and porcine CDCs exhibit intracellular calcium transients synchronous with surrounding myocytes, spontaneous action potentials, and fast inward sodium currents, consistent with a mature ventricular phenotype (Smith et al. 2007). In a preclinical murine model of myocardial infarction, CDC therapy demonstrated superior functional benefits when compared with BMCs and MSCs (Li et al. 2012). In contrast to the experience with ESC‐CMs, large‐animal studies of transplanted cardiospheres or CDCs have failed to demonstrate significant spontaneous or inducible ventricular arrhythmias – although these effects may in part be related to modest long‐term engraftment (Johnston et al. 2009; Lee et al. 2011). Finally, the phase I randomized CADUCEUS trial demonstrated safety and efficacy of CDC delivery by intracoronary infusion, showing a 12.3% reduction in infarct size in the cell‐treated group after 12 months (Makkar et al. 2012; Malliaras et al. 2014).
Owing to divergent culture methods and cell products, therapeutic comparisons of CSC therapy effects are difficult. However, taken together these data provide strong evidence for the therapeutic efficacy of ex vivo proliferated CSCs when delivered to the ICM heart. To date, only CDCs have demonstrated electrophysiological evidence of differentiation to a mature myocyte phenotype that electrically couples with surrounding cells – albeit within inductive culture conditions and not in vivo (Smith et al. 2007).
Theoretical model of stem cell autonomous effects on ventricular arrhythmias
Emerging data from preclinical and clinical studies have provided greater insight into the effects of cardiac cell therapy on ventricular arrhythmias. Figure 1 highlights our current understanding of the interrelated mechanisms by which transplanted stem cells provide cardiac repair and how these functions contribute to either net pro‐ or anti‐ arrhythmic effects. Consider the example of SkMs (left), engrafting to directly replace damaged myocardium. As these cells lack Cx43 expression, tissue inhomogeneities arise in the form of electrically uncoupled grafts, providing a substrate for the formation of reentrant arrhythmias. Conversely, MSCs (right) through the release of paracrine factors, induce cell cycle re‐entry in resident cardiomyocytes and attenuate the effects of negative remodeling conferring an anti‐arrhythmic benefit‐ while their transient retention limits the adverse effects direct coupling to form electrical sinks or paracrine mediated electrophysiological remodeling.
Figure 1. Schematic model of stem cell autonomous effects on ventricular arrhythmias .
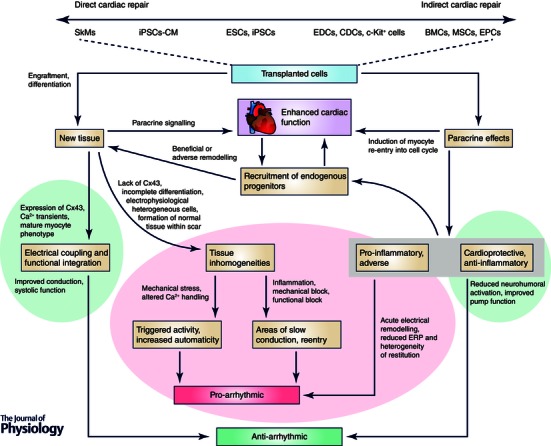
Candidate cell types are positioned along a continuum at the top of the figure, according to the relative extent of direct repair (engraftment + differentiation) vs. indirect repair (paracrine signalling) they provide. These two modes of repair are also shown (blue rectangles) below, with cardiac function serving as a mediator in between. Pro‐arrhythmic contributions are highlighted in red, with anti‐arrhythmic contributions in green.
Modification of stem cells to maximize therapeutic repair
The modest engraftment and functional improvements conferred by first generation autologous therapies have prompted a number of groups to explore the effects of augmenting the regenerative potential of existing cell products. To date, several studies have used direct genetic modification to enhance stem cells before transplantation (Song et al. 2010; Davis & Stewart, 2011). One such study, however, demonstrated reduced cell clearance via apoptosis and increased long‐term retention of CSCs by overexposing Pim‐1 kinase, a key modulator of Akt signalling (Muraski et al. 2008). Parallel efforts using genetic overexpression have been made to improve cell survival (SDF‐1, Bcl‐2), electrical integration (Cx43), differentiation (TGF‐β), migration (CXCR4, eNOS), and vasculogenesis (HIF‐1, VEGF). Attempts to translate these approaches to the clinic have been limited to date. In the phase II ENACT AMI (Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction) trial, early EPCs are transfected with human endothelial nitric oxide synthase (eNOS) prior to intracoronary delivery (Taljaard et al. 2010). This is the first clinical trial to employ a combination gene and cell therapy for the treatment of cardiac disease.
The most significant improvements in CSC retention and long‐term engraftment have come through biomaterial approaches. Low acute retention of injected cells reflects a combination of mechanical extrusion, lymphatic and venous clearance, and off‐target disbursement. Initial biomaterial approaches used matricellular materials to anchor transplanted cells within the site of injection. In addition to structural support, biosynthetic materials provide adhesion stimuli and trophic support that increase the paracrine secretion of cardioprotective cytokines and prevent cell loss due to apoptosis (Zhang et al. 2008). Similarly, synergistic benefits were observed when CSCs have been co‐administered with synthetic platelet gels, supportive matrigel, suggestive of an enhanced paracrine profile with a superior capacity for myocardial repair (Cheng et al. 2012 a,b). Although cells need not be embedded within a collection of excess (potentially prothrombotic material) as intra‐myocardial delivery of cells encapsulated within a protective agarose supplemented cocoon have been shown to improve cell viability and proliferation, indicating that capsules supplemented with key ECM binding proteins re‐establish vital cell‐matrix attachments lost during mobilization for transplant (Mayfield et al. 2014 b). Furthermore embedding cells within biomaterials itself may not be necessary as significant improvements in acute cell retention and functional recovery have been demonstrated following simply applying fibrin glue at the site of injection to presumably limit mechanical extrusion (Terrovitis et al. 2010).
Ultimately, different cell types may have specific propensities to proceed down pro‐ or anti‐arrhythmic pathways. For this reason, it is important to understand cell characteristics when targeting candidate therapies for improvement. For example, efforts to boost engraftment in cell types with a robust paracrine signature may be unwarranted if these cells lack the capacity for differentiation and electrical integration. Combination therapy may provide an attractive means of harnessing both direct and indirect mechanisms of repair without introducing pro‐arrhythmic risk (Latham et al. 2013). As demonstrated by the iterative translational research progress of many cell therapy strategies (Davis & Stewart, 2014), the path from bench to bedside is rarely linear, stressing the need for vigilance to predict and treat inadvertent effects of regenerative cell therapies on the electrophysiological stability of the ICM heart.
Future directions
Since the emergence of stem cell transplantation as a means of repairing and revascularizing damaged hearts, several cell types have been explored in search of an ideal cellular candidate. Through a combination of direct (via differentiation) or indirect (via paracrine effects) repair, many of these technologies have yielded promising results in clinical and preclinical studies. Yet despite the existence of cells capable of forming mature cardiomyocytes, many of these cells lack the persistence and long‐term engraftment required for differentiation. As efforts are made to boost cell persistence and engraftment, increasing consideration should be given to the structural and electrophysiological properties of transplanted cells and the potential for pro‐arrhythmic complications. In the case of less cardiotrophic therapies, engraftment may be unnecessary or even detrimental, outweighing the cardioprotective effects of cell transplantation. As next generation therapies are developed, emphasis should also be placed on understanding possible anti‐arrhythmic contributions of transplanted cells, as this may offer future therapeutic targets. The understanding of pro‐ and anti‐arrhythmic contributions, particularly in the dynamic and unstable setting of ICM, is critical to developing safe and effective regenerative therapies.
Additional information
Competing interests
The authors have no competing interests.
Author contributions
Both S.M. and D.R.D. drafted the article and revised it critically. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Canadian Stem Cell Network and the Canadian Heart and Stroke Foundation (NA‐7346). D.R.D. is funded by the Canadian Institutes of Health Research (Clinician Scientist Award MC2‐121291).
Biography
Darryl R. Davis is a clinician–scientist and cardiac electrophysiologist in the Division of Cardiology at the University of Ottawa Heart Institute. His research focuses upon the culture of explant‐derived cardiac stem cells from routine myocardial biopsies with an emphasis on developing novel means of enhancing long‐term engraftment and therapeutic repair of ischaemic cardiomyopathy. Seth Mount is a Masters candidate in Cellular and Molecular Medicine at the University of Ottawa. His research focuses upon overcoming barriers to the clinical translation of cardiac explant‐derived stem cells for ischaemic cardiomyopathy.

This review was presented at the symposium “Cardiac Arrhythmias: Challenges for Diagnosis and Treatment. A symposium in honour of George Ralph Mines (1886–1914)”, which took place at McGill University, Montreal, QC, Canada, between 6–7 November 2014.
References
- Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, Li RA, O'Rourke B & Marban E (2005). Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res 97, 159–167. [DOI] [PubMed] [Google Scholar]
- Ai X & Pogwizd SM (2005). Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res 96, 54–63. [DOI] [PubMed] [Google Scholar]
- Alter DA, Ko DT, Tu JV, Stukel TA, Lee DS, Laupacis A, Chong A & Austin PC (2012). The average lifespan of patients discharged from hospital with heart failure. J Gen Intern Med 27, 1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barts & The London NHS Trust (2014). BAMI. The effect of intracoronary reinfusion of bone marrow‐derived mononuclear cells (BM‐MNC) on all cause mortality in acute myocardial infarction. ClinicalTrials.gov, NCT01569178. [Google Scholar]
- Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa‐Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A & Anversa P (2007). Human cardiac stem cells. Proc Natl Acad Sci USA 104, 14068–14073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal‐Ginard B & Anversa P (2003). Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe‐Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S & Frisen J (2009). Evidence for cardiomyocyte renewal in humans. Science 324, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobis‐Wozowicz S, Kmiotek K, Sekula M, Kedracka‐Krok S, Kamycka E, Adamiak M, Jankowska U, Madetko‐Talowska A, Sarna M, Bik‐Multanowski M, Kolcz J, Boruczkowski D, Madeja Z, Dawn B & Zuba‐Surma EK (2015). Human induced pluripotent stem cell‐derived microvesicles transmit RNAs and proteins to recipient mature heart cells modulating cell fate and behavior. Stem Cells 33, 2748–2761. [DOI] [PubMed] [Google Scholar]
- Bode F, Karasik P, Katus HA & Franz MR (2002). Upstream stimulation versus downstream stimulation: arrhythmogenesis based on repolarization dispersion in the human heart. J Am Coll Cardiol 40, 731–736. [DOI] [PubMed] [Google Scholar]
- Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J & Anversa P (2011). Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brodsky MA, Allen BJ, Baron D, Chesnie BM, Abate D, Thomas R & Henry WL (1986). Enhanced survival in patients with heart failure and life‐threatening ventricular tachyarrhythmias. Am Heart J 112, 1166–1172. [DOI] [PubMed] [Google Scholar]
- Carpenter L, Carr C, Yang CT, Stuckey DJ, Clarke K & Watt SM (2012). Efficient differentiation of human induced pluripotent stem cells generates cardiac cells that provide protection following myocardial infarction in the rat. Stem Cells Dev 21, 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, Marban E & Abraham MR (2006). Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation 113, 1832–1841. [DOI] [PubMed] [Google Scholar]
- Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S & Sun JP (2004). Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol 94, 92–95. [DOI] [PubMed] [Google Scholar]
- Cheng K, Blusztajn A, Shen D, Li TS, Sun B, Galang G, Zarembinski TI, Prestwich GD, Marban E, Smith RR & Marban L (2012. a). Functional performance of human cardiosphere‐derived cells delivered in an in situ polymerizable hyaluronan‐gelatin hydrogel. Biomaterials 33, 5317–5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Shen D, Smith J, Galang G, Sun B, Zhang J & Marban E (2012. b). Transplantation of platelet gel spiked with cardiosphere‐derived cells boosts structural and functional benefits relative to gel transplantation alone in rats with myocardial infarction. Biomaterials 33, 2872–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van BB, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA & Murry CE (2014). Human embryonic‐stem‐cell‐derived cardiomyocytes regenerate non‐human primate hearts. Nature 510, 273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P & Bolli R (2012). Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126, S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Kizana E, Terrovitis J, Barth AS, Zhang Y, Smith RR, Miake J & Marban E (2010. a). Isolation and expansion of functionally‐competent cardiac progenitor cells directly from heart biopsies. J Mol Cell Cardiol 49, 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Smith RR & Marban E (2010. b). Human cardiospheres are a source of stem cells with cardiomyogenic potential. Stem Cells 28, 903–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR & Stewart DJ (2011). Autologous cell therapy for cardiac repair. Expert Opin Biol Ther 11, 489–508. [DOI] [PubMed] [Google Scholar]
- Davis DR & Stewart DJ (2014). Cell therapy for cardiac repair – bench to bedside and back. In Cardiac Regeneration and Repair, ed. Li RK. & Weisel RD, pp. 138–162. Woodhead Publishing, Sawston, UK. [Google Scholar]
- Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, Li TS, White A, Makkar R & Marban E (2009). Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One 4, e7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert SN, Taylor DG, Nguyen HL, Kodack DP, Beyers RJ, Xu Y, Yang Z & French BA (2007). Noninvasive tracking of cardiac embryonic stem cells in vivo using magnetic resonance imaging techniques. Stem Cells 25, 2936–2944. [DOI] [PubMed] [Google Scholar]
- Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A & Li RK (2006). Cardioprotective c‐kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest 116, 1865–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes S, Amirault JC, Lande G, Nguyen JM, Forest V, Bignolais O, Lamirault G, Heudes D, Orsonneau JL, Heymann MF, Charpentier F & Lemarchand P (2006). Autologous myoblast transplantation after myocardial infarction increases the inducibility of ventricular arrhythmias. Cardiovasc Res 69, 348–358. [DOI] [PubMed] [Google Scholar]
- Grens K (2015). Hearts on trial. Scientist 29 (1 May). [Google Scholar]
- Guerrero PA, Schuessler RB, Davis LM, Beyer EC, Johnson CM, Yamada KA & Saffitz JE (1997). Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest 99, 1991–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagege AA, Carrion C, Menasche P, Vilquin JT, Duboc D, Marolleau JP, Desnos M & Bruneval P (2003). Viability and differentiation of autologous skeletal myoblast grafts in ischaemic cardiomyopathy. Lancet 361, 491–492. [DOI] [PubMed] [Google Scholar]
- Hartman ME, Dai DF & Laflamme MA (2015). Human pluripotent stem cells: Prospects and challenges as a source of cardiomyocytes for in vitro modeling and cell‐based cardiac repair. Adv Drug Deliv Rev Doi: 10.1016/j.addr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JQ, Ma Y, Lee Y, Thomson JA & Kamp TJ (2003). Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res 93, 32–39. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Graf EM, Leutheuser J, Bock M, Balana B, Zahanich I, Christ T, Boxberger S, Wettwer E & Ravens U (2004). Electrophysiological properties of human mesenchymal stem cells. J Physiol 554, 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK & Goodell MA (2001). Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest 107, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevanantham V, Butler M, Saad A, Abdel‐Latif A, Zuba‐Surma EK & Dawn B (2012). Adult bone marrow cell therapy improves survival and induces long‐term improvement in cardiac parameters: a systematic review and meta‐analysis. Circulation 126, 551–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesty SA, Steffey MA, Lee FK, Breitbach M, Hesse M, Reining S, Lee JC, Doran RM, Nikitin AY, Fleischmann BK & Kotlikoff MI (2012). c‐kit+ precursors support postinfarction myogenesis in the neonatal, but not adult, heart. Proc Natl Acad Sci USA 109, 13380–13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R & Marban E (2009). Engraftment, differentiation, and functional benefits of autologous cardiosphere‐derived cells in porcine ischemic cardiomyopathy. Circulation 120, 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC & Lee DS (2006). Tissue distribution of 18F‐FDG‐labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med 47, 1295–1301. [PubMed] [Google Scholar]
- Kannel WB, Plehn JF & Cupples LA (1988). Cardiac failure and sudden death in the Framingham Study. Am Heart J 115, 869–875. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T, Okano T & Sawa Y (2012). Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell‐derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126, S29–S37. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Ohnishi Y, Yoshida A, Okajima K, Azumi H, Ishida A, Galeano EJ, Kubo S, Hayashi Y, Itoh H & Yokoyama M (2002). Heterogeneous loss of connexin43 protein in nonischemic dilated cardiomyopathy with ventricular tachycardia. J Cardiovasc Electrophysiol 13, 865–870. [DOI] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM & Itescu S (2001). Neovascularization of ischemic myocardium by human bone‐marrow‐derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med 7, 430–436. [DOI] [PubMed] [Google Scholar]
- Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, Geisen C, Xia Y, Lu Z, Duan Y, Kettenhofen R, Jovinge S, Bloch W, Bohlen H, Welz A, Hescheler J, Jacobsen SE & Fleischmann BK (2006). Engraftment of engineered ES cell‐derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med 203, 2315–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CS, Munakata K, Reddy CP & Surawicz B (1983). Characteristics and possible mechanism of ventricular arrhythmia dependent on the dispersion of action potential durations. Circulation 67, 1356–1367. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J & Murry CE (2007). Cardiomyocytes derived from human embryonic stem cells in pro‐survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25, 1015–1024. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V & Murry CE (2005). Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol 167, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham N, Ye B, Jackson R, Lam B, Kuraitis D, Ruel M, Suuronen EJ, Stewart DJ & Davis DR (2013). Human blood and cardiac stem cells synergize to enhance cardiac repair when cotransplanted into ischemic myocardium. Circulation 128, S1–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ST, White AJ, Matsushita S, Malliaras K, Steenbergen C, Zhang Y, Li TS, Terrovitis J, Yee K, Simsir S, Makkar R & Marban E (2011). Intramyocardial injection of autologous cardiospheres or cardiosphere‐derived cells preserves function and minimizes adverse ventricular remodeling in pigs with heart failure post‐myocardial infarction. J Am Coll Cardiol 57, 455–465. [DOI] [PubMed] [Google Scholar]
- Lerner DL, Yamada KA, Schuessler RB & Saffitz JE (2000). Accelerated onset and increased incidence of ventricular arrhythmias induced by ischemia in Cx43‐deficient mice. Circulation 101, 547–552. [DOI] [PubMed] [Google Scholar]
- Li GR, Sun H, Deng X & Lau CP (2005). Characterization of ionic currents in human mesenchymal stem cells from bone marrow. Stem Cells 23, 371–382. [DOI] [PubMed] [Google Scholar]
- Li T‐S, Cheng K, Lee S‐T, Matsushita S, Davis DR Malliaras K, Zhang Y, Smith RR & Marban E (2010). Cardiospheres recapitulate a niche‐like microenvironment rich in stemness and cell‐matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells 28, 2088–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L & Marban E (2012). Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere‐derived cells. J Am Coll Cardiol 59, 942–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang F, Song G, Gu W, Chen M, Yang B, Li D, Wang D & Cao K (2013). Intramyocardial injection of pig pluripotent stem cells improves left ventricular function and perfusion: a study in a porcine model of acute myocardial infarction. PLoS One 8, e66688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieu DK, Fu JD, Chiamvimonvat N, Tung KC, McNerney GP, Huser T, Keller G, Kong CW & Li RA (2013). Mechanism‐based facilitated maturation of human pluripotent stem cell‐derived cardiomyocytes. Circ Arrhythm Electrophysiol 6, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grogaard HK, Bjornerheim R, Brekke M, Muller C, Hopp E, Ragnarsson A, Brinchmann JE & Forfang K (2006). Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med 355, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G & Marban E (2012). Intracoronary cardiosphere‐derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379, 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marban L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC & Marban E (2014). Intracoronary cardiosphere‐derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1‐year results of the CADUCEUS trial (CArdiosphere‐Derived aUtologous stem CElls to reverse ventricUlar dySfunction). J Am Coll Cardiol 63, 110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield AE, Tilokee EL & Davis DR (2014. a). Resident cardiac stem cells and their role in stem cell therapies for myocardial repair. Can J Cardiol 30, 1288–1298. [DOI] [PubMed] [Google Scholar]
- Mayfield AE, Tilokee EL, Latham N, McNeill B, Lam BK, Ruel M, Suuronen EJ, Courtman DW, Stewart DJ & Davis DR (2014. b). The effect of encapsulation of cardiac stem cells within matrix‐enriched hydrogel capsules on cell survival, post‐ischemic cell retention and cardiac function. Biomaterials 35, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M & Hagege AA (2008). The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo‐controlled study of myoblast transplantation. Circulation 117, 1189–1200. [DOI] [PubMed] [Google Scholar]
- Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT & Marolleau JP (2001). Myoblast transplantation for heart failure. Lancet 357, 279–280. [DOI] [PubMed] [Google Scholar]
- Menasche P, Hagege AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP & Duboc D (2003). Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol 41, 1078–1083. [DOI] [PubMed] [Google Scholar]
- Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G & Giacomello A (2004). Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95, 911–921. [DOI] [PubMed] [Google Scholar]
- Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A & Drexler H (2006). Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow‐up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST‐elevation infarct regeneration) trial. Circulation 113, 1287–1294. [DOI] [PubMed] [Google Scholar]
- Molgat AS, Tilokee EL, Rafatian G, Vulesevic B, Ruel M, Milne R, Suuronen EJ & Davis DR (2014). Hyperglycemia inhibits cardiac stem cell‐mediated cardiac repair and angiogenic capacity. Circulation 130, S70–S76. [DOI] [PubMed] [Google Scholar]
- Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott‐Flugel L, Dorn T, Goedel A, Hohnke C, Hofmann F, Seyfarth M, Sinnecker D, Schomig A & Laugwitz KL (2010). Patient‐specific induced pluripotent stem‐cell models for long‐QT syndrome. N Engl J Med 363, 1397–1409. [DOI] [PubMed] [Google Scholar]
- Mummery C, Ward‐van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R & Tertoolen L (2003). Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm‐like cells. Circulation 107, 2733–2740. [DOI] [PubMed] [Google Scholar]
- Muraski JA, Fischer KM, Wu W, Cottage CT, Quijada P, Mason M, Din S, Gude N, Alvarez R Jr, Rota M, Kajstura J, Wang Z, Schaefer E, Chen X, MacDonnel S, Magnuson N, Houser SR, Anversa P & Sussman MA (2008). Pim‐1 kinase antagonizes aspects of myocardial hypertrophy and compensation to pathological pressure overload. Proc Natl Acad Sci USA 105, 13889–13894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA & Field LJ (2004). Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664–668. [DOI] [PubMed] [Google Scholar]
- Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD & Lee RT (2004). Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation 110, 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML & Schneider MD (2003). Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA 100, 12313–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani FD, DerSimonian H, Zawadzka A, Wetzel K, Edge AS, Jacoby DB, Dinsmore JH, Wright S, Aretz TH, Eisen HJ & Aaronson KD (2003). Autologous skeletal myoblasts transplanted to ischemia‐damaged myocardium in humans. Histological analysis of cell survival and differentiation. J Am Coll Cardiol 41, 879–888. [DOI] [PubMed] [Google Scholar]
- Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, Fishbein MC, Sharifi BG, Chen PS & Makkar R (2003). Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a swine model of myocardial infarction. J Cardiovasc Electrophysiol 14, 841–848. [DOI] [PubMed] [Google Scholar]
- Povsic TJ, O'Connor CM, Henry T, Taussig A, Kereiakes DJ, Fortuin FD, Niederman A, Schatz R, Spencer R, Owens D, Banks M, Joseph D, Roberts R, Alexander JH & Sherman W (2011). A double‐blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am Heart J 162, 654–662. [DOI] [PubMed] [Google Scholar]
- Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS & Makkar RR (2006). Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol 111, 231–239. [DOI] [PubMed] [Google Scholar]
- Robey TE, Saiget MK, Reinecke H & Murry CE (2008). Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol 45, 567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, Doran R, Becher UM, Hwang SM, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI & Fleischmann BK (2007). Engraftment of connexin 43‐expressing cells prevents post‐infarct arrhythmia. Nature 450, 819–824. [DOI] [PubMed] [Google Scholar]
- Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, Magyar J, Schroder EA, Perlman I & Gepstein L (2004). Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol 559, 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S & Zeiher AM (2004). Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one‐year results of the TOPCARE‐AMI Trial. J Am Coll Cardiol 44, 1690–1699. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Assmus B, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Yu J, Corti R, Mathey DG, Hamm CW, Tonn T, Dimmeler S & Zeiher AM (2009). Intracoronary infusion of bone marrow‐derived mononuclear cells abrogates adverse left ventricular remodelling post‐acute myocardial infarction: insights from the reinfusion of enriched progenitor cells and infarct remodelling in acute myocardial infarction (REPAIR‐AMI) trial. Eur J Heart Fail 11, 973–979. [DOI] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin‐Kern JL, Lechene CP & Lee RT (2013). Mammalian heart renewal by pre‐existing cardiomyocytes. Nature 493, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE & Laflamme MA (2012). Human ES‐cell‐derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla DK, Long X, Glass C, Singla RD & Yan B (2011). Induced pluripotent stem (iPS) cells repair and regenerate infarcted myocardium. Mol Pharm 8, 1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR & Marban E (2007). Regenerative potential of cardiosphere‐derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115, 896–908. [DOI] [PubMed] [Google Scholar]
- Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, Maat AP & Serruys PW (2003). Catheter‐based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six‐month follow‐up. J Am Coll Cardiol 42, 2063–2069. [DOI] [PubMed] [Google Scholar]
- Song H, Song BW, Cha MJ, Choi IG & Hwang KC (2010). Modification of mesenchymal stem cells for cardiac regeneration. Expert Opin Biol Ther 10, 309–319. [DOI] [PubMed] [Google Scholar]
- Soonpaa MH & Field LJ (1997). Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am J Physiol Heart Circ Physiol 272, H220–H226. [DOI] [PubMed] [Google Scholar]
- Takahashi K & Yamanaka S (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Taljaard M, Ward MR, Kutryk MJ, Courtman DW, Camack NJ, Goodman SG, Parker TG, Dick AJ, Galipeau J & Stewart DJ (2010). Rationale and design of Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction (ENACT‐AMI): the first randomized placebo‐controlled trial of enhanced progenitor cell therapy for acute myocardial infarction. Am Heart J 159, 354–360. [DOI] [PubMed] [Google Scholar]
- Terrovitis JV, Smith RR & Marban E (2010). Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res 106, 479–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Editors (2014). Expression of concern: the SCIPIO trial. Lancet 383, 1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS & Jones JM (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- Torella D, Indolfi C & Nadal‐Ginard B (2015). Generation of new cardiomyocytes after injury: de novo formation from resident progenitors vs. replication of pre‐existing cardiomyocytes. Ann Transl Med 3, S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E & Molkentin JD (2014). c‐kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Ponten A, Fleischmann BK & Jovinge S (2010). Cardiomyocyte cell cycle control and growth estimation in vivo–an analysis based on cardiomyocyte nuclei. Cardiovasc Res 86, 365–373. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Chen PS, Qu Z, Karagueuzian HS, Lin SF & Garfinkel A (2002). Electrical restitution and cardiac fibrillation. J Cardiovasc Electrophysiol 13, 292–295. [DOI] [PubMed] [Google Scholar]
- Yeung DF, Boom NK, Guo H, Lee DS, Schultz SE & Tu JV (2012). Trends in the incidence and outcomes of heart failure in Ontario, Canada: 1997 to 2007. CMAJ 184, E765–E773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, Palecek SP, Lyons GE, Thomson JA, Herron TJ, Jalife J & Kamp TJ (2012). Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res 111, 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA & Kamp TJ (2009. a). Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res 104, e30–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SN, Sun AJ, Ge JB, Yao K, Huang ZY, Wang KQ & Zou YZ (2009. b). Intracoronary autologous bone marrow stem cells transfer for patients with acute myocardial infarction: a meta‐analysis of randomised controlled trials. Int J Cardiol 136, 178–185. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Thorn S, DaSilva JN, Lamoureux M, deKemp RA, Beanlands RS, Ruel M & Suuronen EJ (2008). Collagen‐based matrices improve the delivery of transplanted circulating progenitor cells: development and demonstration by ex vivo radionuclide cell labeling and in vivo tracking with positron‐emission tomography. Circ Cardiovasc Imaging 1, 197–204. [DOI] [PubMed] [Google Scholar]
- Zhu WZ, Santana LF & Laflamme MA (2009). Local control of excitation‐contraction coupling in human embryonic stem cell‐derived cardiomyocytes. PLoS ONE 4, e5407. [DOI] [PMC free article] [PubMed] [Google Scholar]


