Abstract
Most of our knowledge regarding glioma cell biology comes from cell culture experiments. For many years the standards for glioma cell culture were the use of cell lines cultured in the presence of serum and 20% O2. However, in vivo, normoxia in many brain areas is in close to 3% O2. Hence, in cell culture, the experimental value referred as the norm is hyperoxic compared to any brain physiological value. Likewise, cells in vivo are not usually exposed to serum, and low-passaged gliomaneurosphere cultures maintained in serum-free medium is emerging as a new standard. A consequence of changing the experimental normoxic standard from 20% O2 to the more brain physiological value of 3% O2, is that a 3% O2 normoxic reference point enabled a more rigorous characterization of the level of regulation of genes by hypoxia. Among the glioma hypoxia-regulated genes characterized using this approach we found VE-cadherin that is required for blood vessel formation, and Filamin B a gene involved in endothelial cell motility. Both VE-Cadherin and Filamin B were found expressed in pseudopalisades, a glioblastoma pathognomonic structure made of hypoxic migrating cancer cells. These results provide additional clues on the role played by hypoxia in the acquisition of endothelial traits by glioma cells and on the functional links existing between pseudopalisades, hypoxia, and tumor progression.
Introduction
Since the establishment of the HeLa cell line [1], cancer cell culture has been linked with cancer research progress. Most of our current knowledge regarding cancer cell biology comes from data issued from cancer cell cultures. Even in vivo experiments, such as tumor xenograft, have extensively used cancer cells which have been amplified in culture in the presence of serum and under 20% O2. Possible drawbacks in the use of these growth conditions are linked to the facts that i) cells in vivo are not usually challenged by serum components [2], ii) 20% O2 is a far higher oxygen concentration than the physiological levels experienced by cells in vivo [3–6]. For example, normoxic pO2 for rat brain tissue is in the range of 19–40 mm Hg (≈2.6 – 5.6% O2) in the grey cortex, 6–16 mm Hg (≈0.8 – 2.2% O2) in the white matter, 11–16 mm Hg (≈1.5 – 2.2% O2) in the hypothalamus, and 20–33 mm Hg (≈2.8 – 4.6% O2) in the hippocampus [3].
The situation becomes increasingly complex for pathologic tissues such as brain tumors. It has long been known that most tumors outgrow their oxygen supply and/or have leaky vessels that are inefficient in oxygen delivery. Therefore, brain tumors, like other solid tumors, exhibit chronic or periodic hypoxic areas. These points are critical since tumor hypoxia has been associated with tumor propagation, malignant progression and resistance to therapy [4]. Hence characterizing the response of glioma cells to hypoxia is a highly relevant field of investigation. This in turn needs defining what a normoxia value is. If pO2 around 1% O2 or below are usually considered as hypoxic, several different values of pO2 ranging from 20% (cell culture “normoxia”) to 6%–1% (tissue normoxia) can be used to define experimental brain cell normoxia [5–8]. The problem of the choice of the standard conditions for cell culture is critical.
In this study, we used two different experimental cell models to determine the transcriptional response of glioma cells to hypoxia (0.3% O2) and normoxia conditions. The first one is the U87 glioma cell line (ATCC HTB-14) which has been established and routinely cultured in normoxia conditions (20% O2) in the presence of serum. This cell line has been considered as a standard glioma cell line for many years (more than 1000 references in PubMed using U87 and glioma as keywords). However, culturing glioma cells from freshly resected tumor as neurospheres and in serum-free medium, usually referred as glioma stem cell cultures, is now considered as a more reliable standard for investigating glioma cell biology [2]. Therefore experiments were also performed on low-passaged gliomaneurosphere cultures constantly maintained in serum-free medium and under physiological brain normoxia (3% O2)conditions [5].
This study aimed at the characterization of relevant hypoxia-inducible genes in glioma cells using new standard conditions of normoxia. The results obtained provide new insights of how hypoxia can induce the expression of endothelial genes in glioma cell culture and in pseudopalisades, a glioblastoma morphological hallmark that links vascular pathology, hypoxia, cancer cell migration and tumor vessel formation [9].
Material and Methods
The study was approved by the Biological Resource Center Ethics Review Board 38043 Hospital of Grenoble. Written consent was obtained from each patient or family.
Cell culture
Glioma cells used were the U87 MG glioma cell line (ATCC: HTB14) and two gliomaneurosphere cultures (Glio5 and Glio6) established from freshly dissociated surgical specimen as previously described [5]. U87 cells were grown as a monolayer in DMEM supplemented with 10% fetal calf serum under standard atmospheric conditions (20% O2). Glio5 and Glio6 cultures were maintained under neurospheres at 3% O2 in serum-free medium (DMEM/F12) supplemented with 0.5 N2, 0.5 B27, 30 ng/ml bFGF and 30 ng/ml EGF, and 0.0002% heparin. These cells have the capacity for self-renewal, express CD133 and are tumorigenic when orthotopically implanted in nude mice [5](Supplementary Material Fig. 1). For experiments with adherent U87 cells, flasks were incubated stationary for 4 hours after seeding to allow cell attachment, then, they were placed for 48 hours on a PS-3D Rotator (Grant-Bio) adjusted to 10 rotations per minute with a platform inclined angle of 7°. This gentle shaking avoided the formation of pO2 gradients observed when monolayer cells are cultured in a stagnant medium. Cells were at 50% confluency at the end of the experiment. Glio6 were grown as floating sphere in serum-free medium. To minimize oxygen gradients in spheroids, cells were seeded at low density so that the sphere size at the end of the experiment had a diameter of less than 100 μm. For hypoxic conditions, cell cultures were maintained in an InVivo2™ hypoxic workstation under a gas mixture of 0.3% O2. Cell cultures under gas mixture of 3% O2 were incubated in a Sanyo MCO-5M multi-gas incubator.
In vitro migration assay
For the in vitro down-regulation of FLNB, we used the validated FLNB-siRNASI02653175 from Qiagen (Qiagen, France) which targets the ACGCATTGACATCCAGATGAA FLNB sequence. Control siRNA was the Negative Control siRNA(cat. no. 1022076)provided by Qiagen. Transfection was performed using the Lipofectamine® RNAi MAX reagent (InVitroGen, France) according to the manufacturer’s instructions. For migration studies, Boyden’s chambers (Becton Dickinson Biosciences, France) with 8-μm pore size polyethylene terephthalate membrane were used according to the manufacturer’s instructions. Briefly, U87 glioma cells were transfected with FLNB siRNA or control siRNA at a final concentration of 10 nM. 72 hours later, transfected cells were harvested and resuspended in DMEM medium with 1% FCS. 2 × 104 were seeded onto the upper compartment of each chamber and placed into wells containing 700μl of DMEM medium with 10% FCS. The migration chambers were incubated 6h at 37°C in hypoxic conditions (0.3%O2). Following incubation, the inserts were fixed in 4% paraformaldehyde (PAF) and stained with Hoescht(Sigma Aldrich, France). Quantitation of migrating cells on the lower surface of each membrane was done by counting ten random fields under a fluorescence microscope. Each assay was performed in triplicate. The data from three independent experiments were pooled for statistical analysis.
Gene expression profiling
Total RNAs were extracted from cells with the MirVana isolation kit™ (Ambion, Applied Biosystems, Foster City, CA) and further controlled (Bio-Analyser, Agilent Technologies, Palo Alto, CA) for quality and concentration. 200 ng of total RNA were amplified with the GeneChip 3′IVT Express Kit (Affymetrix, Santa Clara, CA) and then hybridized on GeneChip® Human Genome U133 Plus 2.0 according to Affymetrix specifications. The expression values of the samples, reported in arbitrary units, were processed and normalized using RMA algorithm. A minimum of three independent analyses using independent cell cultures were performed for each cell line (Glio 6 and U 87) and for each condition of O2 (20%, 3%, 0.3%). The expression changes between two conditions of O2 were validated with the statistical t-test with a p ≤ 0.05 considered as significant. The regulated genes showing significant ratio (i.e., mRNA expression under the lower oxygen level divided by mRNA under the higher oxygen level) less or equal to 0.55 or more or equal to 1.80 were listed. All array data sets are available at GEO under accession number GSE32100 http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=dlkpvsogeqqgkly&acc=GSE32100
RT-qPCR
2 μg of total RNA were transcribed into cDNA using Promega Reverse Transcription reagents with random dN6 primers. PCR primers for each gene were designed using the Universal Probe Library Assay Design Center (https://www.roche-applied-science.com/sis/rtpcr/upl/ezhome.html) and sequences are given as supplementary table IV. Then real-time PCR were performed according to the SYBR Green methodology on an Mx3000™ apparatus (Stratagene, La Jolla, CA). The normalization of the results was realized with ACTB, whose expression is not affected by pO2 according to our Affymetrix data. Finally, the statistical validation of gene changes was checked with the statistical t-test.
Tissue samples and immunohistochemistry
FLNB immunohistochemistry
Two non-tumoral brain tissues and forteen glioblastomas were studied. Tissue samples were taken at surgery for pharmaco resistant epilepsy (control tissue) and for brain tumors respectively. For histological classification, tumor samples were fixed in formalin and the diagnoses were made on paraffin-embedded material using the current World Health Organization classification of tumors of the central nervous system criteria. FLNB immunostaining was carried out on formalin-fixed and paraffin-embedded tissue sections using the Ventana Benchmark Autostainer (Ventana Medical International Inc., Illkirch, France). 4 μm cut sections were boiled for 90 min in citrate buffer for heat-induced epitope retrieval. The primary antibody (rabbit polyclonal ref HPA004747, SIGMA, USA) was applied at a 1:200 dilution for 2 h. Slides incubation with normal rabbit IgG at the same concentration as the primary antibody were used as negative controls.
VE-Cadherin immunohistochemistry was performed on 10 another glioblastomas also diagnosed according the WHO classification. After deparaffinization, 4μm thick sections were boiled for 90 min in citrate buffer for heat-induced epitope retrieval. The primary antibody (rabbit polyclonal ref HPA030562, SIGMA, USA) was applied at room temperature at a dilution of 1:300. Detection was performed using HRP EnVision™ following the manufacturer protocol (DAKO, France).
Results
U87 cells: 20% vs 0.3% O2
In a first set of experiments, we determined the response of U87 glioma cells to hypoxia. The cell response to hypoxia is usually studied by shifting cell cultures from normoxia (20% O2) to less than 1% O2. Accordingly, our transcriptomic analyses were first performed using cell cultures maintained either under atmospheric oxygen or shifted to 0.3% O2 for 48 hours. Using the criteria for selection defined in the material and methods section (variation fold ≤0.55 or ≥1.80; p ≤ 0.05), 265 different genes were found deregulated by hypoxia in glioma U87 cells (218 up-regulated, 47 down-regulated; Supplementary Material Table I, and Fig. 1, set A). However, as previously discussed [5–8], the use of 20% O2 as a normoxic reference is debatable. This value is much higher than the oxygen values experienced by brain cells in vivo. Therefore, when the O2 level was decreased from 20% O2 to0.3% O2 (hypoxia) it is critical to distinguish which part of the observed variations in gene expression is due to hypoxia and which part is due to the withdrawal of experimentally-imposed hyperoxic conditions. To investigate this we analyzed the transcriptomic response of U87 cells when the cultures were shifted from culture normoxia (20% O2) to the median brain pO2 normoxia value (3% O2).
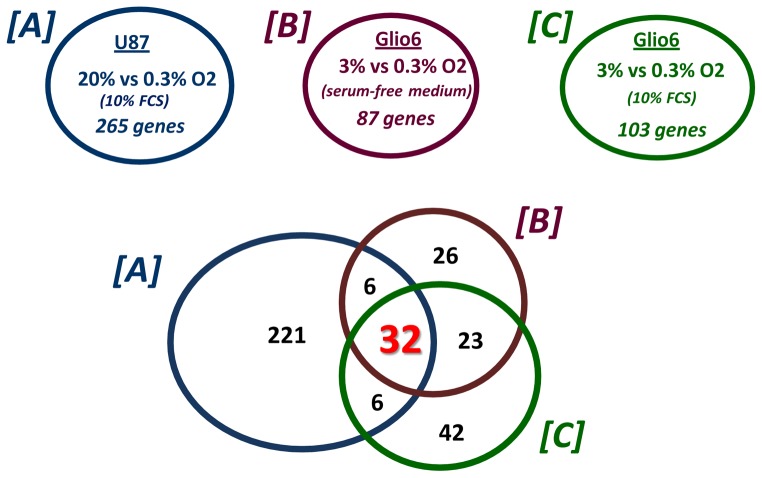
Fig. 1. The glioma cell core response to hypoxia.
Venn diagrams showing the number of genes differentially regulated in U87 cells cultured in 0.3% O2 when 20% O2 is used as the normoxic reference, and the number of genes differentially regulated in Glio6 cells cultured either in the absence or in presence of serum (FCS: Fetal calf serum) in 0.3% when 3% O2 is used as the normoxic reference (cf table II).
U87 cells: 20% vs 3% O2
At least 29 genes can be found differentially regulated (variation fold ≤ 0.55 and ≥ 1.80; p ≤ 0.05)when the U87 cells were shifted from 20% to 3% O2. All of these genes were identified as differentially expressed between 20% and 0.3% O2 (p ≤ 0.05)(Table I). However, the fold-variation of the expression levels of these genes was generally two times lower at 3% compared to 0.3% O2. These results demonstrated that at least several genes already described as hypoxia-regulated can be better considered as oxygen-sensitive, since their expression was significantly increased or decreased at 3% O2 which corresponds to the brain median normoxic level. Consequently, using 20% O2 as a normoxic standard leads to overestimate the variation fold of some genes, since significant changes in gene expression can already occur between 20% and 3% O2. This fact can be evidenced by calculating the ratio between the variation folds obtained at 20 vs 0.3% O2 and the variation folds between 20% and 3% O2 (Table I; calculated hypoxic variation fold). Consequently, in some cases, qualifying a gene as hypoxia-inducible may depend both on the normoxic reference and on the threshold values for significant variations used.
Table I.
Hypoxia regulated genes versus oxygen sensitive genes. A non-exhaustive list of genes regulated (≥ 1,80 or ≤ 0,55; p ≤ 0.05) in U87 cells both under hypoxia 20% O2 vs 0.3% O2, and following return to normoxia (20% O2 vs 3% O2).
| Probe Set ID | Gene Symbol | 3% vs 20% O2 ratio | 0.3% vs 20% O2 ratio | Calculated hypoxic variation fold (0.3 vs 20% O2)/(3% vs 20% O2) |
|---|---|---|---|---|
| 210095_s_at | IGFBP3 | 3.9 | 7.71 | 1.98 |
| 237335_at | ZP1 | 3.14 | 8.27 | 2.63 |
| 219888_at | SPAG4 | 2.94 | 7.21 | 2.45 |
| 236180_at | --- | 2.77 | 3.87 | 1.40 |
| 205832_at | CPA4 | 2.73 | 5.2 | 1.90 |
| 218149_s_at | ZNF395 | 2.55 | 5.6 | 2.20 |
| 232693_s_at | FBXO16 /// ZNF395 | 2.48 | 5.4 | 2.18 |
| 236480_at | --- | 2.36 | 4.17 | 1.77 |
| 225342_at | AK3L1 | 2.35 | 4.5 | 1.91 |
| 201250_s_at | SLC2A1 | 2.32 | 5.69 | 2.45 |
| 219410_at | TMEM45A | 2.29 | 5.03 | 2.20 |
| 221748_s_at | TNS1 | 2.29 | 4.27 | 1.86 |
| 202887_s_at | DDIT4 | 2.29 | 3.99 | 1.74 |
| 201010_s_at | TXNIP | 2.12 | 2.77 | 1.31 |
| 206686_at | PDK1 | 2.06 | 2.75 | 1.33 |
| 228843_at | --- | 2.06 | 4.33 | 2.10 |
| 202022_at | ALDOC | 2.06 | 4.53 | 2.20 |
| 215446_s_at | LOX | 2.03 | 4.26 | 2.10 |
| 236915_at | C4orf47 | 2.02 | 5.49 | 2.72 |
| 204508_s_at | CA12 | 1.95 | 3.49 | 1.79 |
| 230372_at | HAS2 | 1.95 | 3.32 | 1.70 |
| 226348_at | --- | 1.94 | 4.02 | 2.07 |
| 201849_at | BNIP3 | 1.88 | 2.69 | 1.43 |
| 218507_at | C7orf68 | 1.87 | 5.83 | 3.12 |
| 228499_at | PFKFB4 | 1.87 | 3.13 | 1.67 |
| 228483_s_at | TAF9B | 1.82 | 4.91 | 2.70 |
| 206693_at | IL7 | 0.49 | 0.31 | 0.63 |
| 225571_at | LIFR | 0.49 | 0.53 | 1.08 |
| 210662_at | KYNU | 0.45 | 0.35 | 0.78 |
Glio6 cells: 3% vs 0.3% O2
As an alternative to the use of the ATCC U87 glioma cell line, which is routinely cultured in serum-containing medium and under 20% O2, we used a glioma stem cell culture(Glio6) derived from a freshly resected tumor that has been established and consistently cultured as floating spheres in serum-free medium and under 3% O2 [5]. Defining 3% O2 as a normoxic reference point and serum-free medium for cancer cell culture has the advantage to preserve CD133 expression [5]. In addition, this new normoxic standard was a way to avoid the overestimation in gene expression variation folds evidenced above for the U87 cell line. For a transfer of 3% to 0.3% O2 for 48 hours, 87 genes were found differentially expressed in the Glio6 cells (same cut off parameters as described above; Supplementary Material Table II, and Fig. 1, set B).
Glio6 cells: 3% vs 0.3% O2 in the presence of serum
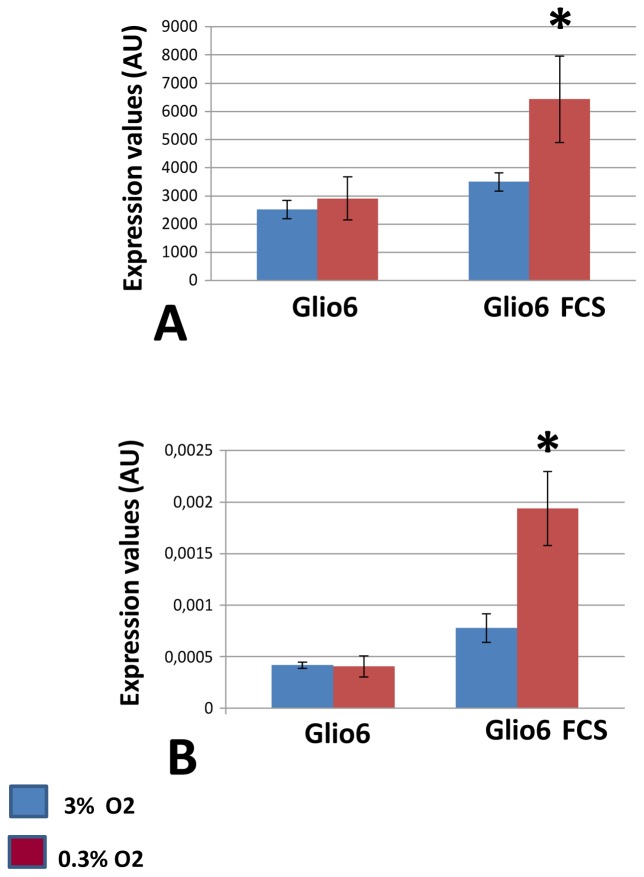
As stated above the Glio6 cells were cultured in serum-free medium, which is the standard culture condition for cancer stem cells. Accordingly, it is commonly assumed that in vivo glioma cells are not naturally exposed to serum and that culturing these cells in the presence of serum may be a source of artifacts[2]. However, intravascular thrombosis and microscopic tumoral hemorrhages are frequently observed in glioma [10,11]. This may cause some glioma cells to be exposed to serum during the course of the disease. A notable point in the context of this study is that since the presence of serum reflects the existence of abnormal or injured vessels, glioma cells challenged with serum components in vivo could also experience low oxygen tensions. Therefore, in the glioma microenvironment, serum and hypoxia can be two concomitant stimuli involved in glioma progression. Therefore, we challenged Glio6 cells to a hypoxic shift (3% to 0.3% O2) in the presence of 10% fetal calf serum for 48 hours, and identified 103 differentially expressed genes. (Fig. 1 set C; Supplementary Material Table III). Interestingly we identified IGFBP3 as a gene whose induction by hypoxia is serum dependent (Fig. 2 A, B)
Fig. 2. IGFBP3, a geneup-regulated by hypoxia in the presence of serum.
RNA were extracted from Glio6 cells cultured in normoxia (Glio6: 3% O2) or hypoxia (0.3% O2) for 48 hours. Glio6 cells were cultured either in the presence or absence of serum. RNA were then processed for transcriptomic analyses (abbreviation used FCS: fetal calf serum).
A:Expression levels are expressed as arbitrary units corresponding to the hybridization signal values on Affymetrix Gene chips. * p≤0.05.
B: RT-qPCR analysis was performed with primers specific for IGFBP3. Data were normalized to β-actin * p≤0.05
Identification of a non-exhaustive core set of hypoxia-regulated genes in glioma cells
A cross-analysis between the data obtained with U87 and Glio6 showed that the intersection of set A (U87 20% vs 0.3% O2, with serum), set B (Glio6 3% vs 0.3% O2, without serum), and set C (Glio6 3% vs 0.3% O2, with serum) gave a list of 32 genes regulated by hypoxia both in Glio6 cells cultured with or without serum and U87 cells cultured with serum (Fig. 1 and Table II). This set can be considered as a non-exhaustive core set of genes regulated by hypoxia in glioma cells. It includes numerous genes that are well known to be regulated by hypoxia (CA9, VEGF, ENO2… ). Three of these genes (HK2, SLC16A3 and TXNIP) are regulated independently by hypoxia and by serum. Five genes code for unidentified proteins or proteins with unknown function and present new potential areas of research. They correspond to the GenBank entries W57613.1, AL110176, AA543084(Affymetrix probe set:236180_at; 232451_at; 236480_at), to the hypothetical locus LOC100506211 (probe set: 230710_at) and to WDR5B (probe set: 235850).
Table II.
A non-exhaustive core set of genes regulated by hypoxia in glioma cells. List of the 32 differentially expressed genes associated with hypoxia (≥ 1,80 or ≤ 0,55 and p≤0.05) in both U87 cells (20% vs 0.3% O2) and Glio6 (3% vs 0.3% O2) in the presence and absence of serum.
| Probe Set ID | Gene Symbol | U87 20 %vs 0.3% O2 |
Glio6 3% vs 0.3% O2 |
Glio6 FCS 3 %vs 0.3% O2 |
|---|---|---|---|---|
| 203438_at | STC2 | 2.97 | 10.02 | 3.94 |
| 226452_at | PDK1 | 3.44 | 5.24 | 3.25 |
| 202912_at | ADM | 2.73 | 4.88 | 2.93 |
| 202934_at | HK2 | 2.19 | 4.37 | 4.18 |
| 202856_s_at | SLC16A3 | 2.50 | 4.33 | 2.49 |
| 228483_s_at | TAF9B | 4.91 | 3.94 | 3.44 |
| 202887_s_at | DDIT4 | 3.99 | 3.91 | 4.09 |
| 236480_at | --- | 4.16 | 3.89 | 2.71 |
| 235850_at | WDR5B | 4.77 | 3.86 | 4.15 |
| 236915_at | C4orf47 | 5.49 | 3.67 | 2.38 |
| 219410_at | TMEM45A | 5.02 | 3.44 | 2.84 |
| 210512_s_at | VEGFA | 1.80 | 3.20 | 2.25 |
| 230710_at | LOC100506211 | 3.76 | 2.99 | 2.31 |
| 220942_x_at | FAM162A | 2.89 | 2.95 | 2.54 |
| 201010_s_at | TXNIP | 2.77 | 2.88 | 3.46 |
| 225342_at | AK3L1 | 4.50 | 2.86 | 3.75 |
| 232693_s_at | FBXO16 /// ZNF395 | 5.40 | 2.68 | 3.06 |
| 205199_at | CA9 | 8.99 | 2.64 | 3.20 |
| 227068_at | PGK1 | 2.72 | 2.64 | 2.18 |
| 221123_x_at | ZNF395 | 5.05 | 2.62 | 3.08 |
| 201849_at | BNIP3 | 2.69 | 2.56 | 2.29 |
| 201250_s_at | SLC2A1 | 5.69 | 2.35 | 2.70 |
| 200632_s_at | NDRG1 | 4.62 | 2.20 | 1.96 |
| 202497_x_at | SLC2A3 | 1.92 | 2.19 | 2.01 |
| 232451_at | --- | 4.39 | 2.15 | 2.42 |
| 223046_at | EGLN1 | 2.44 | 2.08 | 1.95 |
| 203282_at | GBE1 | 1.98 | 2.02 | 1.81 |
| 201313_at | ENO2 | 2.35 | 1.99 | 2.29 |
| 221479_s_at | BNIP3L | 2.12 | 1.97 | 1.91 |
| 236180_at | --- | 3.87 | 1.96 | 2.50 |
| 203710_at | ITPR1 | 2.29 | 1.93 | 1.96 |
| 225750_at | ERO1L | 2.61 | 1.84 | 1.92 |
Identification of novel hypoxia-inducible genes associated to the vascular endothelial phenotype conversion of glioma cells
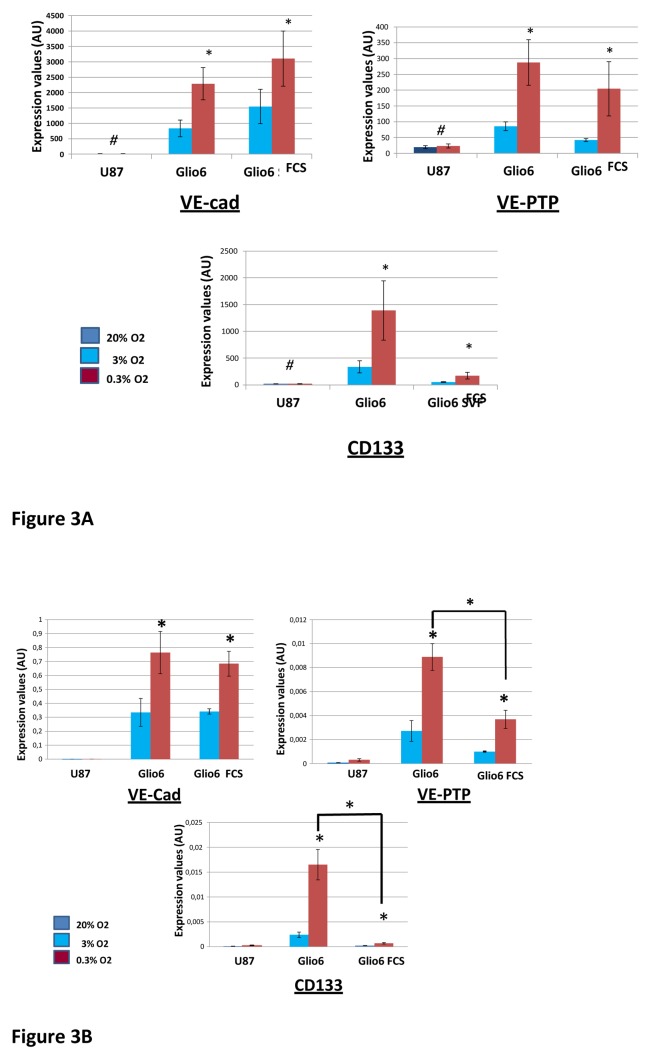
Recent data point to the differentiation of glioblastoma cells into vascular endothelial cells as a crucial process for tumor survival [12–16]. Hypoxia is suggested to be involved in tumor stem cell differentiation into endothelial cells [12]. Importantly, the U87 cell line, although tumorigenic, is unable to generate endothelial phenotypes[13]. Therefore, it can be expected that some genes critical for the acquisition of endothelial traits should be expressed in Glio6 cells and not in U87 cells. Therefore, we looked after genes that were up-regulated by hypoxia in Glio6 cells, but whose expression was not or poorly detectable in U87 cells even under hypoxia. We found only three genes fulfilling these criteria(Fig. 3A). These genes code for the Vascular Endothelial Cadherin (VE-Cad alias CDH5 or CD144), the Vascular Endothelial Protein Tyrosine Phosphatase(VE-PTP or PTPRB), and CD133 (Fig. 3A). VE-cad and VE-PTP are vascular endothelial cell markers. They are necessary for endothelial cell-cell junction formation. CD133 which is usually considered as a cancer stem cell marker, is also expressed by vascular endothelial progenitor cells. RT-qPCR analyses performed for CD133, VE-Cad, VE-PTP on Glio6 and on Glio 5 (another low-passaged glioma cell culture) confirmed the data obtained by hybridization to the Affymetrix chips (Fig. 3B and Supplementary Material: Fig. 2). A notable finding of RT-qPCR analyses was that the levels of induction by hypoxia of CD133 and VE-PTP were significantly lower in the presence of serum (Fig. 3B).
Fig. 3. VE-cad, VE-PTP and CD133, three genes up-regulated by hypoxia in Glio6 cells, but whose expression is poorly detectable in U87 cells even under hypoxia.
RNAs were extracted from cells cultured in normoxia (U87: 20% O2; Glio6: 3% O2) or hypoxia (0.3% O2) for 48 hours. Glio6 cells were cultured either in the presence or absence of serum. RNAs were then processed for transcriptomic analyses.
A: Expression levels are expressed as arbitrary units corresponding to the hybridization signal values obtained with Affymetrix arrays. * p ≤0.05; ** p≤ 0.01; # values below background.
B:RT-qPCR analysis was performed with primers specific for VE-cad, VE-PTP, CD133. Expression levels were normalized relative to the expression level of β-actin. Values are calculated by 2−(Ctgene of interest − Ctbeta actin) * p≤ 0.05; ** p≤0.01.
We then looked after the genes regulated by hypoxia in Glio6 that are constitutively expressed in U87 cells. Using our cut-off values, we found filamin B(flnb) as a gene up-regulated by hypoxia in Glio 6 in the presence and absence of serum and which was expressed at high levels in U87 even under 20% O2 (Fig. 4A). RT-qPCR analyses performed for flnb on Glio6 and Glio5 confirmed the up-regulation of flnB by hypoxia and its expression at high levels in U87 cells (Fig 4B). However, it is noticeable that the Affymetrix array signal values obtained with U87 cells for flnb in hypoxia indicated a 1.3 fold (p<0.05) increase in comparison to the expression level under normoxia. Nevertheless, this ratio was lower than the threshold of significance (≥1.80) we defined for screening our transcriptomic data.
Fig. 4. Filamin B: Regulation of FLNB expression by hypoxia in cultured glioma cells and effect of FLNB siRNA on cell migration.
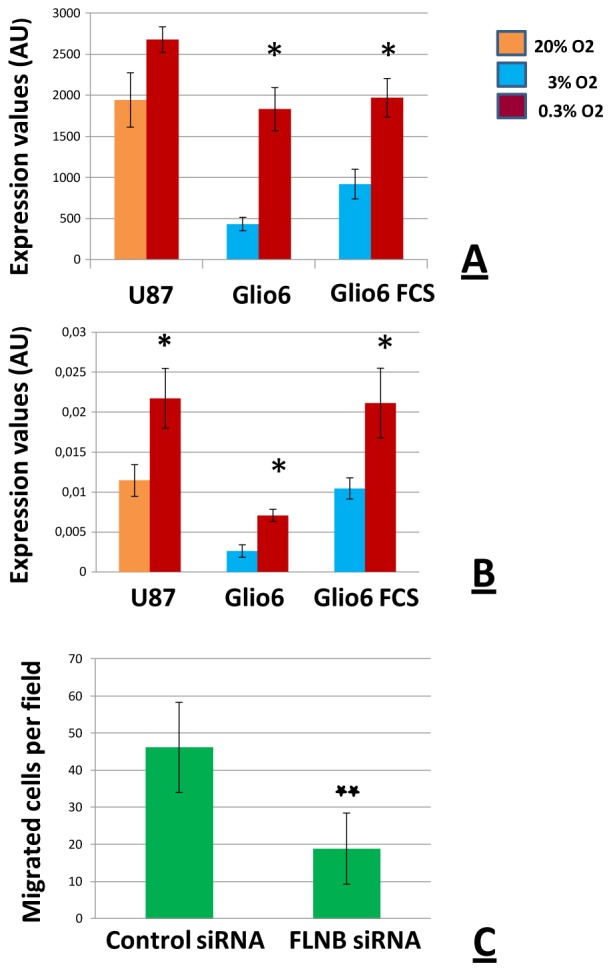
Trancriptomic analyses were performed on RNAs extracted from cells cultured in normoxia (U87: 20% O2; Glio6: 3% O2) or hypoxia (0.3% O2) for 48 hours. Glio6 cells were cultured in the presence or absence of serum.
A:RNAs were processed for analyses by hybridization on Affymetrix chips. Expression levels of FLNB gene are expressed as arbitrary units corresponding to the hybridization signal values. Hybridization signals below 60 are considered as non detected; * p≤0.05.
B: RT-qPCR analysis was performed with primers specific for FLNB. Expression levels of the FLNB gene were normalized relative to the expression level of β-actin. Values are calculated by 2−(CtFLNB − Ctbeta catin);* p≤0.05.
C: FLNB modulates glioma cell migration in vitro. Filamin Bsi RNA significantly reduces glioma cell migration in Boyden’s chamber assays; ** p ≤0.01 (n = 3).
Detection of VE-Cadherin and Filamin B in pseudopalisading cells and functional role of Filamin B inglioma cell migration
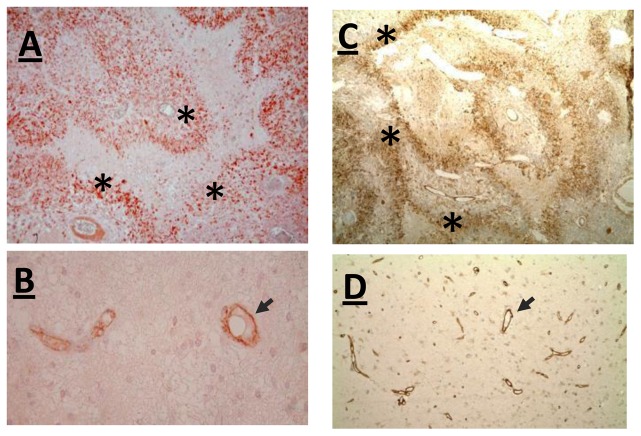
In endothelial cells VE-Cad is involved in several functions including cell-cell adhesion, intracellular signaling and transcriptional regulation [17]. These multiple functions prompted us to investigate the distribution of VE-Cad in patient samples. In addition to endothelial cells of brain blood capillaries (Fig. 5B), an intense VE-Cad immunostaining was detected in pseudopalisading cells which correspond to hypoxic tumor cells surrounding necrotic foci (Fig. 5A). For FLNB similar results were found (Fig. 5C, D). As FLNB is known to be involved in endothelial cell motility [18], and since pseudopalisades are made by actively migrating tumor cells moving away from necrotic hypoxic foci [9,19,20], we investigated the possible function of FLNB in glioma cell motility by transfecting cells with siRNA against Filamin B. We observed a decreased migration of glioma cells treated with ansiRNA-FLNB compared to cells transfected with control si-RNA (Fig. 4C).
Fig. 5. VE-Cadherin and FLNB are expressed in cancer pseudopalisading cells.
An intense VE-Cadherin (A, B) and FLNB (C, D) staining was detected in pseudopalisading guarland-like structure (*) (A, C), and in blood capillaries (black arrow) (B, D) (Magnification folds: A = 100X, C= 40X, B= 160X et D=80X).
Discussion
In tumor tissues such as brain cancer, hypoxia is commonly viewed as a driving force in glioma progression. Therefore, identifying the gene expression modifications of glioma cells in response to hypoxia is a relevant field of investigation. However, in this kind of experiments, the value used to define the experimental normoxia is critical. At the organ level, brain normoxia is highly heterogeneous and varies depending on the anatomical structures from 0.8% O2 to 6% O2[3]. Consequently, in normal brain, oxygen is a micro-environmental variable just as, for example, the extra-cellular matrix. This point is critical not only for cell culture conditions but also for glioma cell plasticity. Since glioma cells are highly invasive, they will experience a wide range of pO2 not only in the tumor mass but also when they invade brain parenchyma. To what extend this physiological brain heteroxia influences tumor progression is a point that remains to be investigated.
Our data provide additional clues on the consequences of using the 20% atmospheric oxygen level as a normoxic standard in experiments dedicated to study the glioma cell response to hypoxia. This overestimates the gene expression variation fold for genes which are already regulated between 20% and 3% O2. This finding is consistent with the observation that the binding activity of the transcription factor HIF that plays a central role in oxygen homeostasis [21] exponentially increases in cell cultures between 20% and 0.5% O2 [22]. One non exclusive mechanism for this fine regulation occurring between 20% and 3% O2 could involve HIFs posttranslational modifications. For example hydroxylation of HIF by two different kinds of hydroxylases (PHDs and FIH) with different affinities for oxygen is a possible way to achieve the goal of regulating both HIF stability (PHD) and transcriptional activity (FIH) in the tissue normoxic range (6%–1% O2) [23,24]. Our analysis was restricted on the transcriptomic response at only one time point (48 hours) and provides a non-exhaustive list of genes regulated between 20% and 3% O2 in the U87 glioma cell line. Further studies investigating the consequences of a 20% to 3% O2 shift at shorter or longer time points on the transcriptome and on the phosphorylation status of proteins are necessary for a better understanding of the consequences of culturing glioma cells at 20% O2. The epigenetic status of glioma cells is also probably modified by long time culture at 20% O2. According to that is the finding that the long term in vitro expansion of human glioblastoma cells at 20% O2 instead of 3% O2 irreversibly alters in vivo aggressiveness and AC133 expression [25].
The use of a physiological normoxic conditions at 3% O2 for brain-derived cell cultures is a mean to circumvent the problem raised by hyperoxic cell culture conditions. Not only it provides a pO2 relevant to the median brain normoxic value, but it also allows a more rigorous characterization of the effects of hypoxia on gene regulation. We found a set of 32 genes that are commonly regulated under 0.3% either in U87 (20 vs 0.3% O2) and Glio6 cells (3% vs 0.3% O2, with and without serum) (Fig. 1). This set of genes can be considered as a non exhaustive core set of genes regulated by hypoxia in glioma cells. An analysis of this list of genes in GEO profile with the keyword hypoxia (http://www.ncbi.nlm.nih.gov/geoprofiles) showed that all these genes have previously been described as hypoxia-regulated either in glioma cells or in other experimental models. Hence, these results validate our experimental approach and analyses. Accordingly, when these 32 significantly differentially expressed genes were uploaded into DAVID for functional annotation clustering, overexpressed GO groups were “glucose metabolic process”, “response to hypoxia” and “response to organic substance”.
hypoxia-induced expression of vascular endothelial genes in glioma cells
Recent data have provided evidence that in glioblastoma, a set of endothelial cells in the tumor vessels can derive from the tumor cells themselves [12,13,16]. In comparison to glioma stem cell cultures established from freshly dissociated surgical specimen, the U87 cell line is unable to generate endothelial phenotypes [13]. Hypoxia is suggested to be involved in tumor cell differentiation into endothelial cells [12]. Therefore, it can be expected that some genes critical for the acquisition of endothelial phenotype are expressed in Glio6 cells and regulated by hypoxia, but poorly expressed in U87 cells either at 20% or 0.3% O2. We found three genes regulated in common by hypoxia in the presence and absence of serum in Glio6 which are not detected as expressed in our transcriptomic analyses in U87 cells (Fig 3). They code for the vascular endothelial cadherin (VE-Cad or CDH5), the vascular endothelial tyrosine phosphatase (PTPRB) and CD133. According to their names VE-Cad (CDH5) and VE-PTP (PTPRB) are vascular endothelial cells proteins. VE-Cad is an endothelial cell-cell adhesion protein that plays critical roles in endothelial cell biology. It is required for maturation, extension and remodeling of vessels [17]. In addition to these properties, VE-cadherin is a signaling molecule interacting with β-catenin and modulating activity of growth factor receptors such as VEGF-R2 and TGFβ receptors [17]. Our finding that VE-Cad is expressed in pseudopalisading cells (Fig. 5), which are known to be in a hypoxic microenvironment, suggests several possible functions for this protein both in the acquisition of endothelial-like features by cancer cells and in cancer progression. The expression of the second identified gene, VE-PTP, has been until now restricted to endothelial cells[17]. It is a phosphatase associated with VE-Cad which enhances VE-Cad mediated cell-cell adhesion [17]. Like VE-Cad, VE-PTP is required for blood vessel development [26,27]. VE-PTP interacts with VE-cadherin to facilitate endothelial cell contacts [28]. Silencing of VE-PTP expression in endothelial cells reduces the adhesive function of VE-Cad and enhances cell proliferation through activation of Tie-2 [29]. It also regulates the spreading and migration of endothelial cells during angiogenesis [30]. Hence, our data provide additional clues on how hypoxia could be associated with the acquisition of an endothelial phenotype by cancer cells and in the formation of mosaic vessels. Regarding CD133, it is usually considered by neuro-oncologists as a brain tumour stem cell marker through its epitope AC133. However, CD133 is also a marker of endothelial progenitor cell[31]. The CD133+ brain cancer stem-cell is a subpopulation of cancer cells with endothelial progenitor features [16]. The fact that CD133 is expressed in the glioblastoma cell population which differentiates into endothelial cells and that this process is crucial for tumor survival [13] is consistent with the use of CD133 as a cancer stem cell marker. Our finding that serum down-regulates the hypoxia-induced CD133 expression could provide an explanation for the existence of CD133-cancer cells. Indeed, a consequence of intravascular thrombosis and microscopic tumoral hemorrhages which are frequently observed in glioma [10,11]is the presence of serum in a hypoxic tumor micro-environment. Thus, this presence of serum could decrease CD133 gene expression. We also found that serum participates to the up-regulation of IGFBP3 by hypoxia (Fig. 2). Therefore, our data provides clues for a synergic role of serum and hypoxia in cancer progression since IGFBP3 promotes TGFβ-mediated cell mesenchymal transition [32]. In this regard it is noteworthy that TGFβ is increased in the plasma of patients with glioblastoma [33]. This led to suggest the existence of an additional model of glioma progression involving a process named endothelial to mesenchymal progression[34].
On the other hand, the use of low passaged glioma cells maintained in serum-free medium under 3% O2 allowed us to characterize FLNB as a new hypoxia-inducible gene. This gene was found hypoxia-inducible in Glio6 and Glio 5, and constitutively highly expressed in U87cells (Fig 4, and Supplementary Material: Fig. 2). Like VE-Cad, FLNB is required for the development of vascular network[35]. Immunodetection of FLNB in tumor samples (Fig. 5), revealed for the first time its overexpression at the level of pseudopalisading cells. Pseudopalisading cells are considered as glioma cells migrating away from hypoxic/anoxic foci [19]. FLNB is known to regulate the initiation of cell migration and plays also a key role in endothelial cell motility and vascular development [18,30,36]. Accordingly we found that FLNB knock-down decreases glioma cell migration in vitro (Fig. 4C). This suggests that FLNB could participate to pseudopalisading cell migration in vivo. The evaluation of the potential of FLNB as a possible therapeutic target in the control of glioma cell invasion from hypoxic/perinecrotic pseudoplisading areas warrants further studies.
In conclusion, we have shown the interest of determining 3% O2 as the normoxic point value for quantifying the transcriptomic response of glioma cells to hypoxia. Using this normoxic value we have been able to characterize a new set of hypoxia-inducible genes which include VE-Cad and FLNB. In addition, we demonstrated the localization of the corresponding proteins in pseudopalisading structures. This provides further evidence to consider pseudopalisade not only as a cellular architecture useful for glioblastoma diagnosis but also as a functional driver for glioblastoma tumor progression linking hypoxia, cancer cell migration and tumor vessel formation [9].
Supplementary Material
Acknowledgments
This work was supported by INSERM and the Ligue Nationale contre le Cancer (National, Rhône-Alpes, Isère and Puy de Dôme sections). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Drs Ashraf, Chabardes, Gay, Hoffman, Seigneuret and Selek for providing tumor samples.
funding: Academic funding (INSERM, University), and the Liguecontre le Cancer.
Footnotes
Conflict of interest: None
References
- 1.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J Exp Med. 1953;97:695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 4.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 5.Platet N, Liu SY, Atifi ME, et al. Influence of oxygen tension on CD133 phenotype in human glioma cell cultures. Cancer Lett. 2007;258:286–290. doi: 10.1016/j.canlet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Csete M. Oxygen in the cultivation of stem cells. Ann N Y Acad Sci. 2005;1049:1–8. doi: 10.1196/annals.1334.001. [DOI] [PubMed] [Google Scholar]
- 7.Wion D, Dematteis M, Nissou MF, et al. Oxygen tension and cancer-cell culture: half a century of artifacts? Med Sci (Paris) 2008;24:1093–1095. doi: 10.1051/medsci/200824121093. [DOI] [PubMed] [Google Scholar]
- 8.Wion D, Christen T, Barbier EL, Coles JA. PO(2) matters in stem cell culture. Cell Stem Cell. 2009;5:242–243. doi: 10.1016/j.stem.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Rong Y, Durden DL, Van Meir EG, et al. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65:529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Brat DJ, Van Meir EG. Vaso-occlusive and prothrombotic mechanisms associated with tumor hypoxia, necrosis, and accelerated growth in glioblastoma. Lab Invest. 2004;84:397–405. doi: 10.1038/labinvest.3700070. [DOI] [PubMed] [Google Scholar]
- 11.Kondziolka D, Bernstein M, Resch L, et al. Significance of hemorrhage into brain tumors: clinicopathological study. J Neurosurg. 1987;67:852–857. doi: 10.3171/jns.1987.67.6.0852. [DOI] [PubMed] [Google Scholar]
- 12.Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci USA. 2011;108:4274–4280. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 14.Sajithlal GB, McGuire TF, Lu J, Beer-Stolz D, et al. Endothelial-like cells derived directly from human tumor xenografts. Int J Cancer. 2010;127:2268–2278. doi: 10.1002/ijc.25251. [DOI] [PubMed] [Google Scholar]
- 15.Shaifer CA, Huang J, Lin PC. Glioblastoma cells incorporate into tumor vasculature and contribute to vascular radio resistance. Int J Cancer. 2010;127:2063–2075. doi: 10.1002/ijc.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 17.Harris ES, Nelson WJ. VE-cadherin: at the front, center, and sides of endothelial cell organization and function. Curr Opin Cell Biol. 2010;22:651–658. doi: 10.1016/j.ceb.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Del Valle-Perez B, Martinez VG, Lacasa-Salavert C, et al. Filamin B plays a key role in vascular endothelial growth factor-induced endothelial cell motility through its interaction with Rac-1 and Vav-2. J Biol Chem. 2010;285:10748–10760. doi: 10.1074/jbc.M109.062984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brat DJ, Castellano-Sanchez AA, Hunter SB, et al. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- 20.Wippold FJ, 2nd, Lammle M, Anatelli F, et al. Neuropathology for the neuroradiologist: palisades and pseudopalisades. AJNR Am J Neuroradiol. 2006;27:2037–2041. [PMC free article] [PubMed] [Google Scholar]
- 21.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol. 1996;271:C1172–1180. doi: 10.1152/ajpcell.1996.271.4.C1172. [DOI] [PubMed] [Google Scholar]
- 23.Dayan F, Roux D, Brahimi-Horn MC, Pouyssegur J, et al. The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1alpha. Cancer Res. 2006;66:3688–3698. doi: 10.1158/0008-5472.CAN-05-4564. [DOI] [PubMed] [Google Scholar]
- 24.Dayan F, Monticelli M, Pouyssegur J, Pecou E. Gene regulation in response to graded hypoxia: the non-redundant roles of the oxygen sensors PHD and FIH in the HIF pathway. J Theor Biol. 2009;259:304–316. doi: 10.1016/j.jtbi.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Bourseau-Guilmain E, Lemaire L, Griveau A, Hervouet E, et al. In vitro expansion of human glioblastoma cells at non-physiological oxygen tension irreversibly alters subsequent in vivo aggressiveness and AC133 expression. Int J Oncol. 2012;40:1220–1229. doi: 10.3892/ijo.2011.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominguez MG, Hughes VC, Pan L, et al. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc Natl Acad Sci USA. 2007;104:3243–3248. doi: 10.1073/pnas.0611510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumer S, Keller L, Holtmann A, et al. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107:4754–4762. doi: 10.1182/blood-2006-01-0141. [DOI] [PubMed] [Google Scholar]
- 28.Nawroth R, Poell G, Ranft A, et al. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winderlich M, Keller L, Cagna G, et al. VE-PTP controls blood vessel development by balancing Tie-2 activity. J Cell Biol. 2009;185:657–671. doi: 10.1083/jcb.200811159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori M, Murata Y, Kotani T, et al. Promotion of cell spreading and migration by vascular endothelial-protein tyrosine phosphatase (VE-PTP) in cooperation with integrins. J Cell Physiol. 2010;224:195–204. doi: 10.1002/jcp.22122. [DOI] [PubMed] [Google Scholar]
- 31.Quirici N, Soligo D, Caneva L, Servida F, et al. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115:186–194. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- 32.Natsuizaka M, Ohashi S, Wong GS, et al. Insulin-like growth factor-binding protein-3 promotes transforming growth factor-{beta}1-mediated epithelial-to- mesenchymal transition and motility in transformed human esophageal cells. Carcinogenesis. 2010;31:1344–1353. doi: 10.1093/carcin/bgq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider T, Sailer M, Ansorge S, et al. Increased concentrations of transforming growth factor beta1 and beta2 in the plasma of patients with glioblastoma. J Neurooncol. 2006;79:61–65. doi: 10.1007/s11060-005-9116-7. [DOI] [PubMed] [Google Scholar]
- 34.Selek L, Dhobb M, van der Sanden B, Berger F, Wion D. Existence of tumor-derived endothelial cells suggests an additional role for endothelial-to- mesenchymal transition in tumor progression. Int J Cancer. 2011;128:1502–1503. doi: 10.1002/ijc.25446. [DOI] [PubMed] [Google Scholar]
- 35.Zhou X, Tian F, Sandzen J, et al. Filamin B deficiency in mice results in skeletal malformations and impaired microvascular development. Proc Natl Acad Sci USA. 2007;104:3919–3924. doi: 10.1073/pnas.0608360104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldassarre M, Razinia Z, Burande CF, et al. Filamins regulate cell spreading and initiation of cell migration. PLoS One. 2009;4:e7830. doi: 10.1371/journal.pone.0007830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.