Abstract:
To prevent thrombotic or bleeding events in patients receiving a total artificial heart (TAH), agents have been used to avoid adverse events. The purpose of this article is to outline the adoption and results of a multi-targeted antithrombotic clinical procedure guideline (CPG) for TAH patients. Based on literature review of TAH anticoagulation and multiple case series, a CPG was designed to prescribe the use of multiple pharmacological agents. Total blood loss, Thromboelastograph® (TEG), and platelet light-transmission aggregometry (LTA) measurements were conducted on 13 TAH patients during the first 2 weeks of support in our institution. Target values and actual medians for postimplant days 1, 3, 7, and 14 were calculated for kaolinheparinase TEG, kaolin TEG, LTA, and estimated blood loss. Protocol guidelines were followed and anticoagulation management reduced bleeding and prevented thrombus formation as well as thromboembolic events in TAH patients postimplantation. The patients in this study were susceptible to a variety of possible complications such as mechanical device issues, thrombotic events, infection, and bleeding. Among them all it was clear that patients were at most risk for bleeding, particularly on postoperative days 1 through 3. However, bleeding was reduced into postoperative days 3 and 7, indicating that acceptable hemostasis was achieved with the anticoagulation protocol. The multidisciplinary, multi-targeted anticoagulation clinical procedure guideline was successful to maintain adequate antithrombotic therapy for TAH patients.
Keywords: multi-targeted antithrombotic therapy, total artificial heart, antithrombosis, aggregometry, thromboelastography
Although some pharmacological and surgical treatments have been used to ameliorate congestive heart failure, ventricular assist devices such as the SynCardia CardioWest Total Artificial Heart (TAH; Syncardia Systems Inc., Tucson, AZ), have proven to be a more beneficial route to bridge patients to transplantation (1,2). Clinical research has supported TAH use as an optimal bridge to transplantation with a 70% survival rate (1–3). Furthermore, the TAH improves both hemodynamic and clinical stability in addition to allowing patients to achieve end-organ recovery and become eligible for cardiac transplantation among many other benefits (3).
However, research also shows that detrimental events such as thromboembolic or sepsis complications can decrease the success rate in TAH patients and at times, lead to death (4,5). Therefore, effective anticoagulation for TAH patients is necessary. In 1933, Szefner et al. were among the first to report the benefits and success of anticoagulation for ventricular assist devices by using multicomponent coagulation monitoring in addition to anticoagulation therapy (6,7).
Thereafter, institutions began to model their protocol and reported clinical data that reflected the findings of Szefner (4,5,8). Acknowledging the importance of anti-coagulation management, we have chosen to use a multi-targeted antithrombotic (MTA) approach for TAH patients. Our targets consist of achieving antiplatelet therapy, developing moderate delays of the intrinsic and extrinsic pathways, and inhibition of clotting factors resulting from vitamin K synthesis. We built our procedure guideline on the reviews and publications of Ensor, Copeland, Nolan, Pavie and their colleagues (2,7,9,10). The purpose of this observational case series is to outline the adoption and results of a MTA therapy clinical procedure guideline (CPG) for TAH patients.
ANTITHROMBOTIC PROTOCOL
The MTA approach that was originally designed by La Pitié institution and later adopted by the Texas Heart Hospital was also applied to 13 TAH patients between the dates of April 2013 to February 2015, with minor changes (6–8,10). On review of Minnesota patient research authorizations, all 13 patients gave permission to use their medical records for research. Patient demographics show that most were within the range of 33–63 years of age, 12 were male while only 1 was female, and they ranged from 75 to 112 kg (Table 1).
Table 1.
TAH patient demographics.
| Patient | Age | Gender | Weight (kg) | Pre-ECMO | VAD | Infection | Transplant (days) | Alive |
|---|---|---|---|---|---|---|---|---|
| 1 | 58 | Male | 75 | No | No | No | Yes (110) | No |
| 2 | 63 | Male | 76 | Yes | No | No | Yes (218) | Yes |
| 3 | 33 | Female | 92 | Yes | No | No | Yes (18) | Yes |
| 4 | 42 | Male | 75 | No | HM II | No | Yes (51) | Yes |
| 5 | 62 | Male | 77 | No | No | No | No | Yes |
| 6 | 60 | Male | 100 | No | No | No | Transferred | Yes |
| 7 | 52 | Male | 82 | No | cRVAD | No | Yes (129) | Yes |
| 8 | 55 | Male | 85 | No | No | No | Yes (98) | Yes |
| 9 | 61 | Male | 87 | No | HM II | Yes | Wait List (185) | No |
| 10 | 49 | Male | 81 | Yes | No | No | Yes (276) | Yes |
| 11 | 58 | Male | 99 | Yes | Impella | No | Yes (159) | Yes |
| 12 | 57 | Male | 112 | No | HM II | No | Wait list (208) | Yes |
| 13 | 61 | Male | 91 | No | No | Yes | Wait list (63) | Yes |
| Percent Yes | 31% | 38% | 15% | 62% | 77% |
Pre-ECMO Yes denotes ECMO support before TAH implant. VAD is ventricular support device use before implantation. Infection Yes is septicemia. HM II is Thoratec Heart Mate II (Thoratec Corporation, Pleasanton, CA). cRVAD is centrifugal pump right ventricular assist and Impella (Abiomed, Danvers, MA) is AbioMed left ventricular assist. Numbers in parenthesis denote number of days of support.
Our current protocol allows for close monitoring of bleeding and anticoagulation management at our institution. This is accomplished with the use of the Thromboelastograph (TEG) and light transmission aggregometry (LTA). The TEG was chosen because of its ability to sensitively analyze the entire clot generation and dissolving process. LTA was chosen because of its ability to assess platelet function. Our current MTA regimen, which models previous research findings involves Heparin (Hospira Inc., Lake Forest, IL), Coumadin (Sodium Warfarin, Bristol Myers Squibb, New York, NY), Aspirin (Cayman Chemical, Ann Arbor, MI), Dipyridamole (Persantine, LGM Pharma, Nashville, TN), and Pentoxifylline (Trental, LGM Pharma).
The transfusion threshold for packed red blood cell (PRBC) administration was <6.3 g/dL. On postimplant days (POD) 1–3 and when the chest tube estimated blood loss is <30 mL/h over a period of 4 hours, heparin is administered intravenously at a rate of 2–5 U/kg/h. The results are analyzed using the TEG and its parameters: coagulation index (CI), reaction (R) time, alpha angle, and maximum amplitude (MA). For the kaolin TEG (KTEG), the therapeutic goal for the CI is −5 to −10 and −3 to 0 for the kaolin-heparinase TEG (HTEG). In addition, labs are monitored for activated partial thromboplastin time (aPTT), platelet count, and fibrinogen levels. In cases in which a patient is positive for heparin-induced thrombocytopenia, .005–.02 mg/kg/h of bivalirudin is given and adjusted until the HTEG is within a normocoagulable range (R time of 3–8 minutes, kinetics of the clot [K] time of 1–3 minutes, CI of −3 to 3). Warfarin is then started within POD's 1–7 once bleeding has ceased. The international normalized ratio (INR) is monitored and the normocoagulable ranges for the HTEG is an R time of 3–8 minutes, and a CI of −3 to 0. A mild hypocoagulable state is also acceptable when the INR is within 2.5–3.5. Warfarin therapy is adjusted as needed according to the INR results. In addition, the TEG alpha angle range of 47–74° is used to determine whether the antiplatelet function and fibrinogen activity are normal.
Aspirin (ASA) therapy can be started on POD 1, if the platelet count is >50 × 109/L. The goal for this regimen is 81–325 mg per 250 × 109/L platelets. Monitoring during ASA administration is performed using LTA to guide therapy. The therapeutic goal for the agonists arachadonic acid (AA), adenosine-5-diphosphate (ADP), and epinephrine (EPI) is 20–40% maximum aggregation. As for LTA agonist collagen, greater than 50% aggregation is desired. ASA administration is adjusted according to the LTA results. Persantine, the next drug in line for the MTA protocol is started at 75–100 mg every 8 hours per 100 × 109/L platelets. It may also be started when the platelets are > 50 × 109/L. If the patient is found to have 300 × 109/L platelets, 300 mg of Persantine is given every 8 hours. Monitoring for this mode of therapy is done using LTA as a guide to reach therapeutic goals of 20–40% for the agonists. The last drug in the MTA protocol is pentoxifylline and it is started at 200–800 mg every 8 hours with the goal of reducing hemolysis and blood viscosity. This drug can be used upon the patient's arrival to the intensive care unit if signs of hemolysis are present.
RESULTS
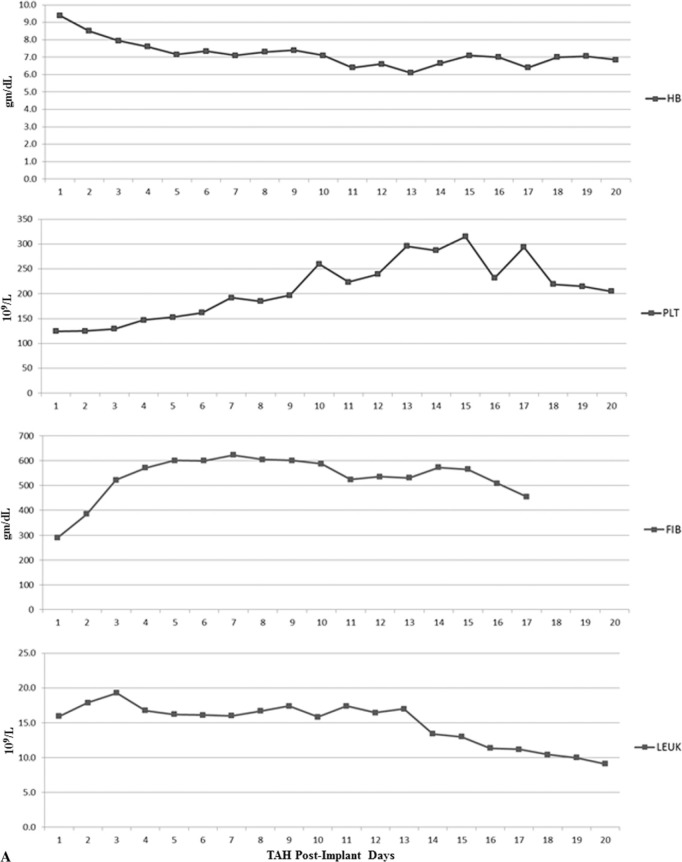
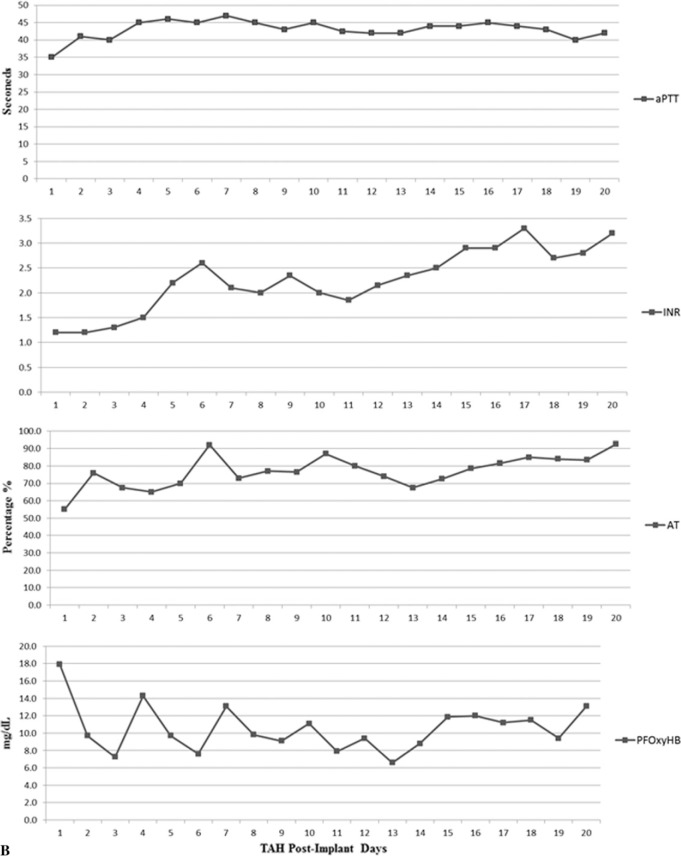
Ventricular assist device (VAD) specialists, critical care specialists, perfusionists, pharmacists, and physicians composed the multidisciplinary team that was able to successfully follow the protocol for the 13 TAH patients. Coagulation lab results were collected and median values were calculated (Figure 1A). Figure 1A demonstrates the acute phase response that is evident through the elevation in the leukocyte count, platelet count, and fibrinogen levels (5). Median results for hemoglobin concentration (g/dL) trend downward and platelet levels increase with time as antiplatelet and anticoagulation management is initiated. Leukocyte count is high during the first days after TAH implantation and fibrinogen levels increase. Medians were also calculated for INR, a PTT, antithrombin (AT) concentration, and plasma free hemoglobin (PFOxyHB) as well as monitored and analyzed postoperatively (Figure 1B). Noting that aPTT results were above the normal range of 28–38 seconds, it is clear that anticoagulation was achieved. INR results show an upward trend after POD 3, indicating anticoagulation and that a mild hypocoagulable state was also maintained.
Figure 1.
(A) Laboratory values of hemoglobin concentration (HB, gm/dL), platelet count (PLT, 109/L), fibrinogen concentration (FIB, gm/dL), and leukocyte count (LEUK, 109/L). (B) Laboratory values of aPTT (sec), INR, AT (%), and PFOxyHB (mg/dL).
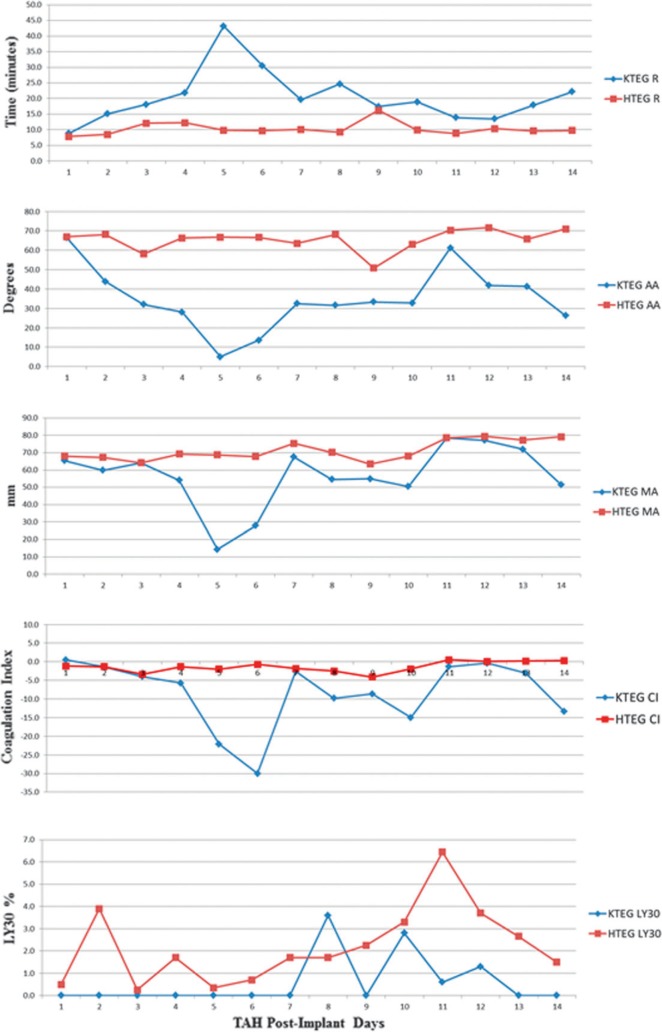
Resulting median laboratory values for postoperative days 1, 3, 7, and 14 were collected (Figure 2). The target for kaolin-heparinase TEG (HTEG) R time is 3–8 minutes and the median (interquartile range: Q1, Q3) results were 8.2 (7.6, 11.8), 10.9 (7.9, 14.9), 10.8 (6.8, 24.4), and 11.3 (8.5, 18.9). As for the HTEG MA data, the target is 54–72 mm and the results were 67.2 (59.7, 68.7), 66.3 (61.2, 73.8), 73.5 (49.1, 78.0), and 77.3 (54.9, 81.9). The target for KTEG R time is 15–18 minutes and the results were 8.9 (6.4, 11.8), 27.1 (10.7, 148), 19.7 (10.5, 64.9), and 22.2 (11.6, 43.9). More than half the patients had a tendency toward hypercoagulability consistent with an acute phase response despite the goal to keep the TEG CI lower than −3.0.
Figure 2.
Thromboelastography values. Red lines connect median values for heparinase + kaolin TEG values and blue lines connect KTEG values. Points are median values for 5–13 patient results per total artificial heart implant day.
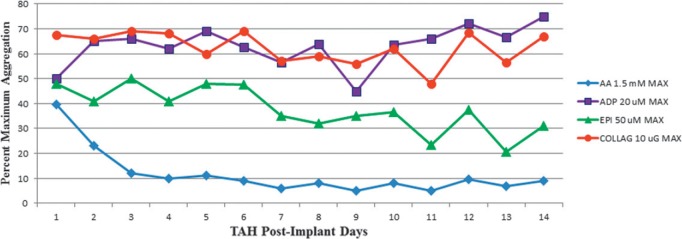
Using LTA with 1.5 mM AA and 20 mM ADP agonists, the target was to achieve <50% aggregation. LTA AA results for postoperative days 3 and 7 were 12 (4, 31) and 9 (4, 32) and for LTA ADP, the results were 62% (36%, 70%) and 63% (54%, 69%) aggregation (Figure 3). Normal LTA by 10 μG of collagen was preserved with the procedure guideline, achieving >50% aggregation. The estimated blood loss was also recorded with the target <12mL/kg/day (Figure 4). The results were 13.3 (10.1, 28.1), 6.1 (2.9, 9.3), 1.4 (0, 3.8), and 0 (0, 2.0) for postoperative days 1, 3, 7 and 10 (Table 2). The median time to chest tube drainage stopping and tube removal was 9.5 (4.5, 12) days. Heterologous blood donor exposures that patients had within the first 2 weeks postimplantation were noted (Table 3).
Figure 3.
LTA platelet maximum aggregation by agonists. COLLAG is collagen. Therapeutic target agonist inhibition is less than 50%.
Figure 4.
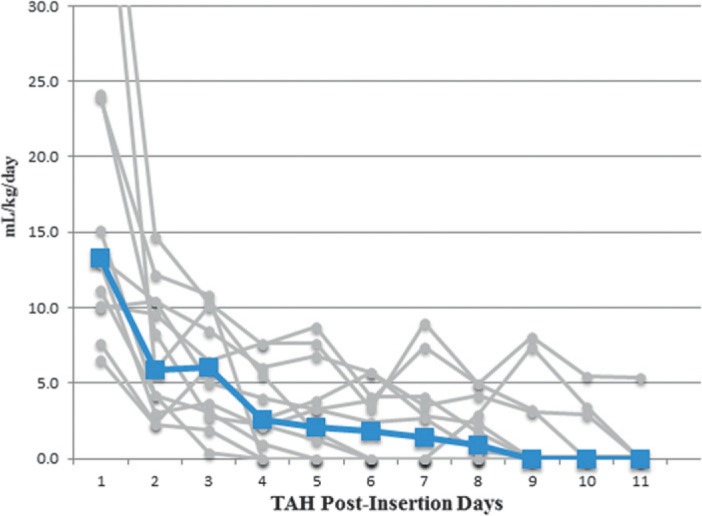
Chest tube drainage estimated blood loss. Blood loss is mL/kg/day. The median blood loss is denoted by the blue markers and line.
Table 2.
Daily estimated blood loss by chest tubes mL/kg/day.
| Postimplant Day | Median | Q1 | Q3 | p Value |
|---|---|---|---|---|
| 1 | 13.3 | 10.1 | 28.1 | <.001 |
| 3 | 6.1 | 2.9 | 9.3 | NS |
| 7 | 1.4 | 0 | 3.8 | NS |
| 10 | 0 | 0 | 2.0 | NS |
The median estimated blood loss in the chest tube drainage system of 13 patients. Median blood loss was significantly higher on postimplant day 1 than days 3, 7, or 10. There was no significant difference between postimplant days 3, 7, and 10. Q1 is the 25th percentile and Q3 is the 75th percentile.
NS, nonsignificant.
Table 3.
Heterologous blood donor exposures in first two implant weeks.
| Product | Median | Q1 | Q3 | Mean | Standard Deviation |
|---|---|---|---|---|---|
| PRBC 330 mL/U | 3 | 1 | 8 | 5.2 | 5.9 |
| Platelet Packs | 2 | 2 | 2.5 | 2.1 | .9 |
| FFP 250 mL/U | 4 | 1.5 | 6.2 | 4.6 | 4.1 |
| Cryo 60 mL/U | 0 | 0 | 0 | .2 | .6 |
| Albumin gm/kg | 1.2 | .6 | 2.4 | 1.6 | 1.2 |
| Cell Process mL/kg | 5.1 | 2.8 | 6.5 | 5.0 | 3.3 |
The heterologous blood product donor exposures descriptive statistics for 13 TAH patients during the first 2 weeks of implantation. Platelets are pooled apheresed units about 250 mL. Cryoprecipitate is about 60–70 mL per unit. Albumin is reported as gm/kg administered. Cell process is the volume of autologous red cells per kg transfused on the first implant day. FFP, fresh frozen plasma.
DISCUSSION
We report our results from the first 14 days of TAH support for 13 patients employing a MTA clinical procedure guideline. The guideline was designed to achieve three main goals: antiplatelet therapy, moderate delays of the intrinsic and extrinsic pathways, and inhibition of clotting factors resulting from vitamin K synthesis in the liver. Reaching each of these targets allowed for adequate anticoagulation and a lower rate of adverse thromboembolic events for TAH patients.
Of the 13 patients, none were observed to have a stroke in the first 14 days. This is similar to findings reported by La Pitié, and the University of Arizona. Together these two institutions only had one incidence of stroke within 25 patient years (2). The patients in this case series were of New York Heart Association class IV and in need of a heart transplant. Some patients relied on other forms of support before receiving a TAH (Table 1). Of these patients, 31% were supported by extracorporeal membrane oxygenation (ECMO), whereas 38% were supported by a VAD, and 62% of the patients were successfully transplanted while 77% survived post transplantation. The average amount of time for TAH support for bridge to transplantation was 129 days. Overall, our data correlate with international findings published by CardioWest, La Pitié, and Copeland, which show 70%, 74%, and 79% of patients who successfully made it to transplantation (2,8).
Based on the clinical data it is clear that patients were most susceptible to bleeding, specifically on postoperative days 1 through 3 (Figure 4). However, drainage was reduced in a timely manner, allowing for adequate adherence to the protocol and administration of the regimens as directed. Of PODs 1, 3, 7 and 10, the most significant day for blood loss/chest tube drainage was POD 1. This correlates with the administration of blood products that were used on the first postoperative day (Table 3). Most importantly, there was no device thrombus or thromboembolic events observed in the first weeks of TAH support. Before using the MTA CPG, a ring of fibrin at the site of the quick connect would be found upon removal of some of the TAH devices. Since implementation of the protocol, the transplant surgical team has not found fibrin during inspection of the explanted TAH devices. Findings from the national experience for CardioWest patients report infection in 12%, we report a similar rate of 15% of infection for our patients.
With transplant candidates the fewer blood donor exposures, the better. Exposures within the first 2 weeks of postimplantation were analyzed. Patients received several forms of blood products, particularly in the perioperative period. Mostly albumin and PRBCs were given throughout the postoperative days if needed. Results from Table 3 allow us to conclude that bleeding in patients was adequately maintained, and therefore, reducing the need for blood products as PODs continued.
Heparin administration was managed according to aPTT target values (Table 4). The median (Q1, Q3) reveals the POD heparin start results of 1 (1, 2) days. The heparin bridge to warfarin or days until heparin was discontinued was 13 (9.25, 17.5) days. Furthermore, coagulation lab results demonstrate that anticoagulation was achieved while we maintained a mild hypocoagulable state acceptable to our guidelines (Figure 1A). The upward trend in platelet count and fibrinogen concentration and the downward trend in hemoglobin within the first PODs are explained by the acute phase response (5). It is also important to note that about 40% of patients received AT supplement within their first week after TAH implantation (Figure 1B). Median values for PFOxyHB indicate that hemoglobin levels were within normal range. The preservation of AT suggests that thrombus was not generated and demonstrates the effectiveness of the MTA CPG.
Table 4.
Heparin administration.
| TAH Postimplant Days (U/kg/h) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
| 5 | 8 | 10 | 9 | 8 | ||||||||||||||||
| 7 | 5 | 13 | 19 | 21 | 20 | |||||||||||||||
| 5 | 9 | 11 | 13 | 14 | 14 | 14 | 15 | 14 | 13 | |||||||||||
| 10 | 11 | 11 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | 12 | ||||||||||
| 6 | 6 | 6 | 4 | 4 | 4 | 4 | 6 | 8 | 8 | 8 | 8 | 8 | ||||||||
| 5 | 3 | 11 | 12 | 13 | 14 | 15 | 16 | 16 | ||||||||||||
| 4 | 5 | 7 | 8 | 9 | 9 | 9 | 10 | 11 | ||||||||||||
| 4 | 7 | 8 | 7 | 7 | 6 | 5 | 5 | 6 | 7 | 7 | 7 | 7 | 7 | 10 | 10 | 10 | 10 | 10 | 10 | 8 |
| 4 | 4 | 5 | 9 | 11 | 11 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | |||
| 5 | 7 | 5 | 10 | 13 | 10 | 10 | 12 | 12 | 12 | 13 | 16 | 16 | 16 | 16 | 16 | |||||
| 10 | 14 | 16 | 17 | 17 | 16 | 16 | 15 | 15 | 15 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
The daily average heparin administration rate for each patient for the first 3 weeks of implantation. Heparin administration was titrated by aPTT, and the difference between HTEG and KTEG R times and alpha angles. Some patients required heparin on later implant days when warfarin therapy was not well controlled. Median start POD 1 (1, 2). Median POD discontinued 13 (9.25, 17.5).
Median values for KTEG and HTEG were collected (Figure 2). The KTEG R time on postoperative day one was below the goal of >15–18 minutes but once heparin was started, KTEG R times surpassed the target. HTEG MA values which were <54–72 mm demonstrated that most patients produced clots weaker than normal. Lastly, aspirin achieved the LTA AA target while the doses of dipyridamole were not effective to reduce LTA by ADP.
TEG “is most useful in patients on heparin” (5). Through TEG monitoring, we were able to adequately dose TAH patients with heparin in the range of 5–10 U/kg/h Copeland et al. (5) had similar findings and stated “Patients become hypercoagulable when the dose of heparin is too low, but that they usually need only small doses of heparin (700 U/h) to be normocoagulable”. We also acknowledge that the TEG may not be useful to monitor patients treated with Warfarin (5). Therefore, we do not solely rely on the TEG but also incorporate the INR and LTA results to assess platelet function and long-term Warfarin therapy.
LTA results proved to be beneficial for our study. Using the agonists AA, ADP, EPI, and collagen allowed us to determine the effectiveness of each drug regimen within our protocol. Figure 3 shows <50% aggregation for AA and EPI. These findings revealed that aspirin was effective in preventing the activation of cyclooxygenase, which is normally responsible for a cascade of events that leads to platelet activation (9). However, ADP revealed that dipyridamole did not achieve antagonistic effects on phosphodiesterase, which is normally responsible for yet another pathway that leads to platelet activation. It is because <50% aggregation was not achieved by dipyridamole that clopidogrel is in consideration for future TAH patient(s).
The multidisciplinary team was able to successfully follow the MTA guidelines for 13 TAH patients. The protocol led to successful avoidance in device thrombus formation and thromboembolic events. Our case series demonstrates that a MTA protocol is an effective means for TAH patients reducing adverse thromboembolic events.
ACKNOWLEDGMENTS
The authors acknowledge Paul Henke, Mike Timmons, and Joe Groteboer for their technical assistance and the Perfusion Team at Mayo Clinic in Rochester, Minnesota, for their support.
REFERENCES
- 1.Copeland JG III, Arabía FA, Banchy ME, et al. The CardioWest total artificial heart bridge to transplantation: 1993 to 1996 national trial. Ann Thorac Surg. 1998;66:1662–9. [DOI] [PubMed] [Google Scholar]
- 2.Copeland JG, Smith RG, Arabia FA, et al. Total artificial heart bridge to transplantation: A 9-year experience with 62 patients. J Heart Lung Transplant. 2004;23:823–31. [DOI] [PubMed] [Google Scholar]
- 3.Copeland JG, Tsau PH, Arabia FA, Xie T.. Correlation of clinical embolic events with coagulability in a patient with a total artificial heart. J Heart Lung Transplant. 1995;14:990–8. [PubMed] [Google Scholar]
- 4.Copeland JG, Arabia FA, Tsau PH, et al. Total artificial hearts: Bridge to transplantation. Cardiol Clin. 2003;21:101–13. [DOI] [PubMed] [Google Scholar]
- 5.Copeland J, Copeland H, Nolan P, Gustafson M, Slepian M, Smith R.. Results with an anticoagulation protocol in 99 SynCardia total artificial heart recipients. ASAIO J. 2013;59:216–20. [DOI] [PubMed] [Google Scholar]
- 6.Szefner J.. Control and treatment of hemostasis in cardiovascular surgery. The experience of La Pitié Hospital with patients on total artificial heart. Int J Artif Organs. 1995;18:633–48. [PubMed] [Google Scholar]
- 7.Pavie A, Leger P, Regan M, et al. Clinical experience with a total artificial heart as a bridge for transplantation: The pitie experience. J Card Surg. 1995;10:552–8. [DOI] [PubMed] [Google Scholar]
- 8.Copeland JG, Smith RG, Arabia FA, et al. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351:859–67. [DOI] [PubMed] [Google Scholar]
- 9.Ensor CR, Paciullo CA, Cahoon WD Jr, Nolan PE Jr.. Pharmacotherapy for mechanical circulatory support: A comprehensive review. Ann Pharmacother. 2011;45:60–77. [DOI] [PubMed] [Google Scholar]
- 10.Ensor CR, Cahoon WD, Crouch MA, et al. Antithrombotic therapy for the CardioWest temporary total artificial heart. Tex Heart Inst J. 2010;37:149–58. [PMC free article] [PubMed] [Google Scholar]