Abstract
The mechanisms responsible for perpetuation of human persistent atrial fibrillation (AF) are controversial and probably vary between individuals. A wide spectrum of mechanisms have been described in experimental studies, ranging from a single localized stable (focal/reentrant) source, to multiple sources, up to diffuse bi‐atrial wavelets. We characterized AF drivers in patients with persistent AF (lasting less than 1 year) using novel high resolution mapping, imaging and modelling approaches with the objective of evaluating their relationship to atrial structural heterogeneities. Using panoramic non‐invasive mapping in humans, focal or reentrant sources driving AF waves were identified, originating from multiple distinct regions and exhibiting short lifespans and periodic recurrences in the same locations. The reentrant driver regions harboured long, fractionated electrograms covering most of the fibrillatory cycle lengths with varying beat‐to‐beat sequences suggestive of unstable trajectories attached to slow conducting heterogeneous tissue. MRI atrial imaging demonstrated that such drivers preferentially clustered at the borders of fibrotic atrial regions. In patient‐specific computer simulations, sustained AF was shown to be driven by meandering transitory reentries attached to fibrosis borders expressing specific metrics in density and extent. Finally, random microstructural alterations devoid of cellular electrical changes were modelled, showing that a percolation mechanism could also explain atrial reentries and complex fractionated electrograms. These data from clinical, imaging and computational studies strongly suggest that intermittent and spatially unstable drivers anchoring to structural heterogeneities are a major pathophysiological mechanism in human persistent atrial fibrillation.

Abbreviations
- AF
atrial fibrillation
- LGE
late gadolinium enhancement
- PsAF
persistent atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common cardiac rhythm anomaly, affecting 3 million people in the USA and 5 million in Europe. With loss of atrial contraction favouring intracardiac thrombus formation, AF is the commonest cause of cardio‐embolic stroke. AF is associated with ventricular dysfunction leading to heart failure and is also known to increase the mortality risk twofold (Benjamin et al. 1998; Camm et al. 2012; Chugh et al. 2014; January et al. 2014). Despite extensive research, AF mechanisms are still controversial but recognized as a complex edifice of incompletely understood pathophysiological processes (Wakili et al. 2011; Nattel & Harada, 2014). They involve electrical remodelling of ion channels, gap junctions and abnormal Ca2+ handling from the sarcoplasmic reticulum at the cell level and a wide spectrum of changes at the organ level affecting the atrial tissue (both myocardium and interstitium) and its variable extra‐atrial extensions into the venous tributaries. In addition there is evidence supporting the role of inflammation and autonomic nervous changes (Hu et al. 2015). Such complex pathophysiology is mainly thought to contribute to the persistent form of AF lasting continuously for less than 12 months or permanent AF lasting for years. However AF may also affect patients in the form of episodic attacks (lasting minutes or hours) and such paroxysms of AF are strongly linked to pulmonary vein sources as confirmed by the lasting efficacy of pulmonary vein isolation (Haissaguerre et al. 1998; Nattel & Harada M, 2014).
The electrophysiological mechanisms maintaining AF involve focal firing and various forms of reentry, some of them first described over a century ago (Mines et al. 1913; Garrey, 1914; Allesie et al. 1973; Spach et al. 1981; Cox et al. 1991; Wijffels et al. 1995). To determine a therapeutic strategy for persistent AF (localized vs. global intervention), the key question is whether the multiple activation waves that characterize AF are generated from few localized sources or whether they are widely distributed across the atria. Below, we have characterized the drivers of persistent AF based on recent clinical experience gathered from a novel high‐density multi‐electrode system for non‐invasive panoramic mapping of AF dynamics combined with MRI imaging of atrial structure and human atrial modelling. The objective was to evaluate the main mechanisms driving persistent AF (lasting less than 1 year) in humans in relation to atrial structural heterogeneities.
Determinants of progressive AF pathology
Persistent AF can be the first clinical presentation of arrhythmia. In these cases, the fibrillatory substrate might have been developing subclinically (silently) in the atria for an unknown period of time, and at a specific stage it surfaces clinically and manifests directly as persistent AF. Alternatively, persistent AF may be the outcome of progression of paroxysmal AF. The latter occurs at a rate of 5–9% annually, affecting globally 30% of paroxysmal AF patients, but the frequency and rapidity of progressive pathological remodelling are not predictable in an individual (Benjamin et al. 1998; Camm et al. 2012; Chugh et al. 2014; January et al. 2014). A recent study has evaluated the rate of electrical and structural remodelling in an ovine model of long‐standing persistent AF. The increase of fibrillatory frequency varied between animals but its slope predicted the time at which AF becomes persistent, reflecting an abbreviation of action potential duration and changes in densities of I CaL, IK1, I Na, and I to, and a progressive increase in atrial dilatation, myocyte hypertrophy and atrial fibrosis (Martins et al. 2014).
In humans, the progression of paroxysmal AF to persistent forms is marked by structural alterations in the atrial tissue brought about by comorbidities like ageing, diabetes and hypertension, chronic obstructive pulmonary disease, cardiomyopathy/heart failure, valvular diseases and prolonged P‐wave duration (> 150 ms). In the absence of these comorbidities, the risk of pathological progression remains low (Martins et al. 2014).
Mechanisms of AF
So far, basic scientists and clinical investigators largely accept the existence of multiple atrial wavelets, macroreentries and localized (focal/reentrant) driving sources as potential electrophysiological mechanisms for the perpetuation of AF (Allessie et al. 1973; Cox et al. 1991; Nattel & Harada, 2014). However the hierarchy or the combination of mechanisms responsible for the perpetuation of human persistent AF are still controversial and they have been debated in this journal (Narayan & Jalife (2014) rebutted by Allessie & De Groot (2014)). The two hypotheses discussed above are radically conflicting as they oppose localized stable drivers versus a diffuse multiwavelet substrate with their corollary in terms of ablation extent.
The focal‐source theory was the outcome of initial experimental studies showing atrial foci or rotors as sources of AF. Shuessler et al. (1992, 1993) used acetylcholine that initially produced multiple unstable drivers until a higher dose, considerably shortening refractory periods, stabilized a reentrant driver at fixed locations, while stable reentries (rotors) producing uninterrupted activity were evidenced by Jalife et al. (2002) associated with the highest dominant frequency and without electrogram fractionation.
In addition, our group reported that the majority of paroxysmal forms of AF and a small percentage of persistent AF are driven by sources lying surprisingly outside the atrial cavity in the pulmonary veins and rapidly firing impulses into the bi‐atrial sink (Haissaguerre et al. 1998).
The multiple‐wavelet hypothesis was the initial proposed mechanism of AF in the continuity of Garrey's and Mines's earlier work. In a computer‐based mathematical model, Moe et al. (1964) proposed that multiple random wavefronts propagating through the atria result in self‐perpetuating ‘daughter’ wavelets maintaining AF. A large atrial mass with a short refractory period and delayed conduction increases the theoretical number of wavelets that prevent AF termination. This work was further substantiated by high density multi‐electrode mapping in animals and patients during surgery by the Maastricht group, reporting a multiplication of randomly circulating waves associated with a decreased atrial refractory period and heterogeneous tissue structure (Schotten et al. 2011). Atrial tissue undergoing widespread electrostructural alterations was considered to be a major factor in association with AF remodelling. Pathologically, the development of persistent AF is characterized by electrical remodelling leading to changes in ion channels, signalling pathways, calcium handling and connexin expression, and to structural changes in the atrial tissue associated with oxidative stress, inflammation and atrial fibrosis due to interstitial myofibroblasts responsible for collagen deposition uncoupling cardiomyocyte bundles (Schotten et al. 2011; Nattel & Harada, 2014; Goldberger et al. 2015).
In chronic AF pacing animal models, AF can be terminated pharmacologically initially but not after 6 months, as shortening of refractory period and slowdown in impulse propagation favouring reentrant mechanisms promotes AF, which in turn begets new changes, making AF a progressive electrostructural abnormality (Wijffels et al. 1995). Further studies confirm that while electrical remodelling is reversible completely within weeks of restoration of sinus rhythm, structural alterations reverse slowly, if at all (Schotten et al. 2011). The link between structure and maintenance of AF has been shown in multiple studies in which AF waves interact with patches of fibrosis (while diffuse fibrosis has little impact) and the orifices of anatomical structures that are critical sites for AF termination during ablation (Haïssaguerre et al. 2005; Tanaka et al. 2007; McDowell et al. 2015).
A recent detailed study used simultaneous endocardial–epicardial optical mapping coupled with 80 μm3 resolution MRI imaging in eight explanted human right atria (Hansen et al. 2015). Sustained AF was induced by pacing during pinacidil perfusion, which stabilizes a reentrant driver at highest doses (30–100 μm), abbreviating refractory periods by –46% to –80% (Fedorov et al 2011). Intramural reentry with 107 ± 50 ms cycle length was anchored on 3D micro‐anatomical tracks (15.4 ± 2.2 × 6.0 ± 2.3 mm 2, 2.9 ± 0.9 mm depth) formed by atrial musculature with increased fibre angle differences and interstitial fibrosis. Targeted ablation terminated most AF episodes (Hansen et al. 2015).
Considering the above mechanisms for the maintenance of AF, the clinical experience indicates that they reflect only a part of the reality spectrum. A distinctly localized driver proven by efficacy of localized ablation has been reported in a minority of persistent AF, while multiple wavelets, on the other end, are an important mechanism for the sustenance of long‐lasting (> 1 year) AF associated with extreme remodelling.
New evidence implicating atrial structure‐based substrate
The following evidence (summarized in Table 1 and detailed in Fig. 1) supports the role of intermittent and spatially unstable drivers clustered in structurally heterogeneous tissue substrate as a dominant mechanism in persistent AF.
Table 1.
Summary of results on the characteristics of AF drivers in relation to structural changes
| Method | Characteristics of AF drivers |
|---|---|
| Non‐invasive mapping | Drivers clustering in distinct regions |
| Electro‐anatomical mapping | AF drivers cluster at atrial areas with complex intermingled fibre architecture: pulmonary veins, septum, left appendage circumference, coronary sinus regions, etc. |
| MRI imaging | Predilection of AF drivers for areas bordering fibrosis |
| Specific fibrosis metrics (density and heterogeneity) | |
| Endocardial multi‐electrode mapping | Long duration electrograms indicating slow and impaired conduction |
| Regional electrograms covering a large part of AF cycle length | |
| No reproducible sequence of electrogram activation indicating variable propagation within the region | |
| Computer modelling | Specific fibrosis metrics – extent and density – are associated with drivers and virtual inducibility |
| Random fibrosis at critical density produces rate‐dependent disruption of propagation and local reentries | |
| Fibrosis creates loosely coupled islands connected by strands |
Figure 1. Mechanisms of atrial fibrillation .

A, schematic presentation of stable single (a) or multiple sources (b) and multiple wavelet (c) mechanisms of AF and the proposed hypothesis (d). In the latter, unstable reentrant drivers are shown to preferentially anchor to structural heterogeneities (black circles) B, snapshots of 5 consecutive AF beats mapped at the septal region from Ad showing the variability in reentry trajectory (a, b and e) focal breakthrough (c) or absence of driver (d) while remaining spatially localized.
Characteristics of drivers on panoramic non‐invasive and intracardiac mapping
Non‐invasive electrical mapping associated with phase mapping is a new technology capable of mapping global beat‐to‐beat AF activation providing new insights into AF dynamics (Rudy & Messinger‐Rapport, 1988; Cuculich et al. 2010; Haissaguerre et al. 2013). We have reported mapping results in 103 patients with persistent AF (Haissaguerre et al. 2014) and our current experience of 415 patients confirms the initial findings.
Multiplicity, instability and anatomical clustering of drivers
Reentrant and focal drivers were observed in a mean of 4.3 atrial regions with this number dependent on the duration of continuous AF. A single driver region was identified only in a minority of persistent AF: 9% in early persistent AF and 0% in long lasting AF. In the initial months, persistent AF was driven from two to three regions and this substrate disseminated progressively with prolongation of AF reaching seven to eight regions for the longer lasting AF (i.e. virtually most of the bi‐atrial surface), indicating that the driver burden is associated with the extent of atrial remodelling. The reentrant drivers were located in the pulmonary vein antra and contiguous structures, left appendage and septum in nearly all patients while left anterior and inferior left atrium–coronary sinus and right appendage were other common locations. Focal breakthroughs originated predominantly from the pulmonary vein ostia and the appendages. These results confirm prior works on the importance of the structures annexed to the left atrium and harbouring complex fibre orientation (Haissaguerre et al. 2005; Rostock et al. 2006).
Driver regions generated a varying set of short‐lasting reoccurring waves occupying part of the AF window span. These drivers were ephemeral but spatially recurrent, thus requiring statistical density mapping. The reentries meandered spatiotemporally for a median of 2.6 repetitive rotations with a spread of trajectory over surface areas of 7 ± 2 cm2. Therefore, the driver maps displayed locations over a certain area with variable extents rather than a discrete spot.
Endocardial multi‐electrode mapping of driver regions
The above data were corroborated by intracardiac mapping using a high density multi‐electrode catheter. Prolonged fractionated electrograms indicating localized slow conduction were more frequently observed at driver versus non‐driver regions. The electrogram sequence covered more of AF cycle length at the driver regions than elsewhere (71% versus 47%, P < 0.001), potentially indicating localized reentry (Haissaguerre et al. 2006) but the same electrogram sequence could only rarely be observed in more than five consecutive beats; indicating instability of local propagation.
Acute impact of driver ablation
Persistent AF could be terminated in 75% of patients with the number of targeted regions increasing with AF duration (3 in AF lasting < 3 months, 4 in AF lasting 4–6 months and 6 in AF lasting > 6 months) again indicating a link with the extent of atrial remodelling. The AF termination rate was 15% for long‐lasting AF (> 12 months). Importantly the local ablation sites where persistent AF was slowed (or terminated) in a given driver region demonstrated often long duration electrograms, which could represent small isthmuses in the reentrant path. Finally a subset of patients have presented episodes of atrial tachycardia after AF termination that fulfilled the requirements of localized reentry involving slow conduction in a narrow anatomical isthmus (Sanders et al. 2005). It was found that 68% of these tachycardias occurred at the same sites where AF drivers were mapped earlier suggesting a common structural substrate. An example is illustrated in Fig. 2.
Figure 2. Localized reentry along the right superior pulmonary vein (RSPV) and adjacent region (middle panel) in a region previously involved as an AF driver (left panel) .

The entire tachycardia circuit (370 ms) is present on the sequence of electrograms (mostly long and fractionated, right panel) along a discrete and tortuous path from points 1 to 9 indicating slow and impaired conduction due to severe tissue remodelling. Tachycardia was terminated within seconds of thermoablation.
Extended clinical experience
More recently, eight European centres enrolled 118 persistent AF patients (AF duration < 1 year). They undertook non‐invasive mapping and driver ablation in a prospective manner, and confirmed the initial results and their reproducibility (Sohal et al. 2015).
AF driver and atrial structural imaging
Atrial fibrosis correlates with the persistence of AF, and electroanatomic bipolar voltage mapping has been used as a surrogate to define scar (Jadidi et al. 2013; Malacome‐Lawes et al. 2013; Kapa et al. 2014). Individual variability in the extent of fibrosis can be seen, however, for a similar AF burden, being sometimes massive in short lasting AF or mild in long persistent AF (Kottkamp, 2013; Rolf et al. 2014; Arentz et al. 2015). Gadolinium‐enhanced magnetic resonance imaging has been shown to provide a non‐invasive metric for its quantification and a metric of overall disease progression (Marrouche et al. 2014).
We have investigated the relationship between AF reentrant sources and the burden/location of fibrosis areas by combining MRI and panoramic non‐invasive mapping in 41 patients. The fibrosis burden, particularly left atrial fibrosis, correlated independently with the number of reentrant regions and the great majority of drivers were found at the borders of fibrosis areas (Fig. 3). There was also a positive correlation with left atrial volume, and with uninterrupted AF duration. Importantly, when compared with the rest of the atria, sites with reentrant drivers showed a significantly higher disorganization index (entropy: 44.8 ± 14.3 vs. 27.8 ± 10.3%), and a greater density of local fibrosis (34.6 ± 15.3 vs. 13.6 ± 7.3%). This indicates that not all fibrosis carries the same arrhythmogenic potential and that distinct tissue characteristics allow formation of the substrate favourable for reentrant drivers. This hypothesis was tested in greater detail using modelling studies to specify the intrinsic characteristics of arrhythmogenic fibrosis.
Figure 3. Relationship between reentry trajectories and focal fibrosis .

MRI and driver mapping registration in 4 patients. The trajectories of phase singularities are overlaid on fibrosis distribution (white indicate late gadolinium enhancement). Each trajectory is colour‐coded according to the percentage of time a phase singularity was observed at each location: yellow trajectories indicate sites of anchoring, of reentrant activity, whereas red trajectories indicate transit paths, where reentrant activity simply drifts. Visual analysis clearly shows that anchoring sites (yellow trajectories) are located at the border of fibrotic areas, whereas trajectories observed either inside (bottom left panel) or outside (upper left panel) fibrotic areas appear as drifting sites (red trajectories).
In silico assessment of arrhythmogenic potential of fibrosis
Patient‐specific atrial models
In the continuity of prior work showing that drivers were confined to certain regions regardless of induction modalities (McDowell et al. 2015), personalized MRI‐based models of 13 patients’ atria were constructed to examine how the spatial distribution of fibrosis affects the dynamics of persistent atrial fibrillation (PsAF) and localization of reentrant driver. Fibrotic density was defined within each 2.5 mm spherical atrial volume. After AF induction the centre of reentrant activities was demonstrated to colocate at the borders of fibrotic regions. The latter had a specific spatial pattern with 80% of them being at sites with fibrotic density > 70%, whereas only 10% of non‐driver regions harboured such fibrotic density (Fig. 4). In addition, virtual inducibility was evaluated with patient‐specific fibrosis parameters. Both global metric (percentage of atrial surface with fibrosis) and high‐density index (percentage of atrial surface with > 70% fibrosis) correlated with inducibility, but the density index has a higher predictive value (Boyle et al. 2015).
Figure 4. Locations of phase singularities associated with reentrant drivers .
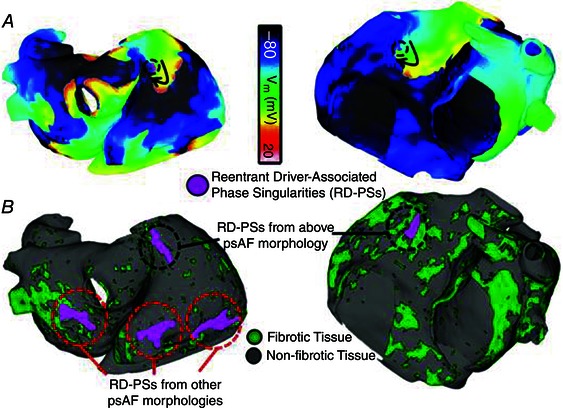
A, transmembrane voltage maps during AF for Patients 1 (left) and 2 (right). Black arrows indicate direction of the propagating reentry. Phase singularities associated with reentrant drivers are indicated by purple dots surrounded by dashed black circles. B, regions anchoring the meandering reentry phase singularities (purple) during the single AF induction in A (dashed black circle) and, in the case of Patient 1 (left), in other observed AF episodes (dashed red circles).
Percolation as a potential mechanism of reentry formation and electrogram fractionation
Further computational studies were performed to investigate in more detail the specific role of microstructural remodelling.
Diffuse fibrosis has been shown to have little effect on propagation in computer simulations (Zhao et al. 2013). However, more severe patchy fibrosis caused severe disruptions to propagating wavefronts (Comtois & Nattel, 2011) and rotor dynamics (Tusscher & Panfilov, 2005). In a combined experimental and modelling study, Bub et al. (2002) showed that reduced coupling, similar to the effects of severe fibrosis, can transform dynamics of the medium from planar wave propagation to reentrant activity. Alonso and Barr recognized that propagation through highly fibrotic tissue was an example of percolation, the movement of a substance through a medium with random structure (Alonso & Bär, 2013).
We performed simulations in two‐dimensional models of atrial tissue with a remodelled central zone, in which myocytes were randomly replaced by collagen with probability P (Fig. 5). Importantly electrophysiological and coupling properties were kept constant throughout the model. The results show that atrial waves pass through the tissue with little disruption for low P values until a critical range of P, where percolation was observed (Fig. 5 A) harbouring severe wavefront disruption, saltatory propagation and small coherent domains. Low amplitude, long lasting electrograms were generated, and decreasing cycle length could lead to wavefronts exiting the remodelled zone with increasing delay and eliciting local reentries. Results were dependent on wavefront direction and fibrotic density. Further increasing P led to conduction block in the remodelled zone. This model shows that a wavefront passing through a medium with random fibrosis (percolation) may produce rate‐dependent disruption of propagation and various reentries within a series of loosely coupled islands connected by strands (Vigmond et al. 2015).
Figure 5. Percolation .

A, percolatory propagation in a computer model of atrial tissue. A central 2 cm × 2 cm fibrotic region is shown in which 55% of the elements have been randomly removed. The tissue was stimulated along the central portion of the left edge. B, clinical electrograms showing increasing fractionation and reentry with increasing pacing frequency. C, simulated electrograms from centre of fibrotic region in panel A also showing increased fractionation and reentry with increased pacing frequency.
Limitations
The proposed hypothesis is based on clinical observations obtained using a combination of novel techniques (non‐invasive mapping, MRI imaging and computer modelling). Our results differ from studies (Narayan et al. 2012; Miller et al. 2014; Swarup et al. 2014; Sommer et al. 2016) that report stable sources rotating permanently while other clinical studies using epicardial (Lee et al. 2014) or endocardial mapping (Alagoz et al. 2015; Zhao et al. 2015) have observed predominantly unstable drivers.
The proposed structure‐based hypothesis applies to a majority of persistent AF but not all. The AF pathophysiological spectrum in humans may vary from a single driving source at one end (Jais et al. 1997) to diffuse multiple wavelets at the other (De Groot et al. 2010). In addition, individual variations in the remodelling process may be observed with varying extent of fibrosis for a similar AF burden, being sometimes massive in short lasting AF or mild in long persistent AF (Kottkamp, 2013; Rolf et al. 2014; Arentz et al. 2015). Electrical remodelling is also variable and some persistent AF patients may present with very short fibrillatory cycle lengths (< 140 ms, i.e. > 420 atrial beats per minute) without apparent low voltage or MRI fibrosis regions, suggesting a predominantly electrical remodelling mechanism of AF with marked action potential abbreviation or arrhythmogenic Ca2+ handling (Martins et al. 2014).
Each technique used in the present study bears some inherent limitations but considered as a group, they concur to on similar findings. The delineation of fibrosis by low voltage mapping or MRI imaging has limitations in individual reproducibility or detection of diffuse (not patchy) homogeneous fibrosis. Atrial structural remodelling consisting of interstitial fibrosis intermingled with surviving fibres of cardiomyocytes produces interstitial expansion, which is the characteristic targeted by late gadolinium enhancement (LGE; extracellular distribution of gadolinium chelates). LGE methods were initially developed to image focal ischaemic scars and regions with lower signal are artificially blackened to increase contrast (Simonetti et al. 2001). In the absence of other causes of interstitial expansion (such as oedema), areas of LGE within the atrium represent areas of most pronounced fibrosis, even if a diffuse and homogeneous interstitial fibrosis component can be present outside LGE areas. Therefore if quantitatively a certain degree of fibrosis may be missed elsewhere in the atrium, LGE imaging is able to distinguish between areas with higher and lower amounts of fibrosis and our results were consistent with prior studies (Jadidi et al. 2013; Marrouche et al. 2014).
The non‐invasive mapping system has also limitations in resolution as it cannot capture the fine details below 7 mm (Rudy et al. 1988, Ramanathan et al. 2004). Thus we cannot exclude that a focal site may be a localized reentry (with small atrial domain) missed by the mapping approach. However, it has a unique panoramic scope showing bi‐atrial interactions, while body filtering does not affect global patterns – the main propagation waves – which may be an essential advantage. In atrial tachycardias non‐invasive mapping thus allowed performance of accurate diagnosis and location of the wave origin (Shah et al. 2013). Despite this limitation, the accuracy of mapping was also confirmed as the same non‐invasive data were used to guide energy delivery and terminate most persistent AF.
Finally, non‐invasive mapping has a preferential scope towards the epicardial side of atria, which may behave as a 3D structure in rapid fibrillatory rhythms. A discordant activity in propagation vectors and phase singularity trajectories may occur across the transmural wall (Schotten et al. 2011; Hansen et al. 2015) resulting in a few millimetres (4.2 ± 0.3 mm in our experience, Gutbrodt et al. 2015 ) discrepancy in endocardial versus epicardial spatial location. In the present study non‐invasive mapping was associated with multi‐electrode endocardial mapping that showed variability in successive electrogram sequences and confirmed instability of driver activation in the mapped regions via different microanatomical paths (Hansen et al. 2015; Vigmond et al. 2015).
Conclusion
This review provides evidence from novel techniques of clinical mapping combined with imaging and computational modelling to support a dominant mechanism of persistent AF in humans, where the drivers are temporally intermittent and spatially unstable within heterogeneous structural/fibrotic atrial regions harbouring specific topological characteristics.
Additional information
Competing interests
M. Haissaguerre, Ashok J. Shah, Hubert Cochet, Meleze Hocini, Remi Dubois, Edward Vigmond and Olivier Bernus have research grants from Medtronic, Biosense Webster. Edward Vigmond is an owner of CardioSolv LLC. Igor Efimov and Natalia Trayanova don't have any conflict of interest.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the grants Agence Nationale de la Recherche (ANR) under Grant Agreements IHU LIRYC ANR‐10‐IAHU‐04.
Biographies
Michel Haïssaguerre, Professor of Cardiology, heads the Department of Cardiology‐electrophysiology at Bordeaux University Hospital. He is also the Director of the Electrophysiology and Heart modelling institute LIRYC. His scientific and clinical work focuses on cardiovascular electrophysiology.
Ashok J. Shah has completed a fellowship in the electrophysiology department of Michel Haissaguerre, focusing on non‐invasive mapping of various simple and complex cardiac arrhythmias.

Hubert Cochet, Associate Professor ‐ Hospital Practitioner, heads the cardiac imaging clinic at the Bordeaux University Hospital, co‐heads the imaging group of LIRYC, and is also the Director of the Cardiology program within TRAIL (Translational Research and Advanced Imaging Laboratory).
Meleze Hocini is Associate Professor ‐ Hospital Practitioner at Bordeaux University Hospital and is deputy director of LIRYC. Her projects are focusing on cardiac stimulation and arrhythmias ablation, in particular ventricular fibrillation and sudden cardiac death.
Olivier Bernus is Professor at the University of Bordeaux, Scientific Director of LIRYC and heads the Tissue Electrophysiology team. His main research interests are in the mechanisms underlying cardiac arrhythmias, especially ventricular fibrillation, using both computational and experimental approaches. Rémi Dubois, Associate Professor, is the Valorization coordinator of LIRYC and heads the signal processing team. His research interests focus on feature extraction of biological signals to help in the diagnosis and the understanding of pathology.
Igor R. Efimov, Visiting Professor at LIRYC, is Chairman of Department of Biomedical Engineering, George Washington University, and Director of NIH‐funded cardiovascular engineering laboratory. He co‐founded Cardialen to develop low energy electrotherapy, with a primary focus on atrial fibrillation.
Edward Vigmond heads the Modelling Group at LIRYC. His research focuses on the use of large‐scale computer modelling to understand and treat cardiac pathologies.
Natalia Trayanova is the Murray B. Sachs Professor of Biomedical Engineering at Johns Hopkins University. She directs the Computational Cardiology Laboratory at the Institute for Computational Medicine. Her research focuses on understanding electrical dysfunction of the heart, and on improving anti‐arrhythmia therapies using a personalized approach.
References
- Alagoz C, Guez A, Cohen A & Bullinga JR (2015). Spiral wave classification using normalized compression distance: towards atrial tissue spatiotemporal electrophysiological behavior characterization. Conf Proc IEEE Eng Med Biol Soc 4503–4506. [DOI] [PubMed] [Google Scholar]
- Allessie MA, Bonke FI & Schopman FJ (1973). Circus movement in rabbit atrial muscle as a mechanism of trachycardia. Circ Res 33, 54–62. [PubMed] [Google Scholar]
- Allessie M & de Groot N (2014). CrossTalk opposing view: Rotors have not been demonstrated to be the drivers of atrial fibrillation. J Physiol 592, 3167–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S & Bär M (2013). Reentry near the percolation threshold in a heterogeneous discrete model for cardiac tissue. Phys Rev Lett 110, 158101. [DOI] [PubMed] [Google Scholar]
- Arentz T, Lehrmann H, Sorrel J, Markstein V, Park C, Allgeier J, Weber R & Jadidi A (2015). Selective substrate based ablation compared to pulmonary vein isolation alone in patients with persistent atrial fibrillation. Heart Rhythm 12, S347, P004‐77. [Google Scholar]
- Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB & Levy D (1998). Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 98, 946–952. [DOI] [PubMed] [Google Scholar]
- Boyle PM, Zahid S, Schwarz EL, Whyte KN, Vigmond EJ, Dubois R, Haïssaguerre M, Hocini M, Jaïs P, Cochet H & Trayanova N (2015). Local complexity of the fibrosis spatial pattern determines the locations of stable reentrant sources in persistent atrial fibrillation: analysis from patient specific models. Heart Rhythm 12, S7‐AB03‐05. [Google Scholar]
- Bub G, Shrier A & Glass L (2002). Spiral wave generation in heterogeneous excitable media. Phys Rev Lett 88, 058101. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines‐CPG; Document Reviewers (2012). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace 14, 1385–1413. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M & Murray CJ (2014). Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 129, 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comtois P & Nattel S (2011). Interactions between cardiac fibrosis spatial pattern and ionic remodeling on electrical wave propagation. Conf Proc IEEE Eng Med Biol Soc 2011, 4669–4672. [DOI] [PubMed] [Google Scholar]
- Cox JL, Schuessler RB, D'Agostino HJ Jr, Stone CM, Chang BC, Cain ME, Corr PB & Boineau JP (1991). The surgical treatment of atrial fibrillation: development of a definitive surgical procedure. J Thorac Cardiovasc Surg 101, 569–583. [PubMed] [Google Scholar]
- Cuculich PS, Wang Y, Lindsay BD, Faddis MN, Schuessler RB, Damiano RJ Jr, Li L, Rudy Y (2010). Noninvasive characterization of epicardial activation in humans with diverse atrial fibrillation patterns. Circulation 122, 1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groot NM, Houben RP, Smeets JL, Boersma E, Schotten U, Schalij MJ, Crijns H & Allessie MA (2010). Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation 122, 1674–1682. [DOI] [PubMed] [Google Scholar]
- Fedorov VV, Glukhov AV, Ambrosi CM, Kostecki G, Chang R, Janks D, Schuessler RB, Moazami N, Nichols CG & Efimov IR (2011). Effects of KATP channel openers diazoxide and pinacidil in coronary‐perfused atria and ventricles from failing and non‐failing human hearts. J Mol Cell Cardiol 51, 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrey WE (1914). The nature of fibrillatory contraction of the heart: Its relation to tissue mass and form. Am J Physiol 33, 397–414. [Google Scholar]
- Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd‐Jones DM, Markl M, Ng J & Shah SJ (2015). Evaluating the atrial myopathy underlying atrial fibrillation: Identifying the arrhythmogenic and thrombogenic substrate. Circulation 132, 278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbrod SR, Walton R, Gilbert S, Meillet V, Jaïs P, Hocini M, Haïssaguerre M, Dubois R, Bernus O & Efimov IR (2015). Quantification of the transmural dynamics of atrial fibrillation by simultaneous endocardial and epicardial optical mapping in an acute sheep model. Circ Arrhythm Electrophysiol 8, 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P & Dubois R (2014). Driver domains in persistent atrial fibrillation. Circulation 130, 530–538. [DOI] [PubMed] [Google Scholar]
- Haïssaguerre M, Hocini M, Sanders P, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Jonsson A, O'Neill MD, Bordachar P, Reuter S, Roudaut R, Clémenty J & Jaïs P (2006). Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation 113, 616–625. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Hocini M, Shah AJ, Derval N, Sacher F, Jais P & Dubois R (2013). Noninvasive panoramic mapping of human atrial fibrillation mechanisms: a feasibility report. J Cardiovasc Electrophysiol 24, 711–717. [DOI] [PubMed] [Google Scholar]
- Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Métayer P & Clémenty J (1998). Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 339, 659–666. [DOI] [PubMed] [Google Scholar]
- Haïssaguerre M, Sanders P, Hocini M, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Bordachar P, Reuter S, Roudaut R, Clémenty J & Jaïs P (2005). Catheter ablation of long‐lasting persistent atrialfibrillation: critical structures for termination. J Cardiovasc Electrophysiol 16, 1125–1137. [DOI] [PubMed] [Google Scholar]
- Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD & Fedorov VV (2015). Atrial fibrillation driven by micro‐anatomic intramural re‐entry revealed by simultaneous sub‐epicardial and sub‐endocardial optical mapping in explanted human hearts. Eur Heart J 36, 2390–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YF, Chen YJ, Lin YJ & Chen SA (2015). Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 12, 230–243. [DOI] [PubMed] [Google Scholar]
- Jadidi AS, Cochet H, Shah AJ, Kim SJ, Duncan E, Miyazaki S, Sermesant M, Lehrmann H, Lederlin M, Linton N, Forclaz A, Nault I, Rivard L, Wright M, Liu X, Scherr D, Wilton SB, Roten L, Pascale P, Derval N, Sacher F, Knecht S, Keyl C, Hocini M, Montaudon M, Laurent F, Haïssaguerre M & Jaïs P (2013). Inverse relationship between fractionated electrograms and atrial fibrosis in persistentatrial fibrillation: combined magnetic resonance imaging and high density mapping. J Am Coll Cardiol 62, 802–812. [DOI] [PubMed] [Google Scholar]
- Jaïs P, Haïssaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M & Clémenty J (1997). A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation 95, 572–576. [DOI] [PubMed] [Google Scholar]
- Jalife J, Berenfeld O & Mansour M (2002). Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res 54, 204–216. [DOI] [PubMed] [Google Scholar]
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (2014). 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 64, e1–e76. [DOI] [PubMed] [Google Scholar]
- Kapa S, Desjardins B, Callans DJ, Marchlinski FE & Dixit S (2014). Contact electroanatomic mapping derived voltage criteria for characterizing left atrial scar in patients undergoing ablation for atrial fibrillation. J Cardiovasc Electrophysiol 25, 1044–1052. [DOI] [PubMed] [Google Scholar]
- Kottkamp H (2013). Human atrial fibrillation substrate: towards a specific fibrotic atrial cardiomyopathy. Eur Heart J 34, 2731–2738. [DOI] [PubMed] [Google Scholar]
- Lee G, Kumar S, Teh A, Madry A, Spence S, Larobina M, Goldblatt J, Brown R, Atkinson V, Moten S, Morton JB, Sanders P, Kistler PM & Kalman JM (2014). Epicardial wave mapping in human long‐lasting persistent atrial fibrillation: transient rotational circuits, complex wavefronts, and disorganized activity. Eur Heart J 35, 86–97. [DOI] [PubMed] [Google Scholar]
- Malcolme‐Lawes LC, Juli C, Karim R, Bai W, Quest R, Lim PB, Jamil‐Copley S, Kojodjojo P, Ariff B, Davies DW, Rueckert D, Francis DP, Hunter R, Jones D, Boubertakh R, Petersen SE, Schilling R, Kanagaratnam P & Peters NS (2013). Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: a 2‐center study. Heart Rhythm 10, 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Daoud E, Wissner E, Bansmann P & Brachmann J (2014). Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA 311, 498–506. [DOI] [PubMed] [Google Scholar]
- Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras‐Rama D, Ennis SR, Takemoto Y, Ponce‐Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero‐Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O & Jalife J (2014). Dominant frequency increase rate predicts transition from paroxysmal to long‐term persistent atrial fibrillation. Circulation 129, 1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell KS, Zahid S, Vadakkumpadan F, Blauer J, MacLeod RS & Trayanova NA (2015). Virtual electrophysiological study of atrial fibrillation in fibrotic remodeling. PLoS One 10, e0117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS & Wheelan KR (2014). Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 25, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mines GR (1913). On dynamic equilibrium in the heart. J Physiol 46, 349–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe GK, Rheinboldt WC & Abildskov JA (1964). A computer model of atrial fibrillation. Am Heart J 67, 200–220. [DOI] [PubMed] [Google Scholar]
- Narayan SM & Jalife J (2014). CrossTalk proposal: Rotors have been demonstrated to drive human atrial fibrillation. J Physiol 592, 3163–3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM & Jalife J (2014). Rebuttal from Sanjiv M. Narayan and José Jalife. J Physiol 592, 3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel WJ & Miller JM (2012). Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM trial. J Am Coll Cardiol 60, 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattel S & Harada M (2014). Atrial remodeling and atrial fibrillation: recent advances and translational perspectives.J Am Coll Cardiol 63, 2335–2345. [DOI] [PubMed] [Google Scholar]
- Ramanathan C, Ghanem RN, Jia P, Ryu K & Rudy Y (2004). Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med 10, 422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, Gaspar T, Bollmann A, Altmann D, Piedra C, Hindricks G & Piorkowski C (2014). Tailored atrial substrate modification based on low‐voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 7, 825–833. [DOI] [PubMed] [Google Scholar]
- Rostock T, Rotter M, Sanders P, Takahashi Y, Jaïs P, Hocini M, Hsu LF, Sacher F, Clémenty J & Haïssaguerre M (2006). High‐density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm 3, 27–34. [DOI] [PubMed] [Google Scholar]
- Rudy Y & Messinger‐Rapport BJ (1988). The inverse problem in electrocardiography: solutions in terms of epicardial potentials. Crit Rev Biomed Eng 16, 215–268. [PubMed] [Google Scholar]
- Sanders P, Hocini M, Jaïs P, Hsu LF, Takahashi Y, Rotter M, Scavée C, Pasquié JL, Sacher F, Rostock T, Nalliah CJ, Clémenty J & Haïssaguerre M (2005). Characterization of focal atrial tachycardia using high‐density mapping. J Am Coll Cardiol 46, 2088–2099. [DOI] [PubMed] [Google Scholar]
- Schotten U, Verheule S, Kirchhof P & Goette A (2011). Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 91, 265–325. [DOI] [PubMed] [Google Scholar]
- Schuessler RB, Grayson TM, Bromberg BI, Cox JL & Boineau JP (1992). Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res 71, 1254–1267. [DOI] [PubMed] [Google Scholar]
- Schuessler RB, Kawamoto T, Hand DE, Mitsuno M, Bromberg BI, Cox JL & Boineau JP (1993). Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation 88, 250–263. [DOI] [PubMed] [Google Scholar]
- Shah AJ, Hocini M, Xhaet O, Pascale P, Roten L, Wilton SB, Linton N, Scherr D, Miyazaki S, Jadidi AS, Liu X, Forclaz A, Nault I, Rivard L, Pedersen ME, Derval N, Sacher F, Knecht S, Jais P, Dubois R, Eliautou S, Bokan R, Strom M, Ramanathan C, Cakulev I, Sahadevan J, Lindsay B, Waldo AL & Haissaguerre M (2013). Validation of novel 3‐dimensional electrocardiographic mapping of atrial tachycardias by invasive mapping and ablation: a multicenter study. J Am Coll Cardiol 62, 889–897. [DOI] [PubMed] [Google Scholar]
- Simonetti OP, Kim RJ, Fieno DS, Hillenbrand HB, Wu E, Bundy JM, Finn JP & Judd RM (2001). An improved MR imaging technique for the visualization of myocardial infarction. Radiology 218, 215–223. [DOI] [PubMed] [Google Scholar]
- Sohal M, Duytschaever M, Tavernier R, Neumann T, Cauchemez B, Albenque JP, Rostock T, Deisenhofer IV, Arentz T, Jadidi A, Ernst S, Packer D, Knecht S (2015). Patient specific distribution of AF drivers prognostic insights from noninvasive mapping of persistent AF. Heart Rhythm 12, S112, P001‐40. [Google Scholar]
- Sommer P, Kircher S, Rolf S, John S, Arya A, Dinov B, Richter S, Bollmann A, Hindricks G (2016). Successful repeat catheter ablation of recurrent longstanding persistent atrial fibrillation with rotor elimination as the procedural endpoint: a case series. J Cardiovasc Electrophysiol 27, 274–280. [DOI] [PubMed] [Google Scholar]
- Spach MS, Miller WT, 3rd Geselowitz DB, Barr RC, Kootsey JM, Johnson EA (1981). The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res 48, 39–54. [DOI] [PubMed] [Google Scholar]
- Swarup V, Baykaner T, Rostamian A, Daubert JP, Hummel J, Krummen DE, Trikha R, Miller JM, Tomassoni GF & Narayan SM (2014). Stability of rotors and focal sources for human atrial fibrillation: focal impulse and rotor mapping (FIRM) of AF sources and fibrillatory conduction. J Cardiovasc Electrophysiol 25, 1284‐1292. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Zlochiver S, Vikstrom KL, Yamazaki M, Moreno J, Klos M, Zaitsev AV, Vaidyanathan R, Auerbach DS & Landas S (2007). Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ Res 101, 839–847. [DOI] [PubMed] [Google Scholar]
- ten Tusscher KHWJT & Panfilov AV (2005). Wave propagation in excitable media with randomly distributed obstacles. Multiscale Model Simul 3, 265–282. [Google Scholar]
- Vigmond E, Pashaei A, Amraoui S & Haissaguerre M (2015). Percolation as a novel mechanism for fractionation of atrial electrograms and reentry. Heart Rhythm 12, S152, P002‐15. [DOI] [PubMed] [Google Scholar]
- Wakili R, Voigt N, Kääb S, Dobrev D & Nattel S (2011). Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest 121, 2955–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijffels MC, Kirchhof CJ, Dorland R & Allessie MA (1995). Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 92, 1954–1968. [DOI] [PubMed] [Google Scholar]
- Zhao J, Stephenson RS, Sands GB, LeGrice IJ, Zhang H, Jarvis JC & Smaill BH (2013). Atrial fibrosis and atrial fibrillation: A computer simulation in the posterior left atrium FIMH’13: Proceedings of the 7th international conference on Functional Imaging and Modeling of the Heart, pp. 400–408. Springer, Berlin, Heidelberg. [Google Scholar]
- Zhao J, Yao Y, Shi R, Huang W, Smaill BH, Lever NA (2015). Progressive modification of rotors in persistent atrial fibrillation by stepwise linear ablation. Heart Rhythm Case Rep 1, 22–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


