Abstract
The mechanisms underpinning human cardiac fibrillation remain elusive. In his 1913 paper ‘On dynamic equilibrium in the heart’, Mines proposed that an activation wave front could propagate repeatedly in a circle, initiated by a stimulus in the vulnerable period. While the dynamics of activation and recovery are central to cardiac fibrillation, these physiological data are rarely used in clinical mapping. Fibrillation is a rapid irregular rhythm with spatiotemporal disorder resulting from two fundamental mechanisms – sources in preferred cardiac regions or spatially diffuse self‐sustaining activity, i.e. with no preferred source. On close inspection, however, this debate may also reflect mapping technique. Fibrillation is initiated from triggers by regional dispersion in repolarization, slow conduction and wavebreak, then sustained by non‐uniform interactions of these mechanisms. Notably, optical mapping of action potentials in atrial fibrillation (AF) show spiral wave sources (rotors) in nearly all studies including humans, while most traditional electrogram analyses of AF do not. Techniques may diverge in fibrillation because electrograms summate non‐coherent waves within an undefined field whereas optical maps define waves with a visually defined field. Also fibrillation operates at the limits of activation and recovery, which are well represented by action potentials while fibrillatory electrograms poorly represent repolarization. We conclude by suggesting areas for study that may be used, until such time as optical mapping is clinically feasible, to improve mechanistic understanding and therapy of human cardiac fibrillation.
Abbreviations
- APD
action potential duration
- AF
atrial fibrillation
- DI
diastolic interval
- FIRM
focal impulse and rotor mapping
- MAP
monophasic action potential
- VF
ventricular fibrillation
Introduction
Cardiac fibrillation encompasses the most complex currently appreciated electrical disorders of the heart, whose rapidly changing spatiotemporal patterns challenge our mechanistic understanding. In many ways, fibrillation represents a ‘final frontier’ in arrhythmia medicine.
Atrial fibrillation (AF) is the most common sustained arrhythmia in the world, affecting over 30 million individuals worldwide (Chugh et al. 2014), and is a major cause of hospitalizations, stroke and death. Therapy includes drugs to modulate cellular or membrane electrical function, or ablation to eliminate pro‐arrhythmic tissue, yet both remain suboptimal due to uncertain mechanistic targets and side‐effects on bystander myocardium (January et al. 2014).
Ventricular fibrillation (VF) is a major cause of sudden cardiac arrest, which afflicts over 300,000 individuals per year in the USA (Chugh et al. 2004) and a similar number in Europe. Pharmacological therapy is limited, and even drugs with sound scientific rationale can increase mortality, highlighting dangerous gaps in our understanding (The Cardiac Arrhythmia Suppression Trial (CAST) Investigators, 1989). Non‐pharmacological therapies are also suboptimal and primarily comprise ablation of potentially large areas of viable tissue with modest success rates or cessation of an episode of fibrillation by high‐energy electric field‐shock that does not prevent future episodes.
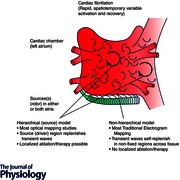
Improved mechanistic definition is essential to improve therapy. A central debate in human atrial or ventricular fibrillation is whether disordered activity is driven by organized processes (hierarchical model) or is self‐sustaining (non‐hierarchical model). Fibrillatory wavelets have a stochastic probability of terminating upon encountering a boundary, and must therefore be replenished to sustain fibrillation (Chen et al. 2000 a). In the first model wavelets are replenished by spatially localized mechanisms, at preferred ‘source’ regions, whereas in the second model wavelets self‐replenish, indicating no preferred regional source(s). This dichotomy is of fundamental importance because it dramatically influences mechanistic thinking at the genetic and cellular levels, structure–function relationships, imaging and therapy.
It is striking not only how little overlap exists between camps, but that this dichotomy may also be divided by mapping technique. Focusing on AF, whilst optical mapping studies of regional activation and recovery (action potentials) mostly show stable source regions that drive disordered activity, most studies that map surrogates for local activation (bipolar or unipolar electrograms) do not, as summarized in Table 1.
Table 1.
Differences in atrial fibrillation mechanisms by mapping modality
| Surface (field | Spatial | Quoted mechanism | ||||
|---|---|---|---|---|---|---|
| Study | Species | Chamber | of view) | resolution | Signals | in original study |
| Atrial – electrogram | ||||||
| Cox et al. (1991) | Human | RA and LA | Epicardial (narrow field) | 6 mm | EGM (bipolar) | ‘Single reentrant circuit’ in RA, LA ‘reentry could not be detected’. |
| Schuessler et al. (1992) | Dogs | RA | Epicardial (narrow field) | 5 mm | EGM (bipolar) | ‘Multiple reentrant circuits stabilized into a single small circuit by ACh’. |
| Konings et al. (1994) | Human | RA | Epicardial (narrow field) | 2.25 mm | EGM (unipolar) | (1) ‘Reentry (random/leading circle)’. (2) ‘Epicardial breakthrough’. |
| Holm et al. (1997) | Human | RA | Epicardial (narrow field) | 3 mm | EGM (bipolar) | (1) ‘Organized (non‐random) re‐entry’. (2) ‘Focal’. |
| Schilling et al. (2000) | Human | RA | Endocardial (wide field) | N/A | Virtual EGM from non‐contact balloon | (1) ‘Single reentry’. (2) ‘Multiple wavefronts’. |
| Wu et al. (2002) | Human | RA and LA | Epicardial (narrow field) | 3 mm | EGM (bipolar) | (1) ‘Large wavefront in RA’. (2) ‘Rapid repetitive activities in LA’. |
| Sahadevan et al. (2004) | Human | RA and LA | Epicardial (series of plaques) | 1.2 mm | EGM (bipolar) | (1) ‘Driver with fibrillatory conduction’. (2) ‘No observable pattern’. |
| Houben et al. (2004) | Human | RA | Epicardial (narrow field) | 2.25 mm | EGM (unipolar) | (1) ‘Multiple wavefronts’. (2) ‘A stable reentrant circuit was not seen’. |
| Allessie et al. (2010) | Human | RA | Epicardial (narrow field) | 2.25 mm | EGM (unipolar) | (1) ‘We failed to find any rotors or foci that could explain the persistence of AF’. (2) ‘Longitudinal dissociation’. |
| de Groot et al. (2010) | Human | RA | Epicardial (narrow field) | 2.25 mm | EGM (unipolar) | (1) ‘Complete reentrant circuits in the epicardial plane were extremely rare’. (2) ‘Epicardial breakthrough’. |
| Narayan et al. (2012 d) | Human | LA and RA | Endocardial (wide field) | 4 mm | Computational physiological filtering of EGMs (FIRM) | (1) ‘Localized rotors’*. (2) ‘Focal impulses’*. |
| Eckstein et al. (2013) | Goat | LA | Endocardial and epicardial (narrow field) | 1.6 mm | EGM (unipolar) | (1) ‘Full 360° rotation was found in < 1% of all 3944 waves’. (2) ‘Endo‐epi dissociation’. |
| Haissaguerre et al. (2013) | Human | LA and RA | Epicardial (wide) | 5–10 mm | Virtual EGM from body surface | (1) ‘Unstable rotors’. |
| Lee et al. (2013) | Canine | RA and LA | Epicardial (narrow field) | 1.2 mm | EGM (bipolar) | (1) ‘Multiple foci’. (2) ‘No random reentry’. (3) ‘Ordered reentry infrequent’. |
| Miller et al. (2014) | Human | LA and RA | Endocardial (wide) | 4 mm | Computational physiological filtering of EGMs (FIRM) | (1) ‘Patient specific rotor and focal sources’*. |
| Lee et al. (2014) | Human | LA | Epicardial (narrow field) | 2.5 mm | EGM (bipolar) | (1) ‘Multiple unstable wavefronts’. (2) ‘Disorganized activity’. (3) ‘Transient rotational circuits’. |
| Walters et al. (2015) | Human | LA | Epicardial (narrow field) | 2.5 mm | EGM (bipolar) | (1) ‘Transient rotors and focal activations’. |
| Atrial – optical | ||||||
| Gray et al. (1996) | Sheep | RA | Epicardial and transmural | 0.5 mm | Optical action potentials | (1) ‘Incomplete reentry’. (2) ‘Epicardial breakthrough’. |
| Skanes et al. (1998) | Sheep | RA and LA | Epicardial | 0.12 mm | Optical action potentials | (1) ‘Stationary rotors’*. (2) ‘Periodic breakthroughs’. |
| Berenfeld et al. (2000) | Sheep | RA and LA | Epicardial | 0.5 mm | Optical action potentials | ‘Microreentrant sources in the LA with fibrillatory conduction’*. |
| Sarmast et al. (2003) | Sheep | RA and LA | Epicardial | 0.5 mm | Optical action potentials | ‘LA and RA rotors’*. |
| Po et al. (2005) | Canine | LA/PV | Endocardial | 0.11 mm | Optical action potentials | ‘Single stationary PV reentrant circuits conforming with rotor hypothesis’*. |
| Chou et al. (2011) | Canine | LA/PV | Epicardial | 0.24 mm | Optical action potentials | ‘Epicardial ablation of LA rotor anchoring sites suppresses AF’*. |
| Gutbrod et al. (2015) | Sheep | LA | Epicardial and transmural | 0.5 mm | Optical action potentials | (1) ‘Short lived meandering rotors’. (2) ‘Transmural discordance’. |
| Hansen et al. (2015) | Human | RA | Epicardial and transmural | 0.33 mm | Optical action potentials | (1) ‘Single localized micro‐anatomic re‐entry can sustain AF in the human heart ex vivo and support the localized driver hypothesis’*. |
| Zhao et al. (2015) | Human | LA | Epicardial and transmural | 0.33 mm | Optical action potentials | (1) ‘Stationary reentrant AF drivers’*. |
*Studies showing stable fibrillatory sources. Endo, endocardium; epi, epicardium; LA, left atrium; PV, pulmonary vein; RA, right atrium.
This review sets out to reconcile how mapping techniques may diverge in fibrillation due to the functional information they convey. We first review results from optical mapping (Gray et al. 1998), often considered the gold standard (Efimov et al. 2004). These studies highlight re‐entry (Spach, 2001; Kléber & Rudy, 2004) that emphasize Mines's seminal work on dynamic equilibrium (Mines, 1913), which defined basic criteria for re‐entrant arrhythmias and introduced the concept of a vulnerable period. The centenary of this work was marked in the The Journal of Physiology (Paterson, 2013) and its continued importance is clear from the depth and breadth of articles in this special issue. Second, we discuss how various mapping approaches that are concordant in organized rhythms may diverge in fibrillation, focusing on the commonly mapped and ablated rhythm of AF. Third, given recent optical mapping studies of human cardiac fibrillation, we attempt to reconcile the results of clinical mapping in human AF and VF. We conclude by suggesting fruitful areas for future study.
Dynamic equilibrium and human cardiac fibrillation
Dynamic and fixed mechanisms are essential ingredients of paroxysmal arrhythmias (Weiss et al. 2015). Under certain conditions, a single ectopic beat can initiate supraventricular tachycardia using stable anatomical pathways, yet under different conditions the ectopic beat may remain orphaned. These concepts also apply to AF or VF, in which dynamic triggers such as ectopic beats, short bursts of tachycardia or varying autonomic balance may initiate fibrillation despite relatively unchanging cardiac architecture, surface curvature, fibre angles, fibrosis and other ‘fixed’ substrates (Engelman et al. 2010).
Dynamic factors can be considered in the context of Mines's dynamic equilibrium of activation and recovery. His original definition describes what we may currently call restitution of these fundamental determinants of reentry: ‘For the beating heart is in a complex dynamic equilibrium. The character of each individual beat depends, inter alia, upon the lapse of time between that beat and its predecessors.’
This review uses this concept as a foundation to understand fibrillation – in deference to Mines, but also because this equilibrium is mostly neglected in studies of human cardiac fibrillation. Few clinical studies have defined the rate response (restitution) of repolarization or conduction leading up to or during fibrillation, and fewer still incorporate these concepts dynamically into mapping.
The relevance of dynamic equilibrium to clinical fibrillation is shown in Fig. 1, illustrating mechanisms enabling a trigger to initiate human AF. In Fig. 1 A, a single ectopic beat initiates human AF during dramatic oscillations of left atrial action potential duration (APD) (Narayan et al. 2002, 2008 b, 2011 a). Figure 1 B show that APD oscillations can be explained by rate response of electrical recovery – i.e. restitution curves of APD against diastolic interval (DI, time between successive activations) (Franz et al. 1988; Franz, 2003). APD shortens with DI, but in this case its slope of > 1 enabled an early beat (i.e. short DI) to amplify subsequent APD shortening, which amplified DI lengthening for the next beat and so on. Such APD alternans has been shown in animal models (Elzinga et al. 1981), and has been shown in patients to enable initiation of AF in proportion to arrhythmic risk (Fig. 1) (Narayan et al. 2002, 2008 b, 2011 a), ventricular tachycardia (Koller et al. 2005; Narayan et al. 2007) and VF (Karagueuzian et al. 1993; Gelzer et al. 2008).
Figure 1. Dynamic balance between activation and recovery in initiating human atrial fibrillation .
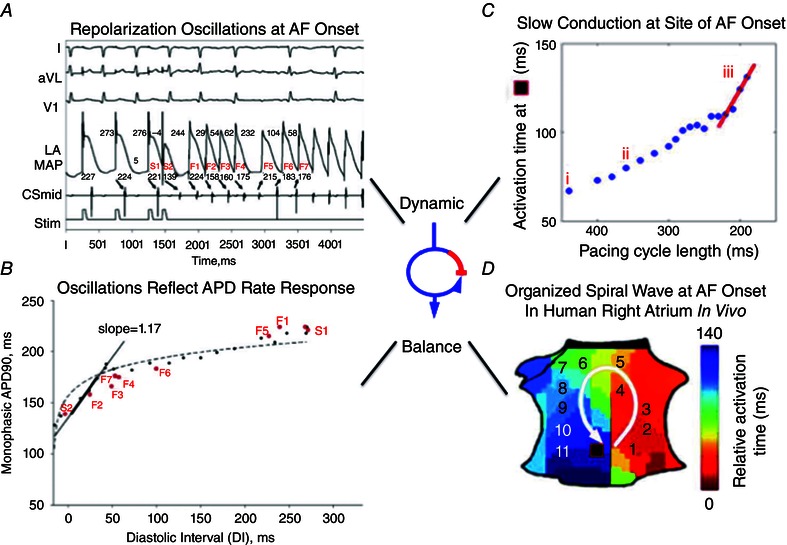
A, electrograms show ectopic beat (S2) causing AF (varying cycle lengths). B, steep APD restitution curve enables S2 to produce repolarization oscillations preceding AF. C, conduction restitution curve shows dynamic slowing at site of AF onset just prior to AF onset (iii, red slope). D, critical activation delay enables block and formation of a counter‐clockwise spiral initiating AF in right atrium. (From Narayan et al. 2008 b; Schricker et al. 2014.)
Conduction velocity is also rate dependent, and Fig. 1 C shows that triggers (in this case, tachycardia) may dramatically slow conduction imminently before AF initiation. Thus, the dynamic equilibrium of APD (repolarization) dispersion and conduction slowing can enable a trigger to produce spiral‐wave re‐entry and initiate human AF (Fig. 1 D) (Schricker et al. 2014).
This concept can also explain the arrhythmic impact of interventions. For instance, cholinergic stimulation (an AF trigger) shortens APD (Po et al. 2005), flattens APD restitution in canine atria (Burashnikov & Antzelevitch, 2005) and slows conduction (Shumaker et al. 1993) – particularly if spatially non‐uniform. Mines's concept of dynamic equilibrium therefore provides a useful model for dissecting tractable components of cardiac fibrillation.
Mapping cardiac fibrillation using dynamics of activation and recovery, and using electrograms
Fibrillation is a rapid spatiotemporally varying arrhythmia that operates at the limits of activation and recovery. Ideally, therefore, mapping of fibrillation should identify activation and recovery at high temporal resolution and at multiple spatial sites without contamination (cross‐talk) from adjacent sites. Figure 2 summarizes the concept of cross‐talk between an electrode and its reference (Fig. 2 A). In spatially coherent rhythms such as flutter where atrial regions activate 1:1 (Fig. 2 B), cross‐talk may have limited impact such as altering activation onset time, but as adjacent regions start to reflect unrelated, out‐of‐phase (incoherent) wavefronts in fibrillation (Figs 2 C and 3) the impact of cross‐talk is more dramatic and may not only alter activation onset times but even spuriously introduce signals or obscure local activation (Fig. 3).
Figure 2. Quantitative analysis of electrogram morphology .
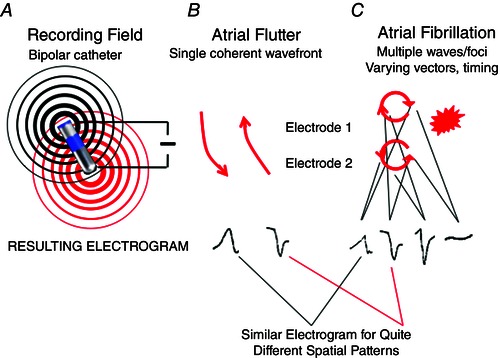
A, poles of a catheter (bipolar – close, unipolar – distant) record from distinct mapping fields. B, electrograms reflect single wavefront of an organized rhythm (e.g. atrial flutter). C, fibrillation, characterized by an uncertain number of wavefronts of uncertain rate, relative timing (phase) and spatial size in undefined recording fields. Summation of these waves may produce variable electrograms from the same spatiotemporal mechanism, or similar electrograms from variable mechanisms. Accordingly, ‘qS’, ‘rR’, or other electrogram rules in fibrillation are not specific for any particular mechanism. The same argument may apply to unipolar electrograms, which summate across wider regions of tissue.
Figure 3. Limitations of electrogram based activation mapping in AF .
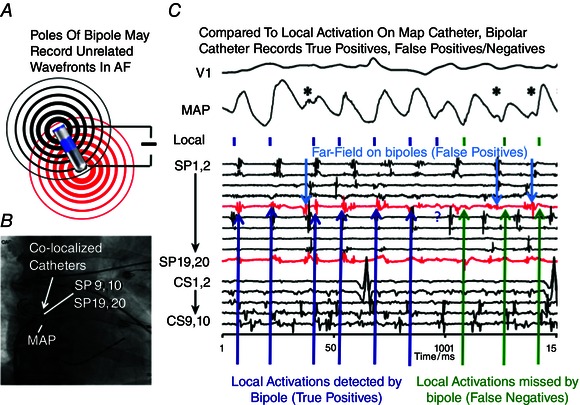
A, poles of a clinical bipolar electrode may record unrelated wavefronts in AF. B, fluoroscopic view of co‐localized MAP, bipolar catheters in human atrium. C, MAP in human right atrium indicate local activation (small vertical bars) from far field (asterisks). Notably, bipolar signals (in red) can indicate actual local activation (true positives), show no deflection (false negatives) and show signals that reflect far‐field electrograms (false positives). (Modified from Narayan et al. 2011 b.)
Optical mapping of voltage‐sensitive dyes currently provides the highest spatial resolution of both activation and recovery, with minimal cross‐talk. The approach illuminates the biological field with light to excite voltage‐sensitive dyes, that fluoresce in proportion to membrane potential, i.e. optical action potentials (OAPs) (Fig. 4). Successive images thus map propagation of activation (wavefront) and recovery (waveback). Crucially, optical maps show minimal cross‐talk – activity is represented by visually defined pixels relatively uninfluenced by remote regions. Limitations of optical mapping include the need to immobilize tissue, to minimize absorption of reflected light (e.g. by blood) and toxicity of fluorescent dyes. As a result, optical mapping is not yet clinically feasible, although proof of concept studies in small animals suggest that this may be on the horizon (Lee et al. 2012).
Figure 4. Spiral wave re‐entry as drivers of cardiac fibrillation .

A, schematic diagram of spiral wave, showing wavefront curvature as conduction velocity slows towards core (*), where wave front meets wave back. Action potentials from sites 1–3 show varying APD, allowing re‐entry around the unexcited, yet excitable core. (From Pandit & Jalife, 2013.) B, first experimental demonstration of spiral waves in rabbit VF. Phase is depicted in colour with spiral wave chirality indicated by + (clockwise) or – (counter‐clockwise). Three phase singularities (PS) are seen. (From Gray et al. 1998.) C, optical mapping of human atria shows stable micro‐reentrant sources on the endocardium sustaining AF, anchored to endocardial fibre complexity, yet passive, transient activity on the epicardium and elsewhere in the periphery. Optical action potentials (OAPs) at sites 1–4 on the endocardium show activation and recovery over multiple sequential cycles, yet electrograms (Cath 1 EG) vary due to cross‐talk and other factors. The authors concluded that stable endocardial micro‐reentrant sources produce unstable epicardial activations. (From Hansen et al. 2015.)
In clinical studies, activation and recovery (action potentials) can be measured from an extracellular monophasic action potential (MAP) catheter. Activation and repolarization measurements from MAPs are validated against intracellular recordings (Franz et al. 1990), reflect minimal far‐field activity (Knollmann et al. 2002), and their dynamics provide insights into human AF (Fig. 1) (Narayan & Franz, 2007; Narayan et al. 2011 b) or VF (Swartz et al. 1993). However, challenges in repeatedly recording MAPs at multiple sites in humans has limited this approach to a small number of experienced centres (Kim et al. 2002; Watanabe et al. 2002; Narayan & Franz, 2007).
In the clinical setting, for practical reasons, cardiac fibrillation is thus mostly mapped from electrograms – voltage–time series at one electrode relative to an adjacent (bipolar) or remote (unipolar) electrode. Electrogram analyses developed for coherent rhythms provide high temporal resolution that is easily repeated at multiple spatial sites. What is frequently overlooked in fibrillatory electrograms, however, is their dramatic potential for intra‐chamber cross‐talk (Narayan et al. 2011 b). In Fig. 3 C, bipolar AF electrograms include unclear components of local (desired) activity and far field (undesired) activity. Accordingly, AF electrograms may not be sensitive nor specific for local activation on adjacent MAPs – indeed, electrograms may even be absent at times when MAPs show clear local activation due to directionality and/or algebraic cancellation (Fig. 3) (Narayan et al. 2011 b). Moreover, action potentials encode information about activation and recovery while electrograms focus on activation, with the activation recovery interval measurable only during tightly controlled conditions (Haws & Lux, 1990) that are absent during fibrillation.
Fibrillatory electrograms are typically analysed using rules developed for organized rhythms (Allessie et al. 2010; Houben et al. 2010; Lee et al. 2014) (Figs 2 and 3), yet resulting maps may be less accurate as rhythms progress from coherent (e.g. flutter) towards non‐spatially coherent fibrillation. Unipolar electrograms may be more accurate than bipolar, since adjacent (non‐coherent) activity is not subtracted (Steinhaus, 1989; Ndrepepa et al. 1995) although, conversely, they summate larger fields. Figure 5 A shows challenges in interpreting unipolar electrograms in AF, which have not been proven to represent local or far field activity by validation from optical maps. This may dramatically influence the results from AF maps.
Figure 5. Human AF maps show differing mechanisms based on mapping technique .
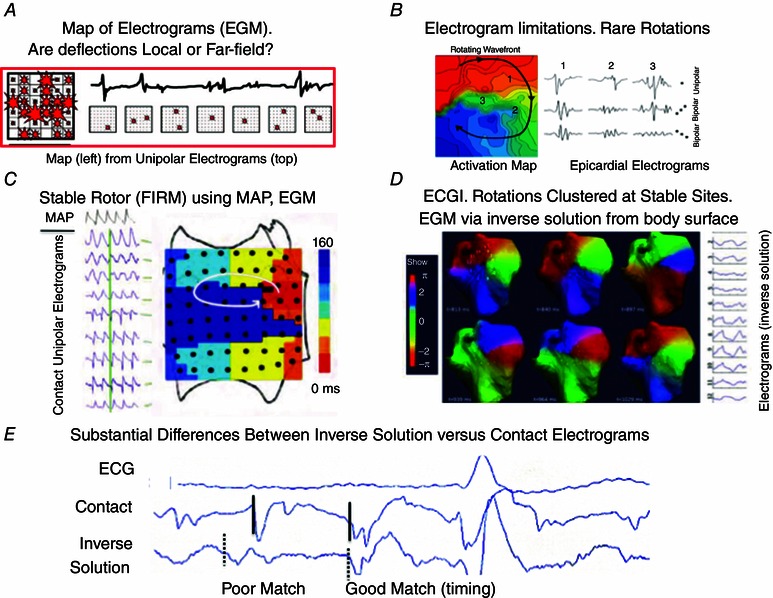
A, electrograms from epicardial plaque produce complex maps in human AF. Electrograms used for maps (top) illustrate the challenge of assigning local activity with confidence. No rotational wavefronts were seen in > 4000 maps. (From de Groot et al. 2010.) B, rotor on activation map (early to late) from same group as A, which were sensitive to electrogram type and unstable in this study. (From Lau et al. 2015.) C, focal Impulse and rotor maps (FIRM) showing rotor in human AF, using computational reconstruction of activation and recovery (from physiological MAP and conduction restitution). A stable rotor is identified from phase mapping (here depicted by early meets late activation) that was eliminated by ablation. FIRM‐guided ablation may improve the results of conventional ablation. (From Narayan et al. 2012 c.) D, non‐invasive mapping using the inverse solution show AF sources (progression of phase colours), clustered in stable spatial regions that were treated by localized ablation. Causes for electrical instability at fixed spatial areas may reflect epicardial variability from stable endocardial rotors, technical limitations or other factors. (From Haissaguerre et al. 2014.) E, errors between virtual inverse solution and real contact electrograms in human AF. The first labelled electrogram poorly matched the contact electrogram in timing and shape; the second matched well in timing but not in shape. Timing and shape both influence activation and phase maps. (From Schilling et al. 2000.) See text for further details.
Optical mapping reveals localized sources for fibrillation (hierarchical model) across species including humans
Localized driving sources for fibrillation were postulated by Mines and Sir Thomas Lewis, then shown computationally (Krinksy, 1966). Spiral waves (rotors) have shown been to drive disordered activity in physical systems such as the Belousov–Zhabotinsky reaction (Winfree, 1972). Rotors have since been shown to be fibrillatory sources in ventricular tissue using optical mapping of fluorescent dyes (Davidenko et al. 1992; Witkowski et al. 1998), and have also been shown to drive AF in animal models (Mansour et al. 2001) and humans (Narayan et al. 2012 d; Hansen et al. 2015).
It is notable that optical mapping studies almost uniformly show that rotors sustain fibrillation, across model systems and species including humans, and sustain disorganized activity through a process termed fibrillatory conduction (Table 1). An optically mapped rotor is illustrated in Fig. 4. A rotor can be defined as a core or phase singularity (see below) from which spiral waves emanate at high speed to cause disorganization in surrounding tissue (Pandit & Jalife, 2013). This disorganization (fibrillatory conduction) may be due to factors such as steep APD restitution (Franz et al. 1988) conduction slowing (Kléber & Rudy, 2004) or tissue anisotropy (Valderrábano et al. 2001).
Optical imaging is well suited to map fibrillation because it simultaneously maps activation and recovery at high spatial resolution, with minimal cross‐talk and at high temporal resolution. Mathematical analyses are typically used to facilitate the quantification of organization within fibrillatory maps. Phase analysis developed by Gray et al. was first used to show organized reentrant sources in ventricular fibrillation (Gray et al. 1998) (Fig. 3 B). Phase represents the progression of tissue from activation onset through repolarization over time, which varies spatially and temporally during fibrillation. Spatial points around which phase exhibits a full rotation (i.e. –π to +π) have indeterminate phase and are termed singularities. Phase mapping is now validated across species for atrium and ventricle (Vaidya et al. 1999; Chen et al. 2000 b; Zaitsev et al. 2003; Noujaim et al. 2007).
This work has catalysed pivotal advances in understanding cardiac fibrillation. In recent decades, many studies have defined how spiral waves arise and drive fibrillation, attach to tissue heterogeneities such as fibrosis (Ikeda et al. 1997; Lim et al. 2006), and how organization breaks down at these edges into unstable fibrillatory activity (Pandit & Jalife, 2013). Rotors may also be detected by other techniques. For instance, periodicity indices such as dominant frequency after Fourier transform, have shown stable gradients in activation consistent with a rotor source (Zaitsev et al. 2000; Wu, 2002; Everett et al. 2004).
Rotors are traditionally considered distinct from functional reentry (leading circle) or anatomical reentry (Comtois et al. 2005), since rotors form due to extreme conduction slowing at the wave tip causing rotation around an ‘unexcited, yet eminently excitable’ core (Pandit & Jalife, 2013). However, spiral waves are well known to anchor to heterogeneities such as fibrosis and scar (Lin et al. 2008), and optical mapping of human AF shows stable endocardial micro‐reentry that sustain AF and precess near regions of micro‐fibrosis where localized ablation terminates AF (Hansen et al. 2015; Zhao et al. 2015). Mechanisms enabling the termination of AF by localized ablation are the subject of ongoing study (Rappel et al. 2015). Thus, the distinction between functional and structural reentry may continue to blur in human cardiac fibrillation (Zaman & Narayan, 2015).
In summary, the vast majority of studies in fibrillation that analysed action potentials – initially in animal ventricles, recently in human atria using optical maps – reveal localized sources that drive disorganization.
Clinically identified mechanisms for human atrial fibrillation
Any proposed mechanistic model for human AF must explain clinical observations in patients including ablation. These include stable gradients of AF rate (Filgueiras‐Rama et al. 2012) and transient linking of activation (Gerstenfeld et al. 1992) within the fibrillating atria. Mechanisms must also explain how limited ablation may on occasion terminate persistent AF (Herweg et al. 2003), as increasingly reported by mechanism‐targeted mapping (Shivkumar et al. 2012; Narayan et al. 2012 a) yet, paradoxically, why extensive empirical ablation may not improve outcomes compared to more conservative approaches (Wynn et al. 2014; Verma et al. 2015).
Optical mapping has heightened the mechanistic debate in human AF. Optical mapping shows that AF in human right and left atria can be sustained by stable endocardial sources at micro‐fibrotic regions and terminated by local ablation (Fig. 3 C) (Hansen et al. 2015; Zhao et al. 2015). While these data agree with the wider optical mapping literature (Table 1) and some clinical studies showing localized sources, they disagree with the majority of clinical reports that support disorganized mechanisms without sources. The next few sections will attempt to reconcile these differences.
The vast majority of mapping studies in human AF over two decades have analysed unipolar (Allessie et al. 2010) or bipolar electrograms (Lee et al. 2014) rather than action potentials (MAP or optical data) (Table 1). In 24 patients with longstanding persistent AF and valvular disease at surgery, Allessie et al. analysed electrograms from atrial plaques and stated ‘… in over 4000 maps of persistent AF… failed to find any rotors or foci that could explain the persistence of AF’ (Allessie et al. 2010). Potential criticisms of this work are that no interventions were tested, the authors simultaneously mapped < 10% of each atrium (areas > 100 cm2 in Jadidi et al. (2013)) so could not exclude sources in remaining atrium, and the authors used electrogram analyses that are challenging (Figs 2, 3 and 5 A). More recently, this group reported rotational activity in AF (Fig. 5 B) (Lau et al. 2015), focusing on the limitations of analysing complex signals. Notably, Fig. 3 illustrates how challenging it may be to analyze signals in AF. Accordingly, some studies that failed to identify sources using wide‐area mapping are difficult to interpret showing, for example, very long (slow) cycle lengths of 250–500 ms (frequency 2–4 Hz) in patients with AF, suggesting analytic errors and the use of bipolar analyses (Shannon's entropy) on unipolar signals (Benharash et al. 2015). Another study of electrograms from epicardial plaques also found rotational circuits, albeit transient (Walters et al. 2015), yet with reproducible vectorial propagation in 62% of cases over prolonged periods that contradict the complexity and lack of rotational circuits in earlier electrogram studies (Allessie et al. 2010).
A novel approach for clinical mapping of AF termed focal impulse and rotor mapping (FIRM) has been designed to circumvent some limitations of traditional electrogram analyses. FIRM records endocardial signals simultaneously from widely sampled clinical electrodes, most practically a basket catheter. Computational analyses are then performed using monophasic APD and conduction restitution data applied to electrograms in human AF to physiologically filter far‐field signals (‘noise’), before phase analysis to identify activation patterns (Narayan et al. 2006, 2011 b, 2012 b; Krummen et al. 2012). Figure 5 C shows a stable endocardial AF rotor mapped at a typical FIRM case, that precesses in 1–2 cm2 areas, controls surrounding activity (Narayan et al. 2013), may be multiple (two to four) in each patient and can be eliminated by localized ablation with high long‐term AF elimination compared to conventional ablation (Narayan et al. 2014; Miller et al. 2014). One major limitation of FIRM is that although baskets are currently the most practical catheter inserted percutaneously to provide wide‐area contact recordings, basket design should be improved to increase resolution and contact. New designs (Lin et al. 2014) may improve upon earlier catheters. It is interesting to note that FIRM mapping results bear many similarities to human optical mapping studies of AF (Hansen et al. 2015; Zhao et al. 2015) (Table 1).
Figure 5 D shows an approach that records from a modified ECG (body surface vest) to mathematically infer unipolar epicardial electrograms, then applies phase to reveal AF drivers (Haissaguerre et al. 2014). These drivers are reported as ‘unstable’ – but cluster in reproducible regions for prolonged times where they can be successfully ablated (Haissaguerre et al. 2014). It is unclear why these results differ from other studies of human AF, i.e. whether wavefront instability in ECGI reflects variable epicardial activity from stable endocardial rotors (Fig. 3 C) (Hansen et al. 2015), or potentially technical factors from the inverse solution. Indeed, inverse solution virtual electrograms in AF may not reflect contact electrograms (Fig. 5 E) (correlation values as low as 0.27, average 0.87 in Schilling et al. (2000); Earley et al. 2006), and projecting epicardial circuits to the chest wall many centimetres away may magnify instability (Rodrigo et al. 2014).
Validation of mapping results in fibrillation is the subject of intense study. Traditionally, termination of an arrhythmia by ablation proves mechanism, but this is more difficult in AF where hundreds of lesions are often applied, and because clinically successful ablation may not acutely terminate AF (Elayi et al. 2010). Nevertheless, there are increasing reports of termination of persistent AF by localized ablation at a predicted location (Shivkumar et al. 2012; Narayan et al. 2012 d; Miller et al. 2014; Haissaguerre et al. 2014) that was previously rare. This observation supports localized sources for AF and is difficult to explain by non‐hierarchical ‘random’ mechanisms; it has stimulated studies on how localized ablation may terminate AF (Rappel et al. 2015) and why termination of AF by ablation may not predict long‐term success (Calkins et al. 2012).
In summary, whereas the action potential encodes information about activation and recovery, the electrogram focuses on activation. It is thus not surprising that different mapping approaches produce divergent results as the density of adjacent non‐coherent wavefronts increases, i.e. in fibrillation. Future studies that compare mapping approaches in clinical AF, particularly against optical maps, will help to further reconcile differences in observed mechanisms. A particularly important direction is to test whether ‘rules’ such as a ‘qS’ deflection indicating a focal source are accurate in AF when validated by MAP or optical data, and more importantly to improve algorithms for future mapping. Improved catheter designs may better represent local activation, and thus complement such algorithms.
Mechanisms in human ventricular fibrillation
There are obvious challenges to mapping human VF, and studies have therefore focused on early VF (Wiggers stage I) prior to defibrillation, or patients on circulatory support. Nevertheless, within these limitations, fibrillatory mechanisms are well studied. At a tissue level, reentry in VF may depend on analogous processes to AF, including APD oscillations in animals with VF (Choi et al. 2001; Pastore et al. 2006) and patients at risk for VF (Koller et al. 2005; Narayan et al. 2008 a) and in VF (Nash et al. 2006 a). Dynamic conduction slowing has also been shown as a mechanism for the propensity to human VF in endocardial clinical (Narayan et al. 2008 a) and Langendorff‐perfused (Nanthakumar et al. 2007) studies.
In parallel with recent studies of human AF, studies of VF have attempted to identify hierarchical mechanisms (localized sources) (Fig. 6). Figure 6 A and B shows FIRM mapping of human VF prior to defibrillation, in which computational electrogram filtering using human APD and conduction restitution data revealed VF rotors in patients. Such rotors often arose in scar border zone (Hayase et al. 2013; Krummen et al. 2014, 2015), and proof of concept reports show that ablation of VF rotors (in sinus rhythm) was able to eliminate clinical VF on long‐term follow up (Hayase et al. 2013; Krummen et al. 2014, 2015). Using other mapping approaches, Fig. 6 C shows an epicardial rotor after 16.8 s of VF, and Fig. 6 D indicates a transmural rotor (scroll wave) in early VF using a combined phase map of endocardial and epicardial electrograms (Nash et al. 2006 b; Nair et al. 2011).
Figure 6. Human VF rotors demonstrated using endocardial and epicardial mapping .

A, FIRM mapping using basket electrograms shows human LV rotor 1. B, localized ablation at rotor rendered VF non‐inducible and eliminated VF on long‐term follow‐up. (From Krummen et al. 2015.) C, human VF epicardial rotor (white arrow) on phase map with complex fibrillatory breakdown. (From Nash et al. 2006 b.) D, transmural LV rotor (scroll wave) in ex vivo early human VF displayed using phase maps of endocardial and epicardial electrograms. (From Nair et al. 2011.)
Structural factors may potentially distinguish the mechanisms for VF and AF, because complex ventricular architecture may anchor rotors, destabilize reentry or facilitate intramural mechanisms (Nash et al. 2006 b; Nair et al. 2011) as shown in simulations (Rogers, 2002). Transmural rotation of ventricular fibres from the epicardium to the endocardium (rotational anisotropy) (Thomas, 1957) may destabilize scroll waves to produce complex dynamics and wavebreak (Rappel, 2001). In patients at risk for sudden cardiac arrest, an ischaemic border zone may present thick–thin transitions and gap junction remodelling, which may also impact wavefront curvature and re‐entry (Macia et al. 2011; Ciaccio et al. 2014).
Thus, many studies of human VF support a hierarchical model driven by sources, yet these mechanisms are less well validated than for AF. First, it is less clear that VF rotors always act as sources, since VF is typically studied for short periods of time and long‐term follow‐up studies from VF rotor ablation, while promising, are presently anecdotal. Secondly, some ventricular studies used techniques that alter the substrate such as recording epicardial electrograms after sub‐endocardial ablation using Lugol's solution (Lee et al. 1996). Future directions in VF mapping include optical mapping studies to better define the structure–function relationship between organized and disorganized regions, and to validate diverse mapping approaches to VF.
Conclusions and future directions
Knowledge in cardiac electrophysiology has progressed significantly in recent decades, yet remarkably, the fundamental mechanisms for human cardiac fibrillation are still debated. One important concept in this debate is that while fibrillation operates at the limits of dynamic activation and recovery, few human studies consider this physiology. Accordingly, mapping techniques that agree in simple rhythms may diverge dramatically in AF. Optical mapping typically shows localized rotors and sources driving fibrillation while activation mapping of electrograms typically simply shows disorganization.
These mechanistic differences have profound clinical implications. Therapy based upon the disorganized model of AF is suboptimal, and cannot easily explain abundant observations of AF modulation by localized intervention or the failure of extensive empiric ablation to improve outcomes. Conversely, novel clinical mapping approaches, including FIRM that computationally combines repolarization with activation data, recapitulate many features of fibrillatory sources found in human optical maps. Therapy based upon novel mechanistic approaches to mapping have shown early promise and randomized trials are underway.
Future directions include, ultimately, the development of clinically useable optical imaging, which circumvents dye‐related toxicity in the beating heart at wavelengths not absorbed by blood. In the interim, optical mapping of human AF ex vivo should be used to validate and compare current mapping modalities, to develop accurate ‘rules’ or algorithms to separate local from distant information for the different recording fields of unipolar or bipolar electrograms. Improved catheter designs may better represent local activation, and complement such algorithms. These approaches may ultimately lead to computational mapping that digitally approximates optical maps.
Recent literature in cardiac fibrillation is replete with studies that are descriptive without mechanistic interventions. For the essential goal of advancing clinical outcomes, it is critical that purely descriptive studies are replaced by studies in which ablation and other interventions are used to establish mechanistic targets for diagnosis and for novel therapies which may include ablation, pacing, genetic or regenerative therapy.
Additional information
Competing interests
S.M.N. reports being co‐inventor on intellectual property owned by the University of California and licensed to Topera Medical, Inc. He has held equity in Topera. S.M.N. also reports having received consulting fees from the American College of Cardiology, Uptodate and Janssen Pharmaceuticals. J.A.B.Z. reports no competing interests.
Author contributions
Both authors contributed substantially to the conceptualization, drafting, revision and assembly of the manuscript. Both authors approved the final version of the manuscript, both persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported in part by grants from the National Institutes of Health (HL70529, HL83359, HL103800) to S.M.N., and a British Heart Foundation Fellowship (FS/14/46/30907) and Fulbright Award to J.Z.
Acknowledgements
We acknowledge Kathleen Mills, BA and Fatemah Shenasa, BA for their assistance in this work.
Biographies
Sanjiv Narayan is Professor of Medicine at Stanford University, where he is Director of Electrophysiology Research and of the Atrial Fibrillation Program, and treats patients with complex arrhythmias. He has postgraduate degrees in software engineering and neuroscience in addition to his clinical training in cardiology and electrophysiology. His translational laboratory develops bioengineering solutions to better understand and treat arrhythmias, and has been continuously funded for 20 years. He has authored over 200 peer‐reviewed articles.
Junaid Zaman is a postdoctoral scholar at Stanford University and Imperial College London. He obtained his medical degree from the University of Oxford, graduating with distinctions and a First Class degree. For his PhD, he received Medical Research Council Chain Florey and British Heart Foundation Fellowships to investigate the relation between electrical signals in atrial fibrillation and underlying tissue structure at the National Heart and Lung Institute. He has won multiple travel awards, most recently from the British Cardiac Society and Stanford Cardiovascular Institute. His research won Best Poster Prize at the European Cardiac Arrhythmia Society Annual Congress 2015. He was recently awarded the inaugural US‐UK Fulbright British Heart Foundation Research Scholar Award to work at Stanford and is a Young Investigator Finalist at the American Heart Association Scientific Sessions 2015.

References
- Allessie MA, de Groot NMS, Houben RPM, Schotten U, Boersma E, Smeets JL & Crijns HJ (2010). Electropathological substrate of long‐standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol 3, 606–615. [DOI] [PubMed] [Google Scholar]
- Benharash P, Buch E, Frank P, Share M, Tung R, Shivkumar K & Mandapati R (2015). Quantitative analysis of localized sources identified by focal impulse and rotor modulation mapping in atrial fibrillation. Circ Arrhythmia Electrophysiol 8, 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenfeld O, Mandapati R, Dixit S, Skanes AC, Chen J, Mansour M & Jalife J (2000). Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the Langendorff‐perfused sheep heart. J Cardiovasc Electrophysiol 11, 869–879. [DOI] [PubMed] [Google Scholar]
- Burashnikov A & Antzelevitch C (2005). Role of repolarization restitution in the development of coarse and fine atrial fibrillation in the isolated canine right atria. J Cardiovasc Electrophysiol 16, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins H, Kuck KH, Cappato R et al (2012). 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace 14, 528–606. [DOI] [PubMed] [Google Scholar]
- Chen J, Mandapati R, Berenfeld O, Skanes AC, Gray RA & Jalife J (2000. a). Dynamics of wavelets and their role in atrial fibrillation in the isolated sheep heart. Cardiovasc Res 48, 220–232. [DOI] [PubMed] [Google Scholar]
- Chen J, Mandapati R, Berenfeld O, Skanes AC & Jalife J (2000. b). High‐frequency periodic sources underlie ventricular fibrillation in the isolated rabbit heart. Circ Res 86, 86–93. [DOI] [PubMed] [Google Scholar]
- Choi BR, Liu T & Salama G (2001). The distribution of refractory periods influences the dynamics of ventricular fibrillation. Circ Res 88, E49–E58. [DOI] [PubMed] [Google Scholar]
- Chou C‐C, Chang P‐C, Wen M‐S, Lee H‐L, Chen T‐C, Yeh S‐J & Wu D (2011). Epicardial ablation of rotors suppresses inducibility of acetylcholine‐induced atrial fibrillation in left pulmonary vein‐left atrium preparations in a beagle heart failure model. J Am Coll Cardiol 58, 158–166. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim Y‐H, McAnulty JH, Z‐J Zheng, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M & Murray CJL (2014). Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129, 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng Z‐J, Mensah G & McAnulty J (2004). Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate‐based review in a large U.S. community. J Am Coll Cardiol 44, 1268–1275. [DOI] [PubMed] [Google Scholar]
- Ciaccio EJ, Ashikaga H, Coromilas J, Hopenfeld B, Cervantes D, Wit AL, Peters NS, McVeigh ER & Garan H (2014). Model of bipolar electrogram fractionation and conduction block associated with activation wavefront direction at infarct border zone lateral isthmus boundaries. Circ Arrhythm Electrophysiol 7, 152–163. [DOI] [PubMed] [Google Scholar]
- Comtois P, Kneller J & Nattel S (2005). Of circles and spirals: bridging the gap between the leading circle and spiral wave concepts of cardiac reentry. Europace 7 Suppl 2, 10–20. [DOI] [PubMed] [Google Scholar]
- Cox JL, Canavan TE, Schuessler RB, Cain ME, Lindsay BD, Stone C, Smith PK, Corr PB & Boineau JP (1991). The surgical treatment of atrial fibrillation. II. Intraoperative electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 101, 406–426. [PubMed] [Google Scholar]
- Davidenko J, Pertsov A, Salomonsz R, Baxter W & Jalife J (1992). Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature 355, 349–350. [DOI] [PubMed] [Google Scholar]
- de Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H & Allessie MA (2010). Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation 122, 1674–1682. [DOI] [PubMed] [Google Scholar]
- Earley MJ, Abrams DJR, Sporton SC & Schilling RJ (2006). Validation of the noncontact mapping system in the left atrium during permanent atrial fibrillation and sinus rhythm. J Am Coll Cardiol 48, 485–491. [DOI] [PubMed] [Google Scholar]
- Eckstein J, Zeemering S, Linz D, Maesen B, Verheule S, van Hunnik A, Crijns H, Allessie MA & Schotten U (2013). Transmural conduction is the predominant mechanism of breakthrough during atrial fibrillation: evidence from simultaneous endo‐epicardial high‐density activation mapping. Circ Arrhythm Electrophysiol 6, 334–341. [DOI] [PubMed] [Google Scholar]
- Efimov IR, Nikolski VP & Salama G (2004). Optical imaging of the heart. Circ Res 95, 21–33. [DOI] [PubMed] [Google Scholar]
- Elayi CS, Di Biase L, Barrett C, et al (2010). Atrial fibrillation termination as a procedural endpoint during ablation in long‐standing persistent atrial fibrillation. Heart Rhythm 7, 1216–1223. [DOI] [PubMed] [Google Scholar]
- Elzinga G, Lab MJ, Noble MI, Papadoyannis DE, Pidgeon J, Seed A & Wohlfart B (1981). The action‐potential duration and contractile response of the intact heart related to the preceding interval and the preceding beat in the dog and cat. J Physiol 314, 481–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman ZJ, Trew ML & Smaill BH (2010). Structural heterogeneity alone is a sufficient substrate for dynamic instability and altered restitution. Circ Arrhythm Electrophysiol 3, 195–203. [DOI] [PubMed] [Google Scholar]
- Everett TH, Verheule S, Wilson EE, Foreman S & Olgin JE (2004). Left atrial dilatation resulting from chronic mitral regurgitation decreases spatiotemporal organization of atrial fibrillation in left atrium. Am J Physiol Heart Circ Physiol 286, H2452–H2460. [DOI] [PubMed] [Google Scholar]
- Filgueiras‐Rama D, Price NF, Martins RP, Yamazaki M, Avula UMR, Kaur K, Kalifa J, Ennis SR, Hwang E, Devabhaktuni V, Jalife J & Berenfeld O (2012). Long‐term frequency gradients during persistent atrial fibrillation in sheep are associated with stable sources in the left atrium. Circ Arrhythm Electrophysiol 5, 1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz MR (2003). The electrical restitution curve revisited: steep or flat slope‐which is better? J Cardiovasc Electrophysiol 14, S140–S147. [DOI] [PubMed] [Google Scholar]
- Franz MR, Chin MC, Sharkey HR, Griffin JC & Scheinman MM (1990). A new single catheter technique for simultaneous measurement of action potential duration and refractory period in vivo. J Am Coll Cardiol 16, 878–886. [DOI] [PubMed] [Google Scholar]
- Franz MR, Swerdlow CD, Liem LM & Schaefer J (1988). Cycle length dependence of human action potential duration in vivo effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady‐state frequencies. J Clin Invest 82, 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelzer ARM, Koller ML, Otani NF, Fox JJ, Enyeart MW, Hooker GJ, Riccio ML, Bartoli CR & Gilmour RF (2008). Dynamic mechanism for initiation of ventricular fibrillation in vivo. Circulation 118, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstenfeld EP, Sahakian AV & Swiryn S (1992). Evidence for transient linking of atrial excitation during atrial fibrillation in humans. Circulation 86, 375–382. [DOI] [PubMed] [Google Scholar]
- Gray RA, Pertsov AM & Jalife J (1996). Incomplete reentry and epicardial breakthrough patterns during atrial fibrillation in the sheep heart. Circulation 94, 2649–2661. [DOI] [PubMed] [Google Scholar]
- Gray RA, Pertsov AM & Jalife J (1998). Spatial and temporal organization during cardiac fibrillation. Nature 392, 75–78. [DOI] [PubMed] [Google Scholar]
- Gutbrod SR, Walton R, Gilbert S, Meillet V, Jais P, Hocini M, Haissaguerre M, Dubois R, Bernus O & Efimov I (2015). Quantification of the transmural dynamics of atrial fibrillation by simultaneous endocardial and epicardial optical mapping in an acute sheep model. Circ Arrhythm Electrophysiol 8, 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissaguerre M , Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P & Dubois R (2014). Driver domains in persistent atrial fibrillation. Circulation 130, 530–538. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Hocini M, Shah AJ, Derval N, Sacher F, Jais P & Dubois R (2013). Noninvasive panoramic mapping of human atrial fibrillation mechanisms: a feasibility report. J Cardiovasc Electrophysiol 24, 711–717. [DOI] [PubMed] [Google Scholar]
- Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RSD, Kilic A, Mohler PJ, Janssen PML, Weiss R, Hummel JD & Fedorov VV (2015). Atrial fibrillation driven by micro‐anatomic intramural re‐entry revealed by simultaneous sub‐epicardial and sub‐endocardial optical mapping in explanted human hearts. Eur Heart J 36, 2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws CW & Lux RL (1990). Correlation between in vivo transmembrane action potential durations and activation‐recovery intervals from electrograms. Effects of interventions that alter repolarization time. Circulation 81, 281–288. [DOI] [PubMed] [Google Scholar]
- Hayase J, Tung R, Narayan SM & Krummen DE (2013). A case of a human ventricular fibrillation rotor localized to ablation sites for scar‐mediated monomorphic ventricular tachycardia. Heart Rhythm 10, 1913–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herweg B, Kowalski M & Steinberg JS (2003). Termination of persistent atrial fibrillation resistant to cardioversion by a single radiofrequency application. Pacing Clin Electrophysiol 26, 1420–1423. [DOI] [PubMed] [Google Scholar]
- Holm M, Johansson R, Brandt J, Luhrs C & Olsson SB (1997). Epicardial right atrial free wall mapping in chronic atrial fibrillation. Eur Heart J 18, 290–310. [DOI] [PubMed] [Google Scholar]
- Houben RPM, de Groot NMS & Allessie MA (2010). Analysis of fractionated atrial fibrillation electrograms by wavelet decomposition. IEEE Trans Biomed Eng 57, 1388–1398. [DOI] [PubMed] [Google Scholar]
- Houben RPM, de Groot NMS, Smeets JLRM, Becker AE, Lindemans FW & Allessie MA (2004). S‐wave predominance of epicardial electrograms during atrial fibrillation in humans: indirect evidence for a role of the thin subepicardial layer. Heart Rhythm 1, 639–647. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Yashima M, Uchida T, Hough D, Fishbein MC, Mandel WJ, Chen P‐S & Karagueuzian HS (1997). Attachment of meandering reentrant wave fronts to anatomic obstacles in the atrium: role of the obstacle size. Circ Res 81, 753–764. [DOI] [PubMed] [Google Scholar]
- Jadidi AS, Cochet H, Shah AJ, Kim SJ, Duncan E, Miyazaki S, Sermesant M, Lehrmann H, Lederlin M, Linton N, Forclaz A, Nault I, Rivard L, Wright M, Liu X, Scherr D, Wilton SB, Roten L, Pascale P, Derval N, Sacher F, Knecht S, Keyl C, Hocini M, Montaudon M, Laurent F, Haïssaguerre M & Jaïs P (2013). Inverse relationship between fractionated electrograms and atrial fibrosis in persistent atrial fibrillation – a combined MRI and high density mapping. J Am Coll Cardiol 62, 802–812. [DOI] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM & Yancy CW (2014). 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64, 2246–2280. [DOI] [PubMed] [Google Scholar]
- Karagueuzian HS, Khan SS, Hong K, Kobayashi Y, Denton T, Mandel WJ & Diamond GA (1993). Action potential alternans and irregular dynamics in quinidine‐intoxicated ventricular muscle cells. Implications for ventricular proarrhythmia. Circulation 87, 1661–1672. [DOI] [PubMed] [Google Scholar]
- Kim B‐S, Kim Y‐H, Hwang G‐S, Pak H‐N, Lee SC, Shim WJ, Oh DJ & Ro YM (2002). Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol 39, 1329–1336. [DOI] [PubMed] [Google Scholar]
- Kléber AG & Rudy Y (2004). Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84, 431–488. [DOI] [PubMed] [Google Scholar]
- Knollmann BC, Tranquillo J, Sirenko SG, Henriquez C & Franz MR (2002). Microelectrode study of the genesis of the monophasic action potential by contact electrode technique. J Cardiovasc Electrophysiol 13, 1246–1252. [DOI] [PubMed] [Google Scholar]
- Koller ML, Maier SKG, Gelzer AR, Bauer WR, Meesmann M & Gilmour RF (2005). Altered dynamics of action potential restitution and alternans in humans with structural heart disease. Circulation 112, 1542–1548. [DOI] [PubMed] [Google Scholar]
- Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC & Allessie MA (1994). High‐density mapping of electrically induced atrial fibrillation in humans. Circulation 89, 1665–1680. [DOI] [PubMed] [Google Scholar]
- Krinksy VI (1966). Excitation propagation in heterogeneous medium (modes similar to cardiac fibrillation). Biofizika 11, 676–683. [PubMed] [Google Scholar]
- Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, Trayanova NA & Narayan SM (2012). Mechanisms for human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ Arrhythm Electrophysiol 5, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummen DE, Hayase J, Morris DJ, Ho J, Smetak MR, Clopton P, Rappel W‐J & Narayan SM (2014). Rotor stability separates sustained ventricular fibrillation from self‐terminating episodes in humans. J Am Coll Cardiol 63, 2712–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummen DE, Hayase J, Vampola SP, Ho G, Schricker AA, Lalani GG, Baykaner T, Coe TM, Clopton P, Rappel W‐J, Omens JH & Narayan SM (2015). Modifying ventricular fibrillation by targeted rotor substrate ablation: proof‐of‐concept from experimental studies to clinical VF. J Cardiovasc Electrophysiol 26, 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau DH, Maesen B, Zeemering S, Kuklik P, van Hunnik A, Lankveld TAR, Bidar E, Verheule S, Nijs J, Maessen J, Crijns H, Sanders P & Schotten U (2015). Indices of bipolar complex fractionated atrial electrograms correlate poorly with each other and atrial fibrillation substrate complexity. Heart Rhythm 12, 1415–1423. [DOI] [PubMed] [Google Scholar]
- Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM & Kalman JM (2014). Epicardial wave mapping in human long‐lasting persistent atrial fibrillation: Transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J 35, 86–97. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Kamjoo K, Hough D, Hwang C, Fan W, Fishbein MC, Bonometti C, Ikeda T, Karagueuzian HS & Chen P‐S (1996). Reentrant wave fronts in Wiggers’ stage II ventricular fibrillation: characteristics and mechanisms of termination and spontaneous regeneration. Circ Res 78, 660–675. [DOI] [PubMed] [Google Scholar]
- Lee P, Taghavi F, Yan P, Ewart P, Ashley EA, Loew LM, Kohl P, Bollensdorff C & Woods CE (2012). In situ optical mapping of voltage and calcium in the heart. PLoS One 7, e42562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Sahadevan J, Khrestian CM, Durand DM & Waldo AL (2013). High density mapping of atrial fibrillation during vagal nerve stimulation in the canine heart: restudying the Moe hypothesis. J Cardiovasc Electrophysiol 24, 328–335. [DOI] [PubMed] [Google Scholar]
- Lim ZY, Maskara B, Aguel F, Emokpae R & Tung L (2006). Spiral wave attachment to millimeter‐sized obstacles. Circulation 114, 2113–2121. [DOI] [PubMed] [Google Scholar]
- Lin JW, Garber L, Qi YR, Chang MG, Cysyk J & Tung L (2008). Region [corrected] of slowed conduction acts as core for spiral wave reentry in cardiac cell monolayers. Am J Physiol Heart Circ Physiol 294, H58–H65. [DOI] [PubMed] [Google Scholar]
- Lin T, Kuck K‐H, Ouyang F & Tilz RR (2014). First in‐human robotic rotor ablation for atrial fibrillation. Eur Heart J 35, 1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Dolmatova E, Cabo C, Sosinsky AZ, Dun W, Coromilas J, Ciaccio EJ, Boyden PA, Wit AL & Duffy HS (2011). Characterization of gap junction remodeling in epicardial border zone of healing canine infarcts and electrophysiological effects of partial reversal by rotigaptide. Circ Arrhythm Electrophysiol 4, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH & Jalife J (2001). Left‐to‐right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation 103, 2631–2636. [DOI] [PubMed] [Google Scholar]
- Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS & Wheelan KR (2014). Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 25, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mines G (1913). On dynamic equilbrium in the heart. J Physiol 46, 349–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair K, Umapathy K, Farid T, Masse S, Mueller E, Sivanandan RV, Poku K, Rao V, Nair V, Butany J, Ideker RE & Nanthakumar K (2011). Intramural activation during early human ventricular fibrillation. Circ Arrhythm Electrophysiol 4, 692–703. [DOI] [PubMed] [Google Scholar]
- Nanthakumar K, Jalife J, Massé S, Downar E, Pop M, Asta J, Ross H, Rao V, Mironov S, Sevaptsidis E, Rogers J, Wright G & Dhopeshwarkar R (2007). Optical mapping of Langendorff‐perfused human hearts: establishing a model for the study of ventricular fibrillation in humans. Am J Physiol Heart Circ Physiol 293, H875–H880. [DOI] [PubMed] [Google Scholar]
- Narayan SM, Bayer JD, Lalani G & Trayanova NA (2008. a). Action potential dynamics explain arrhythmic vulnerability in human heart failure: a clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol 52, 1782–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Baykaner T, Clopton P, Schricker A, Lalani GG, Krummen DE, Shivkumar K & Miller JM (2014). Ablation of rotor and focal sources reduces late recurrence of atrial fibrillation compared with trigger ablation alone: extended follow‐up of the CONFIRM trial (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation). J Am Coll Cardiol 63, 1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Bode F, Karasik P & Franz MR (2002). Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation 106, 1968–1973. [DOI] [PubMed] [Google Scholar]
- Narayan SM & Franz MR (2007). Quantifying fractionation and rate in human atrial fibrillation using monophasic action potentials: implications for substrate mapping. Europace 9 Suppl 6, vi89–vi95. [DOI] [PubMed] [Google Scholar]
- Narayan SM, Franz MR, Clopton P, Pruvot EJ & Krummen DE (2011. a). Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation 123, 2922–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Franz MR, Lalani G, Kim J & Sastry A (2007). T‐wave alternans, restitution of human action potential duration, and outcome. J Am Coll Cardiol 50, 2385–2392. [DOI] [PubMed] [Google Scholar]
- Narayan SM, Kazi D, Krummen DE & Rappel W‐J (2008. b). Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol 52, 1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Krummen DE, Enyeart MW & Rappel W‐J (2012. b). Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PLoS One 7, e46034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Krummen DE, Kahn AM, Karasik PL & Franz MR (2006). Evaluating fluctuations in human atrial fibrillatory cycle length using monophasic action potentials. Pacing Clin Electrophysiol 29, 1209–1218. [DOI] [PubMed] [Google Scholar]
- Narayan SM, Krummen DE & Rappel W‐J (2012. c). Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol 23, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W‐J & Miller JM (2012. d). Treatment of atrial fibrillation by the ablation of localized sources. J Am Coll Cardiol 60, 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Patel J, Mulpuru S & Krummen DE (2012. a). Focal impulse and rotor modulation ablation of sustaining rotors abruptly terminates persistent atrial fibrillation to sinus rhythm with elimination on follow‐up: A video case study. Heart Rhythm 9, 1436–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Shivkumar K, Krummen DE, Miller JM & Rappel W‐J (2013). Panoramic electrophysiological mapping but not electrogram morphology identifies stable sources for human atrial fibrillation: stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ Arrhythm Electrophysiol 6, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Wright M, Derval N, Jadidi A, Forclaz A, Nault I, Miyazaki S, Sacher F, Bordachar P, Clémenty J, Jaïs P, Haïssaguerre M & Hocini M (2011. b). Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm 8, 244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash MP, Bradley CP, Sutton PM, Clayton RH, Kallis P, Hayward MP, Paterson DJ & Taggart P (2006. a). Whole heart action potential duration restitution properties in cardiac patients: a combined clinical and modelling study. Exp Physiol 91, 339–354. [DOI] [PubMed] [Google Scholar]
- Nash MP, Mourad A, Clayton RH, Sutton PM, Bradley CP, Hayward M, Paterson DJ & Taggart P (2006. b). Evidence for multiple mechanisms in human ventricular fibrillation. Circulation 114, 536–542. [DOI] [PubMed] [Google Scholar]
- Ndrepepa G, Caref EB, Yin H, el‐Sherif N & Restivo M (1995). Activation time determination by high‐resolution unipolar and bipolar extracellular electrograms in the canine heart. J Cardiovasc Electrophysiol 6, 174–188. [DOI] [PubMed] [Google Scholar]
- Noujaim SF, Berenfeld O, Kalifa J, Cerrone M, Nanthakumar K, Atienza F, Moreno J, Mironov S & Jalife J (2007). Universal scaling law of electrical turbulence in the mammalian heart. Proc Natl Acad Sci USA 104, 20985–20989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit SV & Jalife J (2013). Rotors and the dynamics of cardiac fibrillation. Circ Res 112, 849–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore JM, Laurita KR & Rosenbaum DS (2006). Importance of spatiotemporal heterogeneity of cellular restitution in mechanism of arrhythmogenic discordant alternans. Heart Rhythm 3, 711–719. [DOI] [PubMed] [Google Scholar]
- Paterson DJ (2013). Arrhythmia: 100 years on from George Ralph Mines. J Physiol 591, 4065–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Po SS, Li Y, Tang D, Liu H, Geng N, Jackman WM, Scherlag B, Lazzara R & Patterson E (2005). Rapid and stable re‐entry within the pulmonary vein as a mechanism initiating paroxysmal atrial fibrillation. J Am Coll Cardiol 45, 1871–1877. [DOI] [PubMed] [Google Scholar]
- Rappel W‐J (2001). Filament instability and rotational tissue anisotropy: A numerical study using detailed cardiac models. Chaos 11, 71–80. [DOI] [PubMed] [Google Scholar]
- Rappel W‐J, Zaman JAB & Narayan SM (2015). Mechanisms for the termination of atrial fibrillation by localized ablation: computational and clinical studies. Circ Arrhythm Electrophysiol doi: 10.1161/CIRCEP.115.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo M, Guillem MS, Climent AM, Pedrón‐Torrecilla J, Liberos A, Millet J, Fernández‐Avilés F, Atienza F & Berenfeld O (2014). Body surface localization of left and right atrial high‐frequency rotors in atrial fibrillation patients: A clinical‐computational study. Heart Rhythm 11, 1584–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JM (2002). Wave front fragmentation due to ventricular geometry in a model of the rabbit heart. Chaos 12, 779–787. [DOI] [PubMed] [Google Scholar]
- Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH & Waldo AL (2004). Epicardial mapping of chronic atrial fibrillation in patients: preliminary observations. Circulation 110, 3293–3299. [DOI] [PubMed] [Google Scholar]
- Sarmast F, Kolli A, Zaitsev A, Parisian K, Dhamoon AS, Guha PK, Warren M, Anumonwo JMB, Taffet SM, Berenfeld O & Jalife J (2003). Cholinergic atrial fibrillation: IK,ACh gradients determine unequal left/right atrial frequencies and rotor dynamics. Cardiovasc Res 59, 863–873. [DOI] [PubMed] [Google Scholar]
- Schilling R, Kadish AH, Peters NS, Goldberger J & Wyn Davies D (2000). Endocardial mapping of atrial fibrillation in the human right atrium using a non‐contact catheter. Eur Heart J 21, 550–564. [DOI] [PubMed] [Google Scholar]
- Schricker AA, Lalani GG, Krummen DE, Rappel W‐J & Narayan SM (2014). Human atrial fibrillation initiates via organized rather than disorganized mechanisms. Circ Arrhythm Electrophysiol 7, 816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuessler RB, Grayson TM, Bromberg BI, Cox JL & Boineau JP (1992). Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res 71, 1254–1267. [DOI] [PubMed] [Google Scholar]
- Shivkumar K, Ellenbogen KA, Hummel John D, Miller JM & Steinberg JS (2012). Acute termination of human atrial fibrillation by identification and catheter ablation of localized rotors and sources: First Multicenter Experience of Focal Impulse and Rotor Modulation (FIRM) Ablation. J Cardiovasc Electrophysiol 23, 1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker JM, Clark JW & Giles WR (1993). Simulations of passive properties and action potential conduction in an idealized bullfrog atrial trabeculum. Math Biosci 116, 127–167. [DOI] [PubMed] [Google Scholar]
- Skanes AC, Mandapati R, Berenfeld O, Davidenko JM & Jalife J (1998). Spatiotemporal periodicity during atrial fibrillation in the isolated sheep heart. Circulation 98, 1236–1248. [DOI] [PubMed] [Google Scholar]
- Spach MS (2001). Mechanisms of the dynamics of reentry in a fibrillating myocardium. Developing a genes‐to‐rotors paradigm. Circ Res 88, 753–755. [DOI] [PubMed] [Google Scholar]
- Steinhaus BM (1989). Estimating cardiac transmembrane activation and recovery times from unipolar and bipolar extracellular electrograms: a simulation study. Circ Res 64, 449–462. [DOI] [PubMed] [Google Scholar]
- Swartz JF, Jones JL & Fletcher RD (1993). Characterization of ventricular fibrillation based on monophasic action potential morphology in the human heart. Circulation 87, 1907–1914. [DOI] [PubMed] [Google Scholar]
- The Cardiac Arrhythmia Suppression Trial (CAST) Investigators (1989). Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med 321, 406–412. [DOI] [PubMed] [Google Scholar]
- Thomas C (1957). The muscular architecture of the ventricles of hog and dog hearts. Am J Anat 101, 17–57. [DOI] [PubMed] [Google Scholar]
- Vaidya D, Morley GE, Samie FH & Jalife J (1999). Reentry and fibrillation in the mouse heart: a challenge to the critical mass hypothesis. Circ Res 85, 174–181. [DOI] [PubMed] [Google Scholar]
- Valderrábano M, Lee MH, Ohara T, Lai AC, Fishbein MC, Lin SF, Karagueuzian HS & Chen PS (2001). Dynamics of intramural and transmural reentry during ventricular fibrillation in isolated swine ventricles. Circ Res 88, 839–848. [DOI] [PubMed] [Google Scholar]
- Verma A, Jiang C, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque J‐P, Nardi S, Menardi E, Novak P & Sanders P (2015). Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 372, 1812–1822. [DOI] [PubMed] [Google Scholar]
- Walters TE, Lee G, Morris G, Spence S, Larobina M, Atkinson V, Antippa P, Goldblatt J, Royse A, O'Keefe M, Sanders P, Morton JB, Kistler PM & Kalman JM (2015). Temporal stability of rotors and atrial activation patterns in persistent human atrial fibrillation: a high‐density epicardial mapping study of prolonged recordings. JACC Clin Electrophysiol 1, 14–24. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Masaki R, Ohkubo K, Okumura Y, Yamada T, Oshikawa N, Saito S, Ozawa Y & Kanmatsuse K (2002). Rate‐dependent changes in atrial action potential duration after short‐ duration rapid atrial pacing in humans. Circ J 66, 874–875. [DOI] [PubMed] [Google Scholar]
- Weiss JN, Garfinkel A, Karagueuzian HS, Nguyen TP, Olcese R, P‐S Chen & Qu Z (2015). Perspective: A dynamics‐based classification of ventricular arrhythmias. J Mol Cell Cardiol 82, 136–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfree AT (1972). Spiral waves of chemical activity. Science 175, 634–636. [DOI] [PubMed] [Google Scholar]
- Witkowski F, Leon L, Penkoske P, Giles W, Spano M, Ditto W & Winfree AT (1998). Spatiotemporal evolution of ventricular fibrillation. Nature 392, 78–82. [DOI] [PubMed] [Google Scholar]
- Wu T‐J (2002). Two types of ventricular fibrillation in isolated rabbit hearts: importance of excitability and action potential duration restitution. Circulation 106, 1859–1866. [DOI] [PubMed] [Google Scholar]
- Wu T‐J, Doshi RN, Huang H‐LA, Blanche C, Kass RM, Trento A, Cheng W, Karagueuzian HS, Peter CT & Chen P‐S (2002). Simultaneous biatrial computerized mapping during permanent atrial fibrillation in patients with organic heart disease. J Cardiovasc Electrophysiol 13, 571–577. [DOI] [PubMed] [Google Scholar]
- Wynn GJ, Das M, Bonnett LJ, Panikker S, Wong T & Gupta D (2014). Efficacy of catheter ablation for persistent atrial fibrillation: a systematic review and meta‐analysis of evidence from randomised and non‐randomised controlled trials. Circ Arrhythm Electrophysiol 7, 841–852. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Berenfeld O, Mironov SF, Jalife J & Pertsov AM (2000). Distribution of excitation frequencies on the epicardial and endocardial surfaces of fibrillating ventricular wall of the sheep heart. Circ Res 86, 408–417. [DOI] [PubMed] [Google Scholar]
- Zaitsev AV, Guha PK, Sarmast F, Kolli A, Berenfeld O, Pertsov AM, de Groot JR, Coronel R & Jalife J (2003). Wavebreak formation during ventricular fibrillation in the isolated, regionally ischemic pig heart. Circ Res 92, 546–553. [DOI] [PubMed] [Google Scholar]
- Zaman JAB & Narayan SM (2015). When is structure, function? – Revisiting an old concept in atrial fibrillation. J Cardiovasc Electrophysiol 26, 1361–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Hansen BJ, Csepe TA, Lim P, Hummel JD & Fedorov VV (2015). Integration of high resolution optical mapping and 3d micro‐ct imaging to resolve the structural basis of atrial conduction in the human heart. Circ Arrhythmia Electrophysiol 8, 1514–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]


