Abstract
Purpose
In the last 8 years, numerous cohort studies have been conducted to estimate the rate of mother-to-child transmission (MTCT) of HIV. Many of these have faced problems in data collection and analysis, making it difficult to compare transmission rates between studies. This workshop on methodological aspects of the study of MTCT of HIV-1 was held in Ghent (Belgium) in February 1992.
Study selection and data extraction
Fourteen teams of investigators participated, representing studies from Central (five) and Eastern Africa (three), Europe (two), Haiti (one) and States (three). A critical evaluation of the projects was carried out, under four headings: (1) enrollment and follow-up procedures, (2) diagnostic criteria and case definitions, (3) measurement and comparison of MTCT rates and (4) determinants of transmission.
Results of data analysis
Reported transmission rates ranged from 13 to 32% in industrialized countries and from 25 to 48% in developing countries. However, no direct comparisons could be made because methods of calculation differed from study to study. Based on this review, a common methodology was developed. Agreement was reached on definitions of HIV-related signs/symptoms, paediatric AIDS and HIV-related deaths. A classification system of children born to HIV-1-infected mothers according to their probable HIV infection status during the first 15 months of life, allowed the elaboration of a direct method of computation of the transmission rate and of an indirect method for studies with a comparison group of children born to HIV-seronegative mothers. This standardized approach was subsequently applied to selected data sets.
Conclusions
The methodology can now be applied to all studies with sufficient follow-up and comparisons made between transmission rates. This step is essential for assessing determinants of transmission and for the development of a common approach for the evaluation of interventions aimed at reducing or interrupting MTCT of HIV.
Keywords: Adult; Female; HIV Infections; HIV-1; Humans; Incidence; Infant, Newborn; Mothers
Introduction
The worldwide spread of HIV through heterosexual contacts and intravenous drug use has lead to a high prevalence of infection among women of child-bearing age, particularly in Africa [1]. Mother-to-child transmission (MTCT) of HIV, often referred to as perinatal transmission, is a direct consequence of this epidemiological pattern. The World Health Organization (WHO) estimates that by 1992, almost 1 million infected children had been born to HIV-infected mothers since the beginning of the HIV epidemic. Almost half a million have already developed AIDS and subsequently died [2]. A larger number of uninfected children are likely to become orphans because of the loss of one or both parents from HIV disease [1,3].
Many researchers have been engaged in the study of MTCT of HIV since the mid-1980s [4–7]. These studies are necessary for at least five reasons: (1) to estimate the MTCT rate of HIV for demographic projections [1,2]; (2) for planning health resources allocation; (3) to obtain a reliable range of values for MTCT of HIV and to compare rates of transmission in various settings; (4) to understand the determinants of MTCT of HIV so that factors amenable to interventions which could decrease transmission can be identified; (5) to improve individual counselling and the case management of mothers and children.
Investigators of MTCT studies confront many practical problems, including difficulties with survey instruments, definitions used for calculating the rate of transmission and the protocol of clinical and biological follow-up of children. The need for a methodological workshop on MTCT of HIV with special reference to Africa was recognized by several investigators, the European Economic Community (EEC) AIDS Task Force and WHO in 1988 [8]. Most of the ongoing studies had insufficient follow-up at that time to provide definite results for the formulation of recommendations for public health and clinical practice.
A meeting was held in 1992 in Ghent (Belgium) under the auspices of the EEC AIDS Task Force, in collaboration with the WHO Global Programme on AIDS and UNICEF. This workshop included 40 scientists from three continents who were experts in paediatrics, obstetrics, virology, epidemiology and biostatistics. The objectives were: (1) to address methodological issues in the estimation of the rate of MTCT of HIV-1 with special reference to developing countries; (2) to present a critical evaluation of selected perinatal studies using a standardized methodological approach. This report provides a summary of the discussions and recommendations made during the workshop for further analysis and follow-up of ongoing studies and for the direction and design of future studies.
Overview of the completed and ongoing studies
The principal investigators of 14 studies participated in this workshop. Five reviewed studies were from Central Africa [9–14], three from Southern and Eastern Africa [15–17], one from the Caribbean [18], three from the USA [19–22] and two from Europe [23–25]. With the exception of one study in Central Africa [13], these were based in large urban centres where HIV seroprevalence is high. In developing countries, there was a wide range in seroprevalence among pregnant women screened, from 3.9% in Brazzaville (Congo) in 1987–1989 [10] to 30% in Kigali (Rwanda) in 1988–1989 [11]. Few seroprevalence figures were available for industrialized countries.
All studies reviewed included as an objective the measurement of the rate of HIV MTCT and the description of the natural history of paediatric HIV infection. However, only about half were able to collect data on potential determinants of HIV MTCT. All studies started between 1985 and 1989. The availability of early diagnostic tools was limited at the start and varied according to site, influencing the design of these studies. Eight studies enrolled livebirths and six enrolled pregnant women, either during pregnancy or at delivery. Enrollment took place in prenatal clinics or maternity wards.
The number of children born to HIV-seropositive mothers enrolled in each study varied from 112 to 1060 in industrialized countries and from 118 to 679 in developing countries. Only two studies from industrialized countries included a comparison group [21,22], compared with the nine studies in developing countries. Various criteria were used to select the comparison group (next delivery, age, parity, geographical origin, etc.). The number of children born to HIV-seronegative mothers enrolled in the comparison group varied from 40 to 3589 per study. Formal sample size calculation was rarely performed before the study was started.
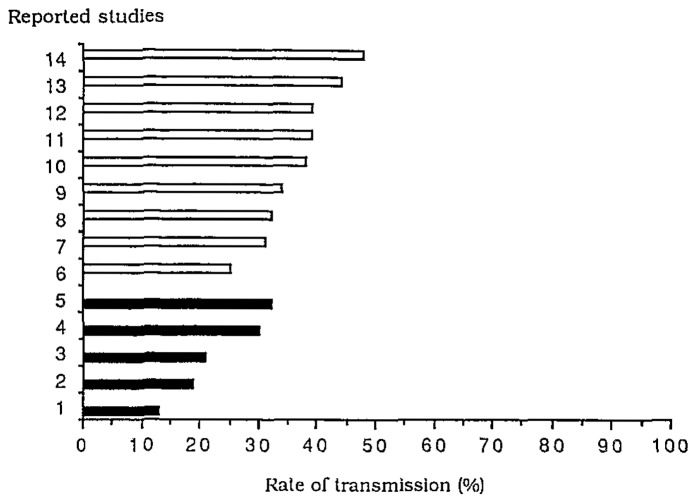
In all studies, there was a regular clinical follow-up throughout the first year of life but there was more variation in the timing and frequency of blood sampling and in the overall length of follow-up. In most studies, mortality in the comparison group (children born to HIV-seronegative mothers) was lower than anticipated, due to surveillance bias. The different estimations of the rate of HIV MTCT reported ranged from 13 to 32% in industrialized countries and from 26 to 48% in developing countries (Fig. 1). However, in each study, the method used to estimate the MTCT rate of HIV was study-specific.
Fig. 1.
Reported rates of mother-to-child transmission of HIV-1 (%) in five studies in industrialized countries (■) and in nine studies in developing countries (□). Ghent, February 1992.
Summary of recommendations
Clinical and laboratory procedures
Enrollment
Women should be identified during pregnancy in preference to delivery to study the timing of transmission and all children enrolled at birth. A strict enrollment definition with clear inclusion criteria should be established and all enrolled children described in any publication. Strictly defined exclusion criteria should be used to reduce the loss to follow-up. Women refusing to participate in the study should be excluded, either at the initial enrollment or if they are unwilling to attend the first follow-up. In addition, it may be appropriate to exclude women and children not permanently resident in the catchment area. Children should be excluded if their mother was not tested at or before the time of delivery. Stillbirths should always be excluded from the calculation of the rate of HIV MTCT.
No specific recommendation with regard to sample size could be made because the sample size depends on study-specific objectives. A comparison group is necessary for the calculation of excess mortality of children born to HIV-seropositive mothers (see Measurement and comparison of rates of transmission). Although it was recognized that the morbidity and mortality of the comparison group may not reflect that of the population from which it was derived, both cohorts have the same access to health care and special services provided by the study. It was recommended that the two groups should be made as comparable as possible in terms of maternal age and parity although individual matching was not considered essential. Another justification for a comparison group is to identify maternal seroconversions and to study postnatal transmission [26].
Follow-up
Clinical information should aim to identify paediatric AIDS, HIV-related signs/symptoms and HIV-related deaths (see proposed definitions in Diagnostic criteria and case definitions). Regular specimen collection (blood or other samples) up to at least 15 months of life is important in the analysis of loss of maternal antibody.
The estimation of mortality should always use survival analysis methods (for example, Kaplan–Meier product limit method [27]). An effort should be made to establish causes of death of children who died at home, using verbal autopsies [28]. A standard verbal autopsy report should be used for this purpose.
Loss to follow-up is a major problem in prospective studies and may bias the estimates of MTCT rates. It should be clearly defined and described. Where possible, comparisons should be made between the clinical and immunological characteristics of children lost to follow-up and those not lost to follow-up, in order to look for potential biases.
Diagnostic criteria and case definitions
Clinical assessment
Two paediatric clinical AIDS definitions applicable to settings with scarce diagnostic facilities have been used in the past (Table 1): the WHO clinical definition [29] (the so-called Bangui definition) and a modified version [8]. In the modified version, persistent cough is not included as a minor sign, but recurrent pneumonia is included as a major sign. These definitions have been evaluated cross-sectionally in various African countries using HIV-antibody test results as the gold standard [30–32]. They have shown low sensitivity and positive predictive value. In cohort studies where repeated cross-sectional assessments are performed, the combination of signs and symptoms necessary to classify a child as fulfilling the AIDS criteria should be identified during the same observation period and not by cumulating morbidity experience over time. A comparative evaluation of the original WHO paediatric AIDS clinical definition and of the modified version should be performed within existing cohort studies. Meanwhile, the use of the original WHO case definition should be recommended.
Table 1.
World Health Organization case definitions for paediatric AIDS* used for the Ghent classification of paediatric HIV infection, 1992.
| WHO clinical case definition for paediatric AIDS [29] |
| Major signs |
| Weight loss or failure to thrive |
| Chronic diarrhoea (> 1 month) |
| Prolonged fever (> 1 month) |
| Minor signs |
| Generalized lymphadenopathy |
| Oro-pharyngeal candidiasis |
| Repeated common infections |
| Persistent cough |
| Generalized dermatitis |
| Confirmed maternal HIV infection |
| Modified WHO clinical case definition for paediatric AIDS [8] |
| Major signs |
| Weight loss or failure to thrive |
| Chronic diarrhoea (> 1 month) |
| Prolonged fever (> 1 month) |
| Severe or repeated pneumonia |
| Minor signs |
| Generalized lymphadenopathy |
| Oro-pharyngeal candidiasis |
| Repeated common infections |
| Generalized pruritic dematitis |
| Confirmed maternal HIV infection |
With both definitions, paediatric AIDS is suspected in a child presenting with a least two major signs and two minor signs in the absence of known causes of immunosuppression.
HIV-related signs and symptoms in children born to HIV-seropositive mothers
It is often not possible to establish persistence of signs and symptoms in young infants. It is for this reason that a scoring system was developed in one study, to take into account the relative weight and the dynamics of the symptoms [9]. This scoring system needs to be evaluated against clinical AIDS definitions and new methods for early diagnosis of HIV infection before it is recommended for use in other research projects. Some signs and symptoms have been shown to be highly predictive of paediatric HIV infection. A list of HIV-related signs and symptoms is summarized in Table 2.
Table 2.
HIV-related signs and symptoms in children born to HIV-seropositive mothers used for the Ghent classification of paediatric HIV infection, 1992.
| Persistent diarrhoea (≥ 15 days) |
| Oral candidiasis (beyond the neonatal period) |
| Generalized lymphadenopathy (enlarged lymph nodes in at least two independent anatomic sites) |
| Failure to thrive (no weight gain for a period of 3 months or crossing two percentiles lines on the growth chart) |
| Chronic parotitis (> 1 month) |
| Herpes zoster infection (‘shingles’) |
| Recurrent pneumonia (two or more episodes) |
HIV-related deaths in children born to HIV-seropositive mothers
For the purpose of calculating the rate of HIV MTCT, it is important to establish whether a child who dies before 15 months is HIV-infected. Three definitions were proposed for children who died before their infection status could be determined by serology (Table 3).
Table 3.
Definitions for children born to HIV-seropositive mothers who died before infection status could be determined by serology. Ghent classification, 1992.
| Probable HIV-related death |
| Either AIDS* |
| or |
| At least one HIV-related sign/symptom** when last seen |
| and |
| Dying from severe infection† or persistent diarrhoea beyond the first 4 weeks of life‡ |
| Probable non-HIV-related death |
| No HIV-related sign/symptoms** when last seen |
| and |
| Dying from cause other than severe infection† or persistent diarrhoea after the first 4 weeks of life |
| Death with indeterminate relation to HIV infection |
| All deaths occurring within the first 4 weeks of life |
| or |
| Beyond this period, all the deaths not classified above |
See definition in Table 1.
See definition in Table 2.
It may be necessary to establish a list of severe paediatric infections for this purpose.
Children who died from persistent diarrhoea but do not present any other HIV-related sign/symptom should be considered as a death with indeterminate relation to HIV infection.
Laboratory assessment
A combination of HIV serum antibody enzyme-linked immunosorbent assay (ELISA) and Western blot (WB) tests was used in most cohort studies. A consensus was reached to assess WB according to the WHO criteria (positive WB if at least the gp41 and gp120/160 bands are reactive). More importantly, a negative WB should be defined by the complete absence of any band. Indeterminate WB consists of all other situations. Some questions were raised about the necessity of using a confirmation test (WB) for longitudinal follow-up of children born to HIV-seropositive mothers [33]. Simpler and cheaper methods, such as two different types of ELISA, have been used in some MTCT studies. Different diagnostic strategies should therefore be compared to WB on serum samples collected between 12 and 18 months of age.
There was a consensus to use 15 months of age as the cut-off point for antibody loss for estimating MTCT rates. However, in infants lost to follow-up before 15 months of age, a negative WB result obtained at or after 9 months could be considered as seroreversion in the absence of clinical AIDS. In the proposed definition, such infants are categorized as uninfected with HIV. The group recognized that a small percentage of HIV-infected infants transiently serorevert before again becoming HIV-seropositive [16,34]. In most studies, the windows of seronegativity have occurred between 3 and 8 months of age with a few exceptions. Omitting the small number of infants with longer windows should not have a significant impact on the overall estimation of the MTCT rate.
Various methods for the early diagnosis of HIV infection in infants born to HIV-seropositive mothers were discussed [viral culture, polymerase chain reaction, p24 antigen, in vitro antibody production, HIV-specific serum immunoglobulin A (IgA)] [35]. It was agreed not to integrate any of these criteria in the proposed methods of estimation of the MTCT rate of HIV at the present time. However, the recommendations made at the Sienna meeting held in January 1992 on the early diagnosis of HIV infection in infants, should be used by the Working Group in the future [36]. These new diagnostic tools, particularly HIV-specific IgA antibody tests [37], should first be evaluated in available stored serum samples from existing studies. The confirmation of the diagnostic value of specific IgA antibody tests on large numbers of serum samples collected at 6 and 9 months of age in children born to HIV-seropositive mothers and performed in a single laboratory facility was considered a research priority. It was felt that this result could be used to validate and revise the Ghent classification.
Measurement and comparison of transmission rates
General approach to the problem
Ideally, the transmission rate (TR) for MTCT of HIV should be computed as: TR = number of HIV-infected children/total number of children enrolled. Based on the experience of the workshop participants, it was decided that calculations should be made using all the available information for the first 15 months of follow-up. In studies that do not have serum samples available at 15 months of age, the 12-month cut-off should be used. There are problems in estimating both the numerator (how to define an HIV-infected child?) and the denominator of this rate (how should deaths before 15 months of age and losses to follow-up be considered?).
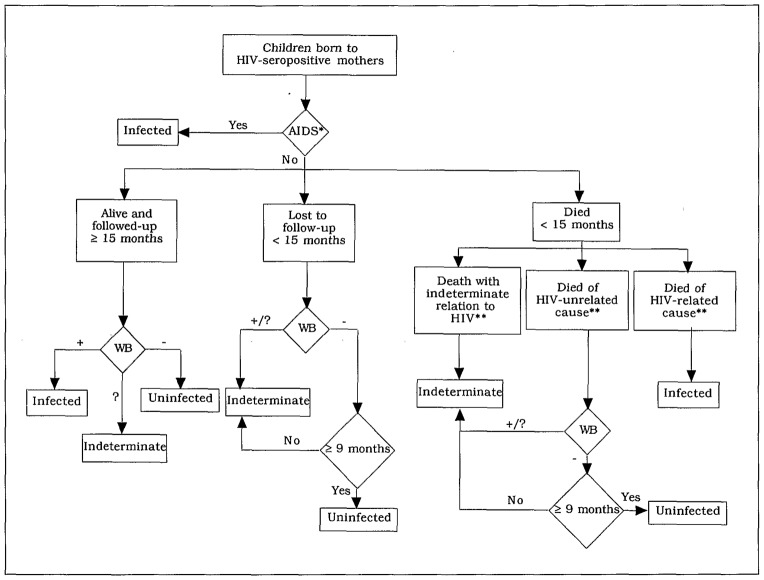
The definitions proposed in Tables 1 and 3 to classify a child as HIV-infected can be recommended. A classification of children according to their probable HIV infection status was developed and is presented in Table 4 and Fig. 2. This classification should be used to compare cohort studies and to minimize the number of children whose HIV infection status remains indeterminate. The risk of misclassification of HIV-infected children was recognized. In some studies, several children who had seroreverted were shown later to have detectable HIV antibodies again [16,34]. Before taking this into account in the present classification, detailed description of these sequential laboratory results, as well as further laboratory testing using new diagnostic tools, were felt to be necessary in those studies reporting this phenomenon frequently.
Table 4.
Ghent classification* of children born to HIV-seropositive mothers according to their probable HIV infection status, 1992.
| HIV-infected |
| HIV WB-antibody positive at 15 months |
| or |
| HIV-related death (see definition in Table 3) |
| or |
| AIDS (see definition in Table 1) |
| HIV-non-infected |
| HIV WB-serum-antibody-negative at 15 months |
| or |
| HIV WB-negative ≥ 9 months in a child lost to follow-up without AIDS |
| or |
| HIV WB negative ≥ 9 months in a child who died from probable non-HIV-related cause (see definition in Table 3) |
| Indeterminate HIV infection status |
| Death before 15 months with indeterminate relation to HIV infection (see definition in Table 3) |
| or |
| Child died of probable not HIV-related cause while WB-positive or indeterminate before 15 months or WB-negative < 9 months (when last seen) |
| or |
| Child lost to follow-up while WB-positive or indeterminate before 15 months or WB-negative < 9 months (when last seen) |
| or |
| Child with indeterminate WB and alive at 15 months |
This classification is designed to be used in the estimation of the rate of mother-to-child transmission of HIV and the comparisons between studies and not in the management of children born to HIV-seropositive mothers. The use of this classification must be restricted to a cohort of children born to HIV-seropositive mothers, all of whom were born more than 15 months before analysis. All children of the cohort should be accounted for using the above classification. In those studies where other methods of diagnosis such as viral culture are used, the results of these methods should be described if modifying the classification, but should be ignored for the purpose of comparisons. WB, Western blot.
Fig. 2.
Algorithm used to classify children born to HIV-seropositive mothers according to their probable HIV infection status. Ghent classification, 1992. *See definition in Table 1; **see definition in Table 3. WB, Western blot.
Methods for estimating the MTCT rate of HIV
Several methods have been used to estimate the MTCT rate, including: (1) the use of antibody tests results only; (2) the use of antibody test results combined with an estimate of excess mortality observed in children born to HIV-seropositive mothers; (3) the use of antibody test results combined with a clinical assessment of HIV infection status in children without definitive serology (either because they died or were lost to follow-up); (4) the use of other virological and immunological methods. The latter will not be considered further in order to allow a standardized methodology to be applied to many of the studies in developing countries without access to advanced virological testing. Also, quantitative antibody assays will not be considered [19] but all the antibody test results should be interpreted as negative, positive or indeterminate.
In method 1, clinical information is not considered. Each child is defined as infected (WB-positive after 15 months), uninfected (WB-negative after 9 months) or indeterminate (otherwise). The number of children in these three categories is denoted as n+, n− and n?, respectively. The total size of the cohort of children born to HIV-seropositive mothers is denoted as N. There are three possible ways to estimate the transmission rate (TR). The lower estimate assumes that all indeterminates are uninfected: TR = n+/N. The upper estimate assumes that all indeterminates are infected: TR = (n+) + (n?)/N. The intermediate estimate assumes that indeterminates provide no information on HIV infection status: TR = (n+)/(n+) + (n−). This first method will yield an unacceptably wide range between the lower and upper estimates if a study has a high background or HIV-specific infant mortality rate or a high rate of loss to follow-up. In these circumstances, the number of indeterminate children will be very high. Consequently, the use of method 1 is not recommended since most of the studies considered suffer from either or both of these problems.
Method 2 requires a comparison group of children born to HIV-seronegative mothers. It will be referred to as the indirect method. There is no need to make a clinical assessment of HIV infection with this approach. It addresses a weakness of method 1, namely that infected children of HIV-seropositive mothers, the exposed group, may be more likely to die before 15 months than uninfected children of HIV-seropositive mothers. To avoid having to determine whether the death was HIV-related, the comparison — unexposed — group is used to model the mortality experience of uninfected children of HIV-seropositive mothers. The indirect method is applicable to settings with a comparison group of at least the same size and similar socio-economic conditions as the exposed group. Let mO (m1) denote the probability of dying before 15 months for children born to HIV-seronegative (seropositive) mothers; these probabilities should be estimated using survival analysis methods, regarding loss to follow-up as a censoring event [27]. If 12 rather than 15 months is used to define definitive serology, then the probabilities of dying before 12 months should be calculated instead. The excess mortality is computed as: (m1 − mO). Let p denote the proportion of children who are HIV-antibody-positive at 15 months (or 12 months when appropriate). The standard way of estimating the TR by this formula has been [18]: TR = (m1 − mO) + p, with p estimating the proportion of HIV-antibody-positive children in the entire cohort, which includes children who died because of HIV infection. However, this effectively counts children who died as a consequence of HIV infection twice. An attempt was made to improve on this method by applying the prevalence of antibody carriage to the total number of children in the cohort minus the estimated number of children who died of causes related to HIV infection. Thus, the preceding formula should be modified as follows:
This indirect method does not resolve the problem that loss to follow-up may be related to HIV infection a status but lost to follow-up is taken into account in the computation of probabilities of dying in both groups. A key assumption for this method is that uninfected children of HIV-infected mothers have the same mortality experience as children of uninfected mothers. It is very likely that maternal HIV infection per se may have an adverse effect on the mortality of a child. Consequently, the excess mortality and thus the MTCT rate of HIV could be overestimated with the indirect method, although the extent of this bias is difficult to quantify. The 95% confidence interval (CI) of TR obtained with the indirect method can be calculated as: . The variance of the transmission rate, Var (TR), can be calculated as (details are available upon request):
SO and S1 are the probabilities of surviving in both groups computed by survival techniques, with their variance usually computed by Rothman’s formula [38]. SR is the survival ratio: SR = S1/SO = (1 − ml)/(1 − mO). Other information required is: n, the denominator upon which the rate of seropositivity (p) is based, i.e., the number of children born to HIV-seropositive mothers and surviving at 15 months of age.
Method 3 uses the classification of children born to HIV-seropositive mothers according to their probable HIV infection status (Table 4 and Fig. 2). Method 3 will be referred to as the direct method. The three formulas under method 1 can be applied, with n+ denoting HIV-infected children, n-denoting HIV-uninfected children and n? denoting children with indeterminate HIV infection status. The success of this method depends on the accuracy of clinical assessment. As most early deaths or early losses to follow-up will be classified as indeterminate HIV infection status, this method will yield disparate lower and upper estimates if there are substantial numbers of such children. The 95% CI of TR obtained with the direct method is easily calculated with the standard methods for proportions:
TR is the transmission rate, ranging between 0 and 1; D is the denominator used for its computation, N or (n+) + (n−) depending of the formula used to calculate TR.
Application of the methods of calculation of the transmission rate
The data collected in Kigali (Rwanda) between 1988 and 1990 during an ongoing MTCT study [12] were used to illustrate the use of the three methods previously described. In this cohort, 218 (N) children born to HIV-seropositive mothers and 218 children born to HIV-seronegative mothers were followed from birth. In the group of children born to HIV seropositive mothers, 27 were alive and HIV-antibody-positive at 15 months (n+). At that time, 136 were alive and HIV-antibody-negative (n−). Seven had been lost to follow-up between 9 and 15 months, at a time when they were already HIV-antibody-negative. The remaining 48 children could not be classified as HIV-antibody-positive or negative at 15 months because of early death in 31 or loss to follow-up in 17.
Using method 1, the minimum value was: TR = 27/218 = 12.4% (CI 8.0–16.8%). The maximum value was TR = (27 + 48)/218 = 34.4% (CI = 28.1–40.7%) and the intermediate value was TR = 27/(27 + 136) = 16.6% (CI = 10.9–22.3%). This shows clearly why the use of method 1 cannot be recommended in a context where the number of indeterminate children is high (48 children or 22% of the cohort).
To use the indirect method (method 2), the following information was obtained using Kaplan–Meier techniques: m1 = 0.1462, mO = 0.0416 and the excess of mortality, m1-mO = 0.1046. The number of children alive at 15 months of age was 174 (n1) in the cohort of children born to HIV-seropositive mothers and 201 (nO) in the comparison group. An estimate of TR can be obtained with the indirect method, using as the prevalence of antibody at 15 months (p), the intermediate value of TR obtained with method 1: p = 27/(27 + 136) = 0.1656. The estimate of TR is therefore TR = 25.7% (CI = 18.8–32.5%). The 95% CI of TR is obtained by computing Var (TR) with the following figures: n = 163, S1 = 0.8539, Var S1 = 0.00059, So = 0.9584 and Var So = 0.00018.
The use of Ghent classification (method 3) for the 218 children born to HIV-seropositive mothers in Kigali gave the following three groups: 46 HIV-infected children, 140 non-HIV-infected and 32 with indeterminate HIV infection status. For the definition of HIV-infected children, the 1989 revision of the WHO clinical definition of paediatric AIDS [8] rather than the 1986 Bangui definition was used. Of the 46 children classified as HIV-infected, 16 were AIDS cases regardless of their HIV serological status (seven of those subsequently died before 15 months of age); 23 were alive, followed up to 15 months and WB-positive at that time; finally, eight died of HIV-related causes before 15 months of age. For the uninfected group, 135 were negative at 15 months and five were lost to follow-up before but were WB-negative at or after 9 months. Thus, the use of the direct method gave the following three estimates of TR: a minimum estimate of 46/218=21.1% (CI = 15.7–26.5%), a maximum estimate of (46 + 32)/218 = 35.8% (CI = 29.4–42.1%) and an intermediate estimate of 46/(218–32) 24.7% (CI = 18.5–30.9%). It is worth noting that the intermediate estimate obtained with the direct method is similar to that obtained with the indirect method, with comparable confidence intervals. However, the range of possible values remains wide with both methods. The investigators chose to report the following range of values around their point estimate of 25.7% (upper and lower limits of the 95% CI obtained by the direct method, 18.8–32.5%) [12].
The direct method was also applied to the European Collaborative Study (ECS) dataset (N = 720 children born to HIV-seropositive mothers) using the 1987 Centers for Disease Control case definition of paediatric AIDS [39]. A cut-off point of 18 months was chosen for the interpretation of the positive WB results. The direct method yielded an intermediate estimate of TR of 12.2% (CI = 9.8–14.5%). This range of values includes the transmission rate of 14.4% reported by these investigators using more sophisticated clinical and laboratory criteria [25]. The indirect method could not be applied in this study, which did not have a comparison group.
Application of the direct and indirect methods to other African data sets gave preliminary results in the range of 24% for the Uganda study [14] to 32% for the Malawi study (data not shown), i.e., comparable to the Kigali figures and twice the ECS intermediate estimate. Table 5 summarizes the different estimates of the transmission rate obtained with three datasets. Looking at the intermediate estimates, one can see a substantial difference between the African studies and the European study which cannot be attributed to differences in methodology and sample size.
Table 5.
Range of possible values for the mother-to-child transmission rate (TR) of HIV-1 using two methods of calculation proposed by the Working Group on Mother-to-Child Transmission of HIV. Ghent, 1992. Kigali, Kampala and European Collaborative Study (ECS) datasets.
| Study | TR(%) | Minimum (s.d.*) | Intermediate (s.d.*) | Maximum (s.d.*) |
|---|---|---|---|---|
| Kigali | IM | – | 25.7% (3.51%) | – |
| DM | 21.1% (2.75%) | 24.7% (3.16%) | 35.8% (3.27%) | |
| Kampala | IM | – | 26.5% (2.51%) | – |
| DM | 18.7% (1.94%) | 24.2% (2.43%) | 41.5% (2.46%) | |
| ECS | DM | 10.0% (1.1%) | 12.2% (1.2%) | 28.3% (1.7%) |
IM, indirect method; DM, direct method (see text for definitions), s.d., standard deviation.
Proposed scheme for data analysis
For the purpose of comparing MTCT rates between studies, the direct method should be applied and results presented with three estimates, each with their 95% CI. For the purpose of internal validation of the measurement of the MTCT rate with several methods in the same dataset, the indirect method should also be applied if an adequate comparison group is available and results presented with a 95% CI. Both methods should be applied to those groups of children born at least 15 months before the date of analysis. This scheme for data analysis can also be applied to MTCT of HIV-2, which has been much less studied until now [40].
Determinants of mother-to-child transmission of HIV-1
Before maternal risk factors for HIV MTCT can be assessed, it is necessary to ensure that the overall estimate of MTCT rate for a particular cohort is reliable, as well as the case-by-case classification of children born to HIV-seropositive mothers according to their probable HIV infection status. For example, a high loss to follow-up could invalidate these results. Few studies in developing countries have included the determination of risk factors for MTCT as a specific objective [41]. Specifically designed studies with systematic and comprehensive data collection in pregnancy, at delivery and in the post-partum period are therefore a priority, with strict enrollment criteria and follow-up procedures designed to minimize loss to follow-up. Finally, the workshop participants recognized the difficulty in determining the exact role of each possible factor during data analysis — confounder, effect modifier, marker or true risk factor. Furthermore, the timing, route(s) and cellular mechanisms by which transmission occurs remained fundamental questions to be answered [42,43].
Factors that have been identified as possible risk factors for HIV MTCT include impaired maternal clinical and immunological status [9,11,25], HIV-seroconversion during pregnancy [26], shortened duration of pregnancy [9,18,44], choriamnionitis [9], vaginal delivery [21], prolonged and/or complicated labour [25] and breast-feeding [45]. Maternal age and parity do not appear to be associated with MTCT in most studies. In Rwanda [12], Kenya [16] and Malawi [17], sexually transmitted diseases were not associated with an increased risk of HIV MTCT. The role of other factors, such as viral characteristics, protective maternal antibodies and genetic factors, is not yet clear [41].
In future, special consideration should be given to the relative importance of each mechanism of transmission and to the study of MTCT of HIV infection through breast-milk [45,46], in particular. Only four studies have included both breast- and formula-fed infants [9,21,23,25]. Three showed an increased transmission rate in breast-fed infants [9,23,25]. However, the magnitude of the risk has not been described accurately and further studies are needed [45–48]. Their design should consider the nutritional and immunological benefits of breast-milk. Post-natal transmission of HIV through breast-mik in women who seroconverted after delivery has been demonstrated clearly [26] and highlights the importance of following-up HIV-seronegative mothers to observe maternal seroconversion and possible subsequent post-natal transmission of HIV.
Acknowledgments
The scientific secretariat acknowledges the help of Dr Davis Hom (Case Western Reserve University, Cleveland) and Paolo Miotti (Johns Hopkins School of Hygiene and Public Health, Baltimore) for providing updated data on their studies. Special thanks to Mr Daniel Commenges (INSERM U 330, Bordeaux) for his invaluable assistance in the development of the statistical aspects of the report. The authors acknowledge the advice and criticisms provided by Professor Neal Halsey (Johns Hopkins School of Hygiene and Public Health, Baltimore), Professor Roger Salamon (INSERM U 330, Bordeaux), Dr Lieve Fransen (EEC AIDS Task Force, Brussels) and Dr Benjamin Nkowane (WHO, Geneva) in their review of the manuscript. This workshop would not have taken place without the encouragement and the support provided by Mr L. Guerrato (Commission of European Communities, Brussels), Dr David Heymann (WHO Global Programme on AIDS, Geneva) and Dr James Sherry (UNICEF, New York).
The authors wish to acknowledge the secretariat assistance of Marijke Bontinck (EEC AIDS Task Force, Brussels), Marie-Pierre Martin (INSERM U 330, Bordeaux) and Dominique Nicolini (Institut du Cerveau, Bordeaux) in the organization of the workshop. Special thanks to Evelyne Bloch, Fara Rafidimanana and Dominique Touchard for their help in the preparation of the manuscript.
Appendix
The Working Group on Mother-to-Child Transmission of HIV comprises the following individuals
Scientific secretariat
François Dabis (France), David Dunn (United Kingdom), Lieve Fransen (EEC AIDS Task Force), Philippe Lepage (Rwanda), Philippe Msellati, coordinator (France), Marie-Louise Newell (United Kingdom), Benjamin Nkowane (WHO Global Programme on AIDS), Catherine Peckham (United Kingdom) and Philippe Van de Perre (Rwanda).
Investigators
Warren Andiman (USA), Ganapati Bhat (Zambia), Stéphane Blanche (France), Reginald Boulos (Haïti), Marc Bulterys (Rwanda), John Chiphangwi (Malawi), Pratibha Datta (Kenya), Joanne Embree (Canada), Carlo Giaquinto (Italy), Neal Halsey (USA), Deo-Gratias Hitimana (Rwanda), David Hom (Uganda), Etienne Karita (Rwanda), Marc Lallemant (Congo), Nsuami Malanda (Zaïre), Marie-Jeanne Mayaux (France), Charles Mitchell (USA), Paolo Miotti (Malawi), Francis Mmiro (Uganda), Samuel Nzingoula (Congo), Felix Omeñaca (Spain), Robert Ryder (Zaïre) and Nathan Shaffer (USA).
Statisticians
Daniel Commenges (INSERM U. 330, Bordeaux, France) and David Dunn (Institute of Child Health, London).
The following persons also participated in the Ghent meeting
Georgette Adjorlolo (Côte d’Ivoire), Jean-Paul Butzler (Belgium), Joan Casanova (EEC AIDS Task Force), Eric Delaporte (France), Joseph Fumbi (UNICEF), William Heyward (WHO Global Programme on AIDS), Normand Lapointe (Canada), Peter Piot (Belgium), Anna-Maria Stevens (Belgium), Marc Tardieu (France) and Marleen Temmerman (Kenya).
References
- 1.Chin J. Current and future dimensions of the HIV epidemic. Lancet. 1990;336:221–224. doi: 10.1016/0140-6736(90)91743-t. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Global Programme on AIDS. Current and Future Dimensions of the HIV-1/AIDS Pandemic: A Capsule Summary. Geneva: WHO; Jan, 1992. WHO/GPA/RES/SF1/92.1. [Google Scholar]
- 3.Quinn TC, Ruff A, Halsey N. Pediatric acquired immunodeficiency syndrome: special considerations for developing nations. Pediatr Infect Dis J. 1992;11:558–568. [PubMed] [Google Scholar]
- 4.Mok JQ, Giaquinto C, De Rossi A, Grosch-Wörner I, Ades AE, Peckham CS. Infants born to HIV-seropositive mothers. Preliminary findings from a multi-centre European study. Lancet. 1987;i:1164–1168. doi: 10.1016/s0140-6736(87)92142-8. [DOI] [PubMed] [Google Scholar]
- 5.Pizzo PA. Pediatric AIDS: problems within problems. J Infect Dis. 1990;161:316–325. doi: 10.1093/infdis/161.2.316. [DOI] [PubMed] [Google Scholar]
- 6.Oxtoby MJ. Perinatally acquired HIV infection. In: Pizzo PA, Wilfert CM, editors. Pediatric AIDS. The Challenge of HIV Infection in Infants, Children and Adolescents. Baltimore: Williams & Wilkins; 1991. pp. 3–21. [Google Scholar]
- 7.Boylan L, Stein ZA. The epidemiology of HIV infection in children and their mothers — vertical transmission. Epidemiol Rev. 1991;13:143–177. doi: 10.1093/oxfordjournals.epirev.a036067. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization Global Programme on AIDS. Report of the Meeting of the Technical Working Group on HIV/AIDS in Childhood; 27 February– 1 March 1989; Geneva: WHO; WHO/GPA/89.2AF. [Google Scholar]
- 9.Ryder RW, Nsa W, Hassig SE, et al. Perinatal transmission of the human immunodeficiency virus type 1 to infants of seropositive women in Zaïre. N Engl J Med. 1989;320:1637–1642. doi: 10.1056/NEJM198906223202501. [DOI] [PubMed] [Google Scholar]
- 10.Lallemant M, Lallemant-Le-Coeur S, Cheynier D, et al. Mother–child transmission of HIV-1 and infant survival in Brazzaville, Congo. AIDS. 1989;3:643–646. doi: 10.1097/00002030-198910000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Lepage P, Dabis F, Hitimana DG, et al. Perinatal transmission of HIV-1: lack of impact of maternal HIV infection on characteristics of livebirths and on neonatal mortality in Kigali, Rwanda. AIDS. 1991;5:295–300. [PubMed] [Google Scholar]
- 12.Lepage P, Van de Perre P, Msellati P, et al. Mother-to-child transmission of human immunodeficiency virus type 1 (HIV-1) and its determinants: a cohort study in Kigali, Rwanda. Am J Epidemiol. 1993;137:589–599. doi: 10.1093/oxfordjournals.aje.a116716. [DOI] [PubMed] [Google Scholar]
- 13.Bulterys M, Chao A, Farzadegan H, et al. Detection of HIV-1 in breast-milk, multiple sexual partners, and mother-to-child transmission of HIV-1: a cohort study. VIII International Conference on AIDS/III STD World Congress; Amsterdam. July 1992; abstract ThC1524. [Google Scholar]
- 14.Hom D, Guay L, Kenya-Mugisha N, et al. Natural history of HIV infection in Ugandan infants. VIII International Conference on AIDS/III STD World Congress; Amsterdam. July 1992; abstract WeC1058. [Google Scholar]
- 15.Hira SK, Kamanga J, Bhat GJ, et al. Perinatal transmission of HIV-1 in Zambia. BMJ. 1989;299:1250–1252. doi: 10.1136/bmj.299.6710.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datta P, Embree J, Ndinya-Achola JO, Kreiss J, Plummer FA. Perinatal transmission in Nairobi, Kenya: 5-year follow-up. VII International Conference on AIDS; Florence. June 1991; abstract MC3. [Google Scholar]
- 17.Liomba G, Miotti P, Canner J, et al. Mortality experience in children of HIV-1-infected mothers in Malawi. VIII International Conference on AIDS/III STD World Congress; Amsterdam. July 1992; abstract PoC4238. [Google Scholar]
- 18.Halsey NA, Boulos R, Holt E, et al. Transmission of HIV-1 infections from mothers to infants in Haiti. Impact of childhood mortality and malnutrition. JAMA. 1990;264:2088–2092. [PubMed] [Google Scholar]
- 19.Andiman WA, Simpson J, Olson B, Dember L, Silva TJ, Miller G. Rate of transmission of human immunodeficiency virus type 1 infection from mother to child and short-term outcome of neonatal infection. Results of a prospective cohort study. Am J Dis Child. 1990;144:758–766. doi: 10.1001/archpedi.1990.02150310026020. [DOI] [PubMed] [Google Scholar]
- 20.Scott GB, Hutto C, Makuch RW, et al. Survival in children with perinatally acquired immunodeficiency virus type 1 infection. N Engl J Med. 1989;321:1791–1796. doi: 10.1056/NEJM198912283212604. [DOI] [PubMed] [Google Scholar]
- 21.Hutto C, Parks WP, Lai S, et al. A hospital-based prospective study of perinatal infection with human immunodeficiency virus type 1. J Pediatr. 1991;118:347–353. doi: 10.1016/s0022-3476(05)82145-6. [DOI] [PubMed] [Google Scholar]
- 22.Parekh BS, Shaffer N, Chou-Pong P, et al. Lack of correlation between maternal antibodies to V3 loop peptides of gp120 and perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 1991;5:1179–1184. doi: 10.1097/00002030-199110000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Blanche S, Rouzioux C, Guihard Moscato ML, et al. A prospective study of infants born to women seropositive for human immunodeficiency virus type 1. N Engl J Med. 1989;320:1643–1648. doi: 10.1056/NEJM198906223202502. [DOI] [PubMed] [Google Scholar]
- 24.European Collaborative Study. Children bom to women with HIV-1 infection: natural history and risk of transmission. Lancet. 1991;337:253–260. [PubMed] [Google Scholar]
- 25.European Collaborative Study. Risk factors for the mother-to-child transmission of HIV-1. Lancet. 1992;339:1007–1012. doi: 10.1016/0140-6736(92)90534-a. [DOI] [PubMed] [Google Scholar]
- 26.Van de Perre P, Simonon A, Msellati P, et al. Postnatal transmission of human immunodeficiency virus type 1 from mother to infant. A prospective cohort study in Kigali, Rwanda. N Engl J Med. 1991;325:593–598. doi: 10.1056/NEJM199108293250901. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Non-parametric estimation from incomplete samples. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 28.Snow RW, Armstrong JRM, Forster D, et al. Childhood deaths in Africa: uses and limitations of verbal autopsies. Lancet. 1992;340:351–355. doi: 10.1016/0140-6736(92)91414-4. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Acquired immunodeficiency syndrome (AIDS): WHO/CDC case definition for AIDS. Wkly Epidemiol Rec. 1986;61:69–73. [Google Scholar]
- 30.Colebunders RI, Greenberg A, Nguyen-Dinh P, et al. Evaluation of a clinical case definition of AIDS in African children. AIDS. 1987;1:151–153. [PubMed] [Google Scholar]
- 31.Lepage P, Van de Perre P, Dabis F, et al. Evaluation and simplification of the World Health Organization clinical case definition for paediatric AIDS. AIDS. 1989;3:221–225. doi: 10.1097/00002030-198904000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Tindvebwa D, Friesen H, Naamara W, George R, Downing R, Tembo G. Development of a clinical case definition for paediatric AIDS in Uganda. VII International Conference on AIDS; Florence. June 1991; abstract WC3231. [Google Scholar]
- 33.European Collaborative Study. How necessary is Western blot in the follow-up of children of HIV-infected mothers? [Letter.] AIDS. 1992;6:1544–1545. [PubMed] [Google Scholar]
- 34.Lepage P, Van de Perre P, Simonon A, Msellati P, Hitimana DG, Dabis F. Transient seroreversion in children born to HIV-1 infected mothers. Pediatr Infect Dis J. 1992;11:892–894. [PubMed] [Google Scholar]
- 35.Husson RN, Comeau AM, Hoff R. Diagnosis of human immunodeficiency virus infection in infants and children. Pediatrics. 1990;86:1–10. [PubMed] [Google Scholar]
- 36.Report of a Consensus Workshop, Sienna (Italy), January 17–18 1992: Early diagnosis of HIV infection in infants. J Acquir Immune Defic Syndr. 1992;5:1169–1178. [PubMed] [Google Scholar]
- 37.Quinn TC, Kline RL, Halsey N, et al. Early diagnosis of perinatal HIV infection by detection of viral-specific IgA antibodies. JAMA. 1991;266:3439–3442. [PubMed] [Google Scholar]
- 38.Rothman KJ. Estimation of confidence limits for the cumulative probability of survival in life table analysis. J Chron Dis. 1978;31:557–560. doi: 10.1016/0021-9681(78)90043-7. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control. Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. MMWR. 1987;36(suppl 1S):3S–15S. [PubMed] [Google Scholar]
- 40.Sibailly TS, Adjorlolo G, Gayle HD, et al. Prospective study to compare HIV-1 and HIV-2 perinatal transmission in Abidjan. Côte d’Ivoire. VIII International Conference on AIDS/III STD World Congress; Amsterdam. July 1992; abstract WeC1065. [Google Scholar]
- 41.Maternal factors involved in mother-to-child transmission of HIV-1. J Acquir Immune Defic Syndr; Report of a Consensus Workshop; Sienna (Italy). January 17–18 1992; 1992. pp. 1019–1029. [PubMed] [Google Scholar]
- 42.Douglas GC, King BF. Maternal-fetal transmission of human immunodeficiency virus: a review of possible routes and cellular mechanisms of infection. Clin Infect Dis. 1992;15:678–691. doi: 10.1093/clind/15.4.678. [DOI] [PubMed] [Google Scholar]
- 43.Borkowsky W, Krasinski K. Perinatal human immunodeficiency virus infection: ruminations on mechanisms of transmission and methods of intervention. Pediatrics. 1992;90:133–136. [PubMed] [Google Scholar]
- 44.Temmerman M, Plummer FA, Mirza NB, et al. Infection with HIV as a risk factor for adverse obstetrical outcome. AIDS. 1990;4:1087–1093. doi: 10.1097/00002030-199011000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breast-feeding. Lancet. 1992;340:585–588. doi: 10.1016/0140-6736(92)92115-v. [DOI] [PubMed] [Google Scholar]
- 46.Van de Perre P, Lepage P, Homsy J, Dabis F. Mother-to-infant transmission of human immunodeficiency virus by breast-milk: presumed innocent or presumed guilty? Clin Infect Dis. 1992;15:502–507. doi: 10.1093/clind/15.3.502. [DOI] [PubMed] [Google Scholar]
- 47.Van de Perre P, Simonon A, Hitimana DG, et al. Infective and anti-infective properties of breast milk from human immunodeficiency virus type 1 infected mothers in relation to mother-to-child transmission. Lancet. 1993;341:914–918. doi: 10.1016/0140-6736(93)91210-d. [DOI] [PubMed] [Google Scholar]
- 48.World Health Organization Global Programme on AIDS. Consensus statement from the WHO/UNICEF consultation on HIV transmission and breast-feeding. WHO-GPA; 30 April–1 May 1992; Geneva: WHO; 1992. WHO/GPA/INF/92.1. [Google Scholar]