Abstract
Nitrative stress has an important role in microvascular degeneration leading to ischemia in conditions such as diabetic retinopathy and retinopathy of prematurity. Thus far, mediators of nitrative stress have been poorly characterized. We recently described that trans-arachidonic acids are major products of NO2•-mediated isomerization of arachidonic acid within the cell membrane, but their biological relevance is unknown. Here we show that trans-arachidonic acids are generated in a model of retinal microangiopathy in vivo in a NO•-dependent manner. They induce a selective time- and concentration-dependent apoptosis of microvascular endothelial cells in vitro, and result in retinal microvascular degeneration ex vivo and in vivo. These effects are mediated by an upregulation of the antiangiogenic factor thrombospondin-1, independently of classical arachidonic acid metabolism. Our findings provide new insight into the molecular mechanisms of nitrative stress in microvascular injury and suggest new therapeutic avenues in the management of disorders involving nitrative stress, such as ischemic retinopathies and encephalopathies.
Keywords: Animals; Animals, Newborn; Nitroarginine; Oxidation-Reduction; Oxidative Stress; Oxygen; Rats; Rats, Sprague-Dawley; Retina; Antioxidants; Glutathione; Isoenzymes; Malondialdehyde; Metalloporphyrins; Microcirculation; Nitric Oxide; Nitric Oxide Synthase
Ischemia-induced proliferative retinopathies such as diabetic retinopathy and retinopathy of prematurity are characterized by microvascular degeneration and retinal ischemia, which can lead to secondary aberrant neovascularization, hemorrhages and blindness1–3. Several mechanisms involved in the loss of retinal vasculature have been identified. A downregulation of the proangiogenic vascular endothelial growth factor (VEGF) by hyperoxia or an inhibition of its receptor signaling leads to microvascular degeneration4–7. Antiangiogenic factors, such as thrombospondin (TSP)-1 are also important because deletion of TSP-1 protects against oxygen-induced vaso-obliteration8. Oxygen-derived free radicals contribute to microvascular injury2,9, and antioxidants inhibit microvascular degeneration in models of diabetic retinopathy10 and oxygen-induced retinopathy (OIR)2,11,12. These reactive oxygen species can react with nitric oxide (NO•) to generate highly reactive nitrogen species13, including peroxynitrite, nitrogen dioxide (NO2•) and dinitrogen trioxide (N2O3), whose deleterious effects on cell function are generally referred to as nitrative stress14. Several studies have indicated a crucial role for NO• and ensuing nitrative stress in ischemic retinopathies. In particular, it has been shown that retinal microvascular degeneration is associated with increased expression of endothelial nitric oxide synthase (eNOS)15–17, increased generation of nitrites, nitrates and peroxy-nitrite, and protein tyrosine nitration16–19, and can be prevented by pharmacological inhibition of NOS16,18 or deletion of Nos3, which encodes eNOS18. The mechanisms by which NO•-derived reactive species participate in microvascular injury, however, are not fully characterized.
We previously described a peroxidation process mediated by NO2• that results in cis-trans-isomerization of arachidonic acid. This reaction produces four stable trans-arachidonic acid (TAA) monoisomers, 5E-AA, 8E-AA, 11E-AA and 14E-AA20, two of them (5E-AA and 8E-AA) being endogenous and not found in diet21. Furthermore, we showed in a model of septic shock that isomerization of arachidonic acid by NO2• occurs in vivo22. Thus far, the pathological relevance and specific biological effects of these TAAs are unknown. We reasoned that the increased nitrative stress upon oxygen exposure in OIR16,18 may predispose to isomerization of arachidonic acid, and therefore investigated the generation of TAAs in an oxygen-induced model of microvascular degeneration and their influence on retinal vasculature and endothelial cell signaling and survival.
RESULTS
TAAs increase after nitrative stress
NO2• is a highly reactive free radical that originates in a number of ways in vivo, including through aerobic oxidation of NO• (refs. 21,23) and homolytic cleavage of peroxynitrite, which is formed by reaction of NO• with superoxide (O2•−)21,23,24. NO•, O2• − and 3-nitrotyrosine, markers of peroxynitrite- and NO2•-induced nitration of proteins23,24, are abundantly generated in endothelial cells exposed to hyperoxia15 and in oxygen-induced retinopathy16,18. To evaluate the hypothesis that these conditions may favor isomerization of arachidonic acid, we exposed postnatal day (P) 7 rat and mouse pups to 75–80% oxygen or room air for 24 h, and measured TAA levels in the retinas. Hyperoxia led to a significant increase in the concentration of free (unesterified to phospholipids, P < 0.001, n = 6–13 animals per group) as well as total (free plus phospholipid-bound, P < 0.01, n = 3–4 animals per group) TAA, reaching levels of 1.65 μM and 4.72 μM, respectively, in O2-exposed retinas (calculated from estimated retinal volumes; Fig. 1a,b). To ascertain that the TAAs originated from an NO•-dependent pathway, we treated oxygen-exposed animals with the specific NO• synthase (NOS) inhibitor N-nitro-L-arginine-methylester (L-NAME)18. Intraocular injection of L-NAME before oxygen exposure prevented the increase in TAA levels (Fig. 1a), indicating that their formation depends on NO• and ensuing nitrative stress in this model. Because eNOS is mainly responsible for the generation of nitrative stress in hyperoxia16,18, as neuronal NOS does not increase and inducible NOS is not expressed16, we tested the contribution of eNOS on generation of TAAs in this model of microvasculopathy using C57BL/6 mice with a disrupted Nos3 gene. TAA levels did not increase in retinas of Nos3−/− mice in contrast to their wild-type counterparts (Fig. 1b).
Figure 1.
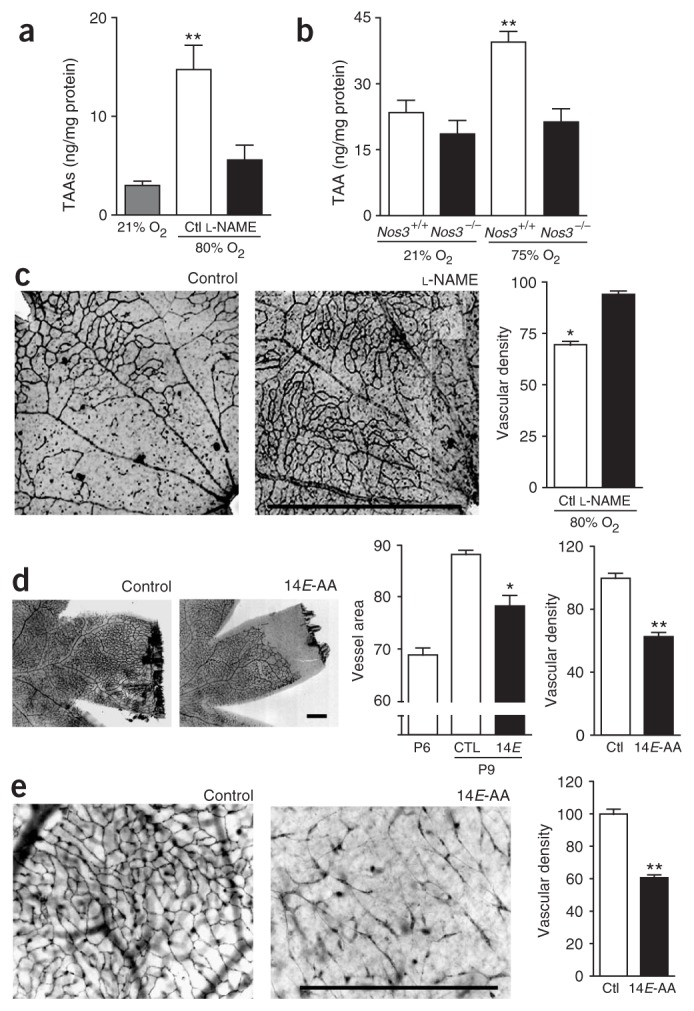
TAA levels rise secondary to nitrative stress and induce retinal microvascular degeneration. (a) Free (unesterified) retinal TAAs in room air–raised and oxygen-exposed rats treated with L-NAME. Experiment performed on P8. (b) Total (free + bound (phospholipid-esterified)) retinal TAAs in room air–raised and oxygen-exposed Nos3+/+ or Nos3−/− mice. Experiment performed on P8. (c) Effect of NOS inhibitor L-NAME on vascular density in oxygen-induced retinal microvascular degeneration in the rat. Experiment performed on P9. Scale bar, 500 μm. (d) In vivo effects of 14E-AA on retinal vascularization and vascular density. Experiment performed on P9. Scale bar, 500 μm. (e) Ex vivo effects of 14E-AA on vascular density of retinal explants. Experiment performed on P4. Scale bar, 500 μm. Values in histograms are mean ± s.e.m. *P < 0.05; ** P < 0.01. Ctl, control; 14E, 14E-AA.
TAAs inhibit angiogenesis and induce vaso-obliteration
We next evaluated the hypothesis that the nitrative stress–evoked increase in TAA concentrations could be involved in the retinal microvascular damage observed in OIR. Concordant with the observations on changes in TAA concentrations in OIR and the role of eNOS presented above, we corroborated that NOS inhibition (using L-NAME in rats) preserves retinal vasculature in the model of oxygen-induced vaso-obliteration in oxygen-exposed rat pups (Fig. 1c, P < 0.01). Using concentrations of TAAs in the same range as those found in OIR (Fig. 1a), we determined their effects on physiologic retinal vascularization in vivo as well as ex vivo on retinal explants. In rodents, retinal angiogenesis starts soon after birth25, and superficial micro-vessels reach the edges of the retina by P9 (ref. 25). We assessed the effects of TAAs on normal angiogenesis using intraocular injections in P6 rat pups. Eyes injected with TAAs showed a reduced vascularized retinal area (P < 0.01, n = 14 eyes per group from three different experiments) and vascular density (P < 0.01) compared to vehicle-treated eyes, clearly showing that TAAs hinder physiological angiogenesis and cause vaso-obliteration (Fig. 1d), similar to the changes observed in the hyperoxic models16,18,26. To investigate the effects of TAAs on microvascular degeneration in situ, we exposed newborn pig retinal explants27 to TAAs and assessed vascular density. Again, exposure to TAAs resulted in a significant decrease in vascular density compared to controls (P < 0.001, n = 14–15 explants per group from three separate experiments; Fig. 1e).
TAAs induce selective cell death by apoptosis
To clarify the cell-type susceptibility thought to be involved in microvascular degeneration, we assessed effects of TAAs on the survival of different types of cultured neuromicrovascular cells. TAAs induced a concentration- and time-dependent microvascular endothelial cell death (Fig. 2a,b; EC50 at 24 h: 3–8 μM, consistent with in vivo concentrations; Fig. 1b,c); concentrations of TAAs within this range were used thereafter. In contrast, smooth muscle, human umbilical vein endothelial cells (HUVECs) and astrocytes were unaffected by exposure to TAAs (Fig. 2c–e), suggesting that TAAs specifically lead to microvascular endothelial cell death.
Figure 2.
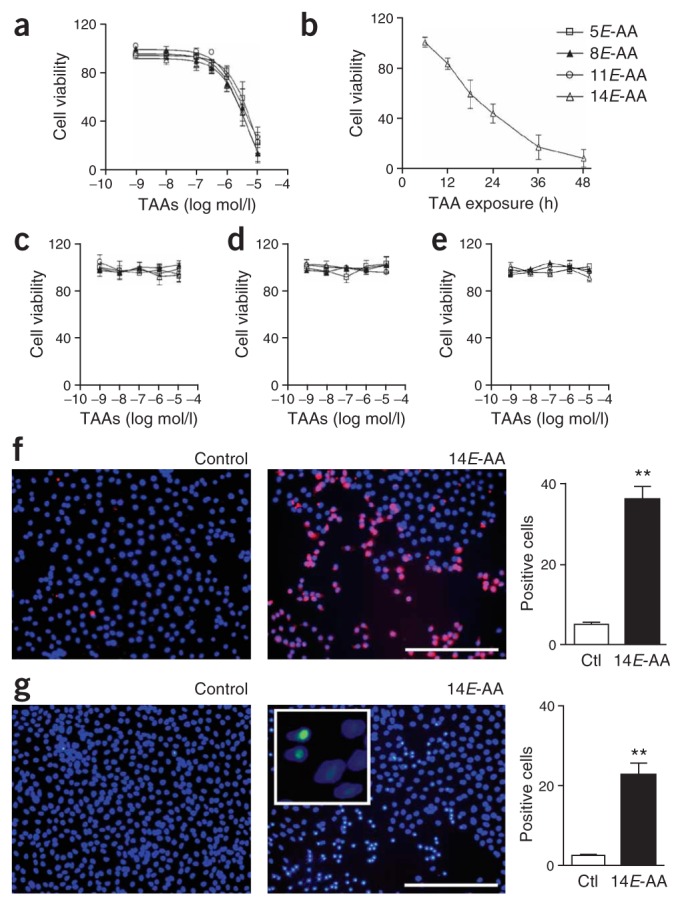
TAAs induce selective neuromicrovascular endothelial cell death by apoptosis. Concentration-dependent effect of TAAs (5E-, 8E-, 11E- and 14E-AA) on cell viability: (a) microvascular endothelial cells, (c) smooth muscle cells, (d) HUVECs and (e) astrocytes. Time-dependent effect (b) of TAA (14E) on microvascular endothelial cell viability. Values (percent of control) are mean ± s.e.m. of five to six experiments (a,b) and three separate experiments (c–e), each performed in triplicate. (f) Caspase activity assay of 14E-AA–treated microvascular endothelial cells. (g) TUNEL assay of 14E-AA–treated microvascular endothelial cells. Inset shows morphological changes in TUNEL-positive cells. Scale bar, 250 μm. Values in histograms are mean ± s.e.m. of percent total cell count from three different experiments performed in duplicate. Ctl, control. **P < 0.01.
We examined the nature of microvascular endothelial cell death using TUNEL staining for detection of nuclear DNA fragmentation and a caspase chromogenic substrate to detect caspase activity, both of which are hallmarks of apoptosis. DNA fragmentation (P < 0.0001) and caspase activity (P < 0.0001) were significantly increased in endothelial cells treated with TAAs (Fig. 2f,g), indicative of apoptotic endothelial cell death. Consistently, the nuclear morphology of Hoechst-stained endothelial cells treated with TAAs was altered, showing a pycnotic appearance in TUNEL-positive cells (Fig. 2g).
TAAs induce apoptosis through upregulation of TSP-1
To investigate the mechanisms underlying TAA-induced endothelial cell apoptosis, we first determined whether arachidonic acid metabolic pathways2 are involved; notably, roles for leukotrienes and prostanoids in angiogenesis and microvascular damage are well documented2,28,29. Inhibition of cyclooxygenase 1, cyclooxygenase 2 and leukotriene receptor did not prevent TAA-induced cell death (Supplementary Fig. 1 online). TAAs only slightly increased concentrations of throm-boxane (Supplementary Fig. 1 online), reported to exert retino-vascular endothelial cytotoxicity28; correspondingly, inhibitors of thromboxane A2 synthase and receptor did not affect TAA-induced endothelial cell death (Supplementary Fig. 1 online). Agonists of prostanglandin I2 and E2 receptors, previously shown to possess prosurvival properties2,29, did not counter the cytotoxic effects of TAAs (Supplementary Fig. 1 online). Furthermore, inhibition of the cytochrome P450 monooxygenase pathway, which can metabolize TAAs30, did not influence TAA-evoked cytotoxicity (Supplementary Fig. 1 online). Thus, pro–cell-death effects of TAAs seemed to be independent of classical major arachidonic acid metabolic pathways.
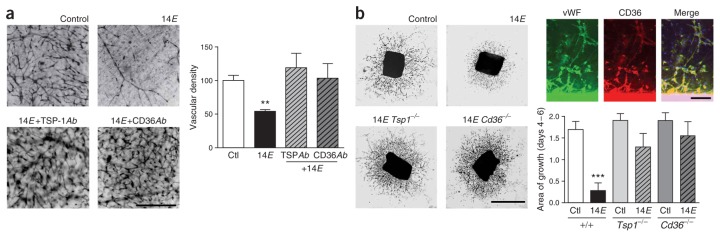
Because pro- and antiangiogenic factors have been shown to have an important role in the pathogenesis of oxygen-induced retinopathy3, we determined whether TAAs influenced their expression in endothelial cells. TAAs caused a rapid (within 2–6 h) increase in the expression of the antiangiogenic TSP-1 in cultured endothelial cells (Fig. 3a). Expression of VEGF receptor 2 (VEGFR2), shown to contribute to endothelial cell apoptosis and vessel loss in ischemic retinopathies4–7, was found to exhibit a relatively delayed (~12 h) time-dependent decrease in response to TAA (Fig. 3a). We investigated the effect of TSP-1 on VEGFR2 expression; TSP-1 depressed expression of VEGFR2 after exposure for 4 h, probably by inducing protease activation and its degradation31 (Fig. 3b). Therefore, the delayed decrease in expression of VEGRF2 is likely to occur secondarily to the TAA-induced upregulation of TSP-1. TSP-1 is a large matricellular protein that is synthesized and secreted by several cell types including endothelial cells32. TSP-1 inhibits angiogenesis by inducing micro-vascular endothelial cell apoptosis31–33 and inhibiting the migration of these cells32. The ability of TSP-1 to trigger endothelial cell apoptosis requires the activation of its transmembrane receptor CD36 (refs. 34,35) and is essential to its antiangiogenic activity in vivo35. To establish a cause-and-effect relationship between TSP-1 upregulation and TAA-induced endothelial cell apoptosis, we used function-blocking antibodies directed against TSP-1 and CD36. Both TSP-1–specific antibody and antibody to CD36 that specifically blocks the TSP-1 binding site fully prevented TAA-induced endothelial cell death (Fig. 3c); in contrast, cell death elicited by TAAs was not altered by an antibody to CD36 that blocks the binding site of oxidized low-density lipoprotein (Fig. 3c). We also tested the involvement of TSP-1 in retinal explants treated with TAAs. The vaso-obliterative effects of TAA under these conditions were prevented by both TSP-1– and CD36-specific antibodies (Fig. 4a). To further corroborate the anti-angiogenic role of TSP-1 and CD36 in response to TAAs, we tested the effects of TAAs on vascular sprouting from Matrigel-embedded aortic rings isolated from TSP-1– and CD36-knockout mice; sprouting aortic endothelium (von Willebrand factor (vWF) positive) contained immunoreactivity to CD36 (Fig. 4b). TAAs interfered with vascular sprouting in aortas of wild-type mice, whereas this effect was virtually undetected in aortas of mice with disruptions in the genes encoding TSP-1 and CD36 (Fig. 4b, P < 0.01). In line with these observations, the absence of an effect of TAAs on HUVECs (Fig. 2d) is consistent with their lack of CD36 receptors36. Taken together, the increase in TSP-1 expression observed before endothelial cell death, the apoptotic nature of endothelial cell death induced by TAAs and abrogation of cytotoxic effects of TAAs with antibodies to either TSP-1 or CD36 as well as in mice deficient in these proteins strongly point to a TSP-1–dependent pathway.
Figure 3.
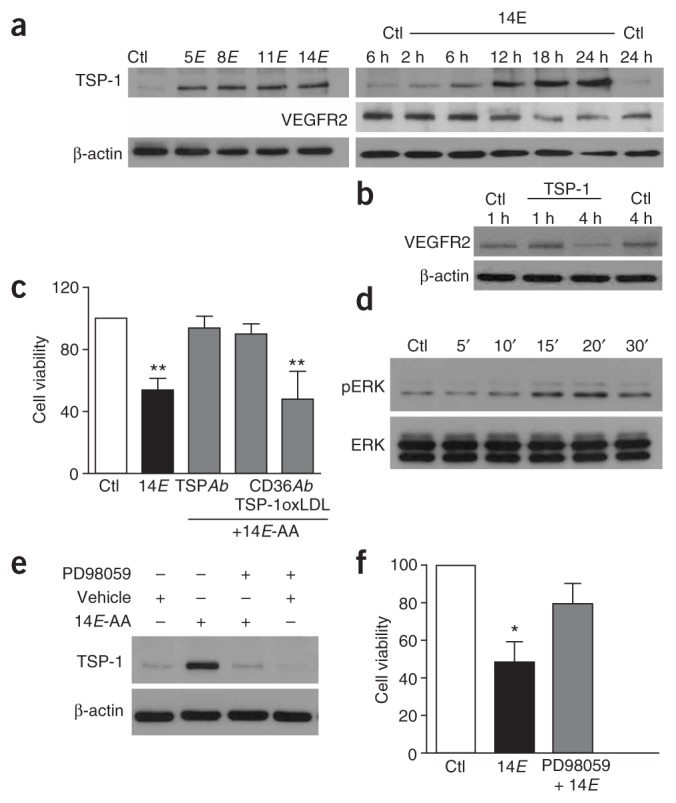
TAAs induce endothelial cell death by an ERK 1/2-dependent upregulation of TSP-1. (a) Representative western blot of TSP-1 expression in endothelial cells exposed to each TAA for 18 h (left), and of TSP-1 and VEGFR2 immunoreactivity in response to 14E-AA for different durations (right). (b) Representative western blot of VEGFR2 expression in TSP-1–exposed endothelial cells. (c) Effects of TSP-1– (TSPAb) and two CD36-specific (CD36Ab) antibodies (blocking either the TSP-1 or oxidized low-density lipoprotein (oxLDL) binding sites of CD36) on TAA-induced endothelial cell viability. Values in histogram are mean ± s.e.m. of three separate experiments performed in triplicate. **P < 0.01 compared with control (Ctl) and other values. (d) Representative western blot of ERK 1/2 phosphorylation in endothelial cells exposed to 14E-AA. (e) Representative western blot of TSP-1 expression in the presence or absence of 14E-AA and the MEKK-ERK1/2 pathway inhibitor PD98059. Immunoblots for a,b,d and e are representative of three separate experiments. (f) Effect of PD98059 on TAA-induced endothelial cell death. Values are mean ± s.e.m. of three separate experiments performed in triplicate. *P < 0.05 compared with other values.
Figure 4.
TAAs induce a TSP-1–dependent microvascular degeneration and inhibit of angiogenesis in tissue explants. (a) Representative retinal explants from newborn pigs treated with 14E-AA in the presence or absence of TSP-1– (TSP-1Ab) and CD36-specific (CD36Ab) antibodies. Scale bar, 250 μm. Values in histogram are mean ± s.e.m. of four to five explants per treatment group. **P < 0.01. (b) Representative microvascular sprouting from Matrigel-embedded aortic rings from wild-type, Tsp1−/− and CD36−/− mice exposed to 14E-AA (left panels). Scale bar, 1 mm. CD36 expression in microvascular sprouts (right colored panels); representative of two experiments. Scale bar, 100 μm. Values in histograms are mean ± s.e.m. of 19–20 explants per group. ***P < 0.01 compared with control (Ctl) and other values.
We also conducted experiments to determine mechanisms underlying TAA-induced upregulation of TSP-1. Involvement of the mitogen-activated protein kinase (MAPK) pathway has been shown in many apoptotic processes37. Recent studies have indicated that a transient activation of ERK1/2 (also known as MAPK p42/44) contributes to induction of apoptosis in several cell types, whereas basal, sustained activity is required for the maintenance of cell survival38,39. We found ERK1/2 to be transiently phosphorylated in response to exposure to TAAs in endothelial cells (Fig. 3d). Furthermore, blockage of the MEKK-ERK1/2 pathway with the specific MAPK kinase inhibitor PD98059 markedly suppressed TAA-induced upregulation of TSP-1 as well as TAA-induced endothelial cell death (Fig. 3e,f); inhibition of the other two MAPK pathways involved in stress and inflammation, namely p38 and JNK, had no effect on these parameters (data not shown). Hence, ERK1/2 seems to be required for TAA signaling.
TAAs induce TSP-1 ensuing vaso-obliteration in vivo
Oxygen-induced retinopathy is associated with vaso-obliteration, resulting in part from nitrative stress16,18 (Fig. 1a), which increases formation of TAAs21,22 (Fig. 1b,c). TAAs in turn cause retinal endothelial degeneration in vitro, ex vivo and in vivo (Figs. 1d,e and 2a,f,g), through induction of TSP-1 (Figs. 3c and 4a,b). We therefore investigated in this oxygen-induced model of ischemic retinopathy the involvement of TSP-1 and CD36 in microvascular degeneration. We first showed that intravitreal injection of TAAs indeed caused an upregulated expression of TSP-1 in vivo, which was specifically localized on the endothelium, as shown by immunohistochemistry (Fig. 5a); on the other hand, there was no change in the expression of total retinal VEGFR2 expression, which is found in numerous cell types at that stage of development7. An increase in TSP-1 expression (protein and mRNA) was also detected in retinas of rats and mice exposed to hyperoxia for 24 h (Fig. 5b,c) along with the rise in TAA levels (Fig. 1b,c); TSP-1 was localized on endothelium in this model also (Fig. 5d). Correspondingly, this increase in TSP-1 (Tsp1 mRNA expression detected by quantitative PCR and corrected for that of Vwf) was significantly attenuated in Nos3−/− compared to wild-type oxygen-exposed mice (Fig. 5c; P < 0.01, n = 6 per group). Finally, to verify that oxygen-induced changes in TAAs (Fig. 1b,c) and ensuing upregulation of TSP-1 (Fig. 5b) are physiologically relevant, we studied retinal vascularization in hyperoxia-exposed mice deficient in CD36; vaso-obliteration was significantly attenuated in Cd36−/− mice (Fig. 5e, P < 0.001, n = 8 per group); a similar attenuation was reported in hyperoxia-exposed Tsp1−/− mice8.
Figure 5.
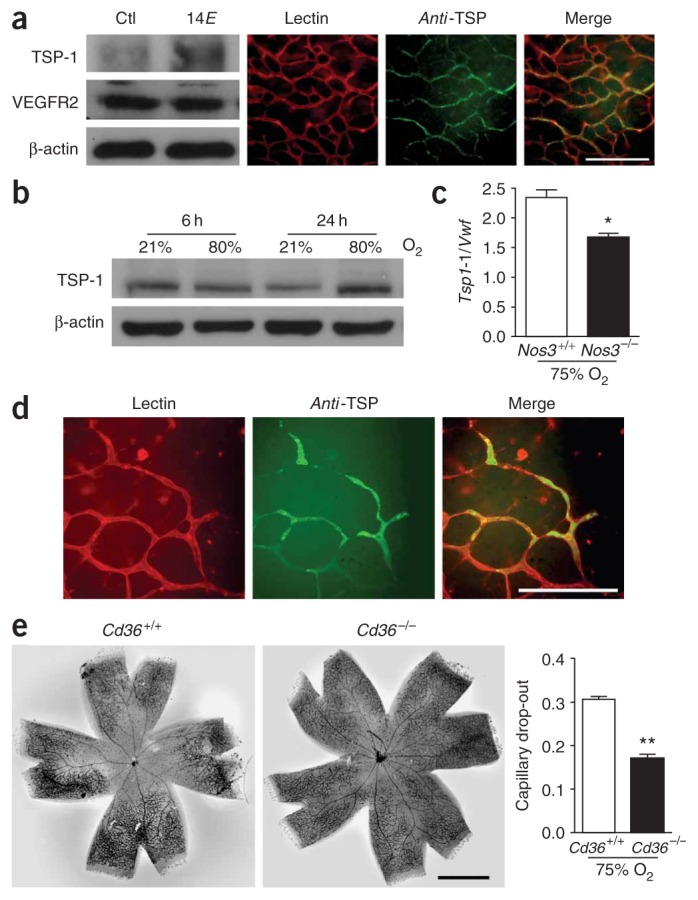
TAA-induced upregulation of TSP-1 in vivo and importance in hyperoxia-induced retinal microvascular degeneration. (a) Representative western blots of TSP-1 and VEGFR2 expression in rat pup retinas injected with TAAs (left). TSP-1 was localized in microvessels (detected by lectin staining) in retinas exposed to TAAs. Scale bar, 250 μm. TAAs were injected on days 1 and 4 and retinas were isolated on day 5 for western blot and immunofluorescent staining. Experiments were performed three times. (b) Western blot of TSP-1 in rat pups exposed to hyperoxia or normoxia for 6 and 24 h; representative of four rats in each group and time. (c) Tsp1 mRNA expression corrected for Vwf (as a measure of endothelialization) in retinas of wild-type and Nos3−/− mice exposed to hyperoxia for 24 h. (d) Microvascular localization of TSP-1 in retinas of wild-type mouse pups exposed to hyperoxia for 24 h. Panel representations of two experiments are similar to those in a. Scale bar, 125 μm. (e) Vaso-obliteration in Cd36+/+ and Cd36−/− mice exposed to hyperoxia for 24 h. Scale bar, 1 mm. *P < 0.05; **P < 0.01.
DISCUSSION
The recently described markers of nitrative stress and major products of NO2•-mediated isomerization of arachidonic acid, TAAs, represent a new aspect of NO2•-induced toxicity. We investigated whether and how endogenous TAAs contribute to endothelial cell degeneration. We found that TAAs are generated in vivo in an experimental model of oxygen-induced microvascular degeneration, reaching concentrations in the micromolar range. The nitrative stress dependency of TAA formation was shown by using the NOS inhibitor L-NAME and Nos3−/− animals, which prevented the increase of TAAs upon exposure to oxygen and associated vaso-obliteration. Moreover, TAAs were found to induce microvascular endothelial cell death in vitro, ex vivo and in vivo.
Previous studies have shown that isomerization of arachidonic acid occurs in vivo. TAAs are found to circulate as free acids in human plasma40 and have been shown to increase in a model of septic shock22, a condition that is correlated with nitrative stress41,42. Our data provide an additional pathological condition in which TAAs are generated in substantial amounts. We now show that these unique lipids are biologically active molecules and important contributing factors to microvascular damage as seen in ischemic retinopathies. This was demonstrated by showing that TAAs induce selective apoptosis of microvascular endothelial cells in vitro, and exert specific, species-independent cytotoxicity on retinal microvessels in both ex vivo and in vivo models.
In vivo, TAAs derive solely from the reaction of the radical NO2• with arachidonic acid21,22, probably involving the binding of NO2• to arachidonic acid followed by a rearrangement of the nitroarachidonyl radical, loss of NO2• and formation of a trans bond21. Thus, they are specifically produced under conditions associated with nitrative stress, such as inflammation and ischemia; this inference is supported in our study using pharmacological inhibitors of NOS and Nos3−/− mice. Under these pathological conditions, NO•-mediated cell death is thought to be caused by the reaction of NO•-derived free radicals, in particular peroxynitrite, N2O3, and NO2•, with biological targets such as DNA, lipids and proteins13. The proposed mechanisms include inactivation of mitochondrial respiration43, modulation of gene expression44, nitration of DNA and protein13,24 and peroxidation of lipids13,23. Although all the previously described effects of NO•-derived free radicals concentrated on direct modifications of target molecules such as protein and DNA, which may alter their function, no secondary signaling molecule has so far been identified. By showing that TAAs are released during nitrative stress and induce microvascular damage mediated by upregulation TSP-1 in retinal microvascular endothelial cells, our results identify TAAs as new mediators of nitrative stress in vivo and unravel a mechanism by which reactive nitrogen species contribute to microvascular degeneration in ischemic retinopathies. Because generation of NO•-derived free radicals has been suggested to be involved in the pathophysiology of numerous diseases41, we propose that our findings could possibly be extrapolated to explain micro-vascular damage occurring in diabetes, ischemic encephalopathies and inflammation.
Our data indicate that TAA-induced cell death and microvascular degeneration are mediated by TSP-1; in addition, upregulation of the latter seems to depend on activation of ERK1/2. Although ERK1/2 is usually regarded as a cytoprotective kinase, whereas the two other main MAPKs, p38 and JNK, are generally considered to be mediators of cell death37, a transient activation of ERK1/2 has been shown to induce apoptosis, whereas sustained activity favors cell survival38,39. Thus, our results provide additional evidence that ERK1/2 can have opposing effects on cell death and survival, depending on the kinetics and amplitude of its activation and the cellular environment, and provide new insight on the regulation of TSP-1 expression. Participation of TSP-1 in oxygen-induced microvascular injury has been shown by the demonstration that TSP-1–deficient mice are less susceptible to oxygen-induced vaso-obliteration8. In agreement with this finding, we found that retinal expression of TSP-1 is enhanced after 24 h of hyperoxia in OIR, before considerable vascular degeneration would normally be observed in this model25, and mice deficient in CD36 show less vaso-obliteration upon exposure to hyperoxia. Together, our data indicate TAA-induced expression of TSP-1 as an important pathway responsible for subsequent microvascular damage, and also provide evidence for a previously undescribed link between nitrative stress and TSP-1, notably involving ERK1/2.
The signaling pathway leading from TAA-induced activation of MAPK to upregulation of TSP-1 remains undefined. We found that expression of p53 and PPARγ, which are thought to be involved in regulation of TSP-1 (refs. 45,46), were not modulated by TAAs. On the other hand, previous studies suggest that trans-fatty acids alter cell membrane function by modifying its fluidity and permeability, leading to activation of membrane-bound channels and enzymes21. Nevertheless, the specificity of the effect of TAAs on expression of TSP-1 suggests that they might exert their effect through an as yet unknown receptor. Further characterization of the molecular mechanisms leading to TAA-evoked induction of TSP-1 is required and is currently under investigation.
The formation of TAAs is subject to the redox potential of the tissue. Accordingly, an immature subject would be more vulnerable as a result of its decreased antioxidant capacity47–49. We have recently shown that nitration, which leads to generation of TAAs20, occurs when the redox state of the developing tissue is shifted toward an oxidative environment16. Our current findings, primarily observed in newborn animals, concur with this inference. There is currently no available effective treatment to prevent microvascular damage in ischemic retinopathies and microangiopathies. By identifying a new mechanism of microvascular injury, our data provide new therapeutic strategies in these seriously debilitating diseases associated with nitrative stress. Thus, interventions targeting the formation of TAAs or NO2• may have potential therapeutic applications in conditions such as ischemic retinopathies and other microangiopathies.
METHODS
Animals
All animals were used according to a protocol of the Hôpital Sainte-Justine Animal Care Committee. We purchased 1–3-d-old piglets from Fermes Ménard and Sprague-Dawley rats from Charles River. Nos3−/−, Cd36−/− and Tsp1−/− mice and corresponding wild-type mice were provided by P. D’Orleans-Juste (Universite de Sherbrooke), M. Febbraio (Weill Medical College, Cornell University) and J. Lawler (Beth Israel Deaconess Medical Center), respectively.
Animal preparation and quantitation of retinal vascularization
We maintained P7 rat pups, Nos3+/+ and Nos3−/− mice, and Cd36+/+ and Cd36−/− mice of C57BL/6 background in room air or exposed them to 80% (rats) or 75% (mice) oxygen for 24 h (Oxycycler A82OCV, Biospherix) to generate an oxygen-induced retinopathy. We intravitreally injected rat pups with L-NAME (Sigma; 10−5 M final intraocular concentration based on estimated eye volume29) before exposure to oxygen. We intravitreally injected other rat pups at P6, P7 and P8 with either vehicle or 14E-AA (estimated final concentration of 5 × 10−6 M)29.
Explants of retinal tissue can be cultured for several days and retain their proliferation and differentiation capacities and their histological architecture27. We cultured retinas of newborn (1–2 d old) pigs for 3 d on a Nucleopore polycarbonate Track Etch membrane (pore size 0.03 μm, Whatman) placed on DMEM (Gibco) containing 1% FBS and 1% penicillin-streptomycin, in the presence or absence of 14E-AA (5 × 10−6 M), CD36-specific (clone FA6-152, 10 μg/ml, Immunotech) or TSP-1–specific (clone A4.1, 30 μg/ml, Neomarkers) antibodies. We performed immunohistochemistry for whole retinas and explants using thrombospondin Ab-3 antibody (1:100, Oncogene) and lectin Griffonia simplicifolia conjugated to TRITC (1:100, Sigma) as previously described29. We flat-mounted and photographed retinas and explants; we measured vascular density as capillary length per retinal surface area (mm/mm2); we measured vascularized areas and expressed them as percent of total retinal area16,29.
TAA measurement
We dissected retinas and placed them into a 1:1 chloroform/methanol solution containing butylated hydroxytoluene to extract lipids. We measured TAA (both esterified to lipids and free) content by mass spectrometry analysis20,21 and normalized it to protein content.
Cell cultures
We cultured endothelial cells and astrocytes from newborn pig brain and retina28,50. We purchased VSMCs and HUVECs from Clonetics.
Assessment of cell viability
We exposed cells to each TAA for 24 h at indicated concentrations or to 14E-AA (5 × 10−6 M) for different durations and assessed cell viability by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, Sigma) assay28. In another set of experiments, we pretreated cells with TSP-1–specific antibody (clone A4.1, 30 μg/ml, Neomarkers), CD36-specific antibodies (clones FA6-152 (which blocks TSP-1 binding), 10 μg/ml, Immunotech, and JC63.1 (which blocks binding of oxidized low-density lipoprotein), 2 μg/ml, Cayman Chemicals) and PD98059 (MEKK inhibitor, 10−5 M, Calbiochem) 30 min before exposing them to 14E-AA (5 × 10−6 M) for 24 h. We calculated cell viability as percent of control values.
Apoptosis assays
We cultured endothelial cells for 18 h in the presence or absence of 14E-AA (5 × 10−6 M). We performed detection of DNA fragmentation with a commercial kit based on TUNEL (TACS TdT kit, R&D Systems). We assessed caspase activity using a commercial kit (Sulforhodamine Multi-caspase Activity kit, Biomol Research Laboratories). We calculated values as positive cells as a percent of total cells.
Western blots
We exposed lysates of endothelial cells to 14E-AA (5 × 10−6 M) in the presence or absence of PD98059 (10−5 M) or to TSP-1 (10−7 M, provided by H. Ong, Université de Montréal) and we used the membrane fraction of rat pup retinas for western blot analysis of protein expression28. We used the following antibodies: monoclonal TSP-1–specific (clone Ab-1, 1:400, Oncogene), VEGFR2-specific (1:250, Chemicon International), polyclonal ERK 1/2- and phosphorylated ERK 1/2-specific (1:5,000, Promega), and mono-clonal β-actin–specific (1:60,000, Abcam).
Microvascular sprouting from aortic explants
We prepared aortae from 10-week-old male C57BL/6 wild-type, Tsp1−/− and Cd36−/− mice of the same background. We covered aortic rings with 50 μl Matrigel and cultured them for 4 d in EGM-2 medium (Clonetics). We exposed explants to 14E-AA (5 × 10−6 M) from day 4 to 6 of culture. We took photomicrographs of individual explants at day 4 and day 6 and measured microvascular sprouting as the surface covered at day 6 minus that at day 4. We characterized outgrowing cells by double-labeling them with monoclonal vWF-specific (1:100, Dako) and polyclonal CD36-specific (1:100, Santa Cruz) antibodies after acetone fixation.
Real-time quantitative RT-PCR
We exposed Nos3+/+ and Nos3−/− mice of C57BL/6 background to 75% oxygen from P7 to P8. We dissected retinas and performed RNA extraction using the Qiagen RNA extraction kit (Qiagen). For cDNA synthesis, we used 2 μg mRNA in all reactions. We performed real-time quantitative PCR on MJ4000 and MJ3005 thermocyclers (Stratagene), using 5′-GTTTGTGCAGCAGAGGAACA-3′ and 5′-CATTCACCCTGGCTCTTCTC-3′ for Vwf and 5′-GCCATGTCCTCTCACAGGGC-3′ and 5′-CTGTGGCCACTGG GAGATTAGC-3′ for Tsp1. We standardized RNA levels to parallel measurements of S16 RNA.
Data analysis
Data were analyzed by Kruskall-Wallis and Mann-Whitney tests according to the number of groups compared. P < 0.05 was considered to be statistically significant.
Acknowledgments
The authors wish to thank H. Fernandez for her technical skills and help. This work was supported by grants from the Canadian Institutes of Health Research, the March of Dimes Birth Defects Foundation, the Heart and Stroke Foundation of Québec, the Fonds de la Recherche en Santé du Québec, Le Réseau de Recherche en Santé de la Vision and La Fondation du NO. E.K.-D. is recipient of a fellowship award from the ‘Association des Juniors en Pédiatrie/Gallia’ (France). F.S. and S.C. are recipients of fellowship and scientist awards, respectively, from the Canadian Institutes of Health Research. S.B. and M.S. are recipients of studentships from the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada, respectively. P.H. is supported by grants from the Hospital For Sick Children Foundation and Fonds de la Recherche en Santé du Québec. M.B. is supported by grants from the US National Institutes of Health (R01 GM62453) and Philip Morris USA, Inc. S.C. also holds a Canada Research Chair (perinatology). The authors thank M. Febbraio and J. Lawler, who provided the CD36 and TSP-1 knockout animals, respectively.
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare that they have no competing financial interests.
Note: Supplementary information is available on the Nature Medicine website.
References
- 1.Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 2.Hardy P, et al. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovasc Res. 2000;47:489–509. doi: 10.1016/s0008-6363(00)00084-5. [DOI] [PubMed] [Google Scholar]
- 3.Campochiaro PA. Retinal and choroidal neovascularization. J Cell Physiol. 2000;184:301–310. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Alon T, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 5.Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–1228. doi: 10.1001/archopht.1996.01100140419009. [DOI] [PubMed] [Google Scholar]
- 6.Smith LE, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med. 1999;5:1390–1395. doi: 10.1038/70963. [DOI] [PubMed] [Google Scholar]
- 7.Shih SC, Ju M, Liu N, Smith LE. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest. 2003;112:50–57. doi: 10.1172/JCI17808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228:630–642. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 9.Spierer A, Rabinowitz R, Pri-Chen S, Rosner M. An increase in superoxide dismutase ameliorates oxygen-induced retinopathy in transgenic mice. Eye. 2005;19:86–91. doi: 10.1038/sj.eye.6701424. [DOI] [PubMed] [Google Scholar]
- 10.Kowluru RA, Tang J, Kern TS. Abnormalities of retinal metabolism in diabetes and experimental galactosemia. VII Effect of long-term administration of antioxidants on the development of retinopathy. Diabetes. 2001;50:1938–1942. doi: 10.2337/diabetes.50.8.1938. [DOI] [PubMed] [Google Scholar]
- 11.Penn JS, Tolman BL, Bullard LE. Effect of a water-soluble vitamin E analog, trolox C, on retinal vascular development in an animal model of retinopathy of prematurity. Free Radic Biol Med. 1997;22:977–984. doi: 10.1016/s0891-5849(96)00479-0. [DOI] [PubMed] [Google Scholar]
- 12.Raju TN, Langenberg P, Bhutani V, Quinn GE. Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal of published trials. J Pediatr. 1997;131:844–850. doi: 10.1016/s0022-3476(97)70031-3. [DOI] [PubMed] [Google Scholar]
- 13.Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 14.Kroncke KD. Mechanisms and biological consequences of nitrosative stress. Biol Chem. 2003;384:1341. doi: 10.1515/BC.2003.151. [DOI] [PubMed] [Google Scholar]
- 15.Gu X, et al. Hyperoxia induces retinal vascular endothelial cell apoptosis through formation of peroxynitrite. Am J Physiol Cell Physiol. 2003;285:C546–C554. doi: 10.1152/ajpcell.00424.2002. [DOI] [PubMed] [Google Scholar]
- 16.Beauchamp MH, et al. Redox-dependent effects of nitric oxide on microvascular integrity in oxygen-induced retinopathy. Free Radic Biol Med. 2004;37:1885–1894. doi: 10.1016/j.freeradbiomed.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 17.El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003;44:3135–3143. doi: 10.1167/iovs.02-1022. [DOI] [PubMed] [Google Scholar]
- 18.Brooks SE, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:222–228. [PubMed] [Google Scholar]
- 19.El-Remessy AB, et al. Experimental diabetes causes breakdown of the blood-retina barrier by a mechanism involving tyrosine nitration and increases in expression of vascular endothelial growth factor and urokinase plasminogen activator receptor. Am J Pathol. 2003;162:1995–2004. doi: 10.1016/S0002-9440(10)64332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H, et al. Nitrogen dioxide induces cis-trans-isomerization of arachidonic acid within cellular phospholipids. Detection of trans-arachidonic acids in vivo. J Biol Chem. 1999;274:16235–16241. doi: 10.1074/jbc.274.23.16235. [DOI] [PubMed] [Google Scholar]
- 21.Balazy M, Poff CD. Biological nitration of arachidonic acid. Curr Vasc Pharmacol. 2004;2:81–93. doi: 10.2174/1570161043476465. [DOI] [PubMed] [Google Scholar]
- 22.Balazy M. Trans-arachidonic acids: new mediators of inflammation. J Physiol Pharmacol. 2000;51:597–607. [PubMed] [Google Scholar]
- 23.Kirsch M, Korth HG, Sustmann R, de Groot H. The pathobiochemistry of nitrogen dioxide. Biol Chem. 2002;383:389–399. doi: 10.1515/BC.2002.043. [DOI] [PubMed] [Google Scholar]
- 24.Prutz WA, Monig H, Butler J, Land EJ. Reactions of nitrogen dioxide in aqueous model systems: oxidation of tyrosine units in peptides and proteins. Arch Biochem Biophys. 1985;243:125–134. doi: 10.1016/0003-9861(85)90780-5. [DOI] [PubMed] [Google Scholar]
- 25.Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division. Evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–1214. [PubMed] [Google Scholar]
- 26.Smith LE. Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res. 2004;14(Suppl A):140–4. doi: 10.1016/j.ghir.2004.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Caffe AR, et al. Mouse retina explants after long-term culture in serum free medium. J Chem Neuroanat. 2001;22:263–273. doi: 10.1016/s0891-0618(01)00140-5. [DOI] [PubMed] [Google Scholar]
- 28.Beauchamp MH, et al. Role of thromboxane in retinal microvascular degeneration in oxygen-induced retinopathy. J Appl Physiol. 2001;90:2279–2288. doi: 10.1152/jappl.2001.90.6.2279. [DOI] [PubMed] [Google Scholar]
- 29.Sennlaub F, et al. Cyclooxygenase-2 in human and experimental ischemic proliferative retinopathy. Circulation. 2003;108:198–204. doi: 10.1161/01.CIR.0000080735.93327.00. [DOI] [PubMed] [Google Scholar]
- 30.Roy U, Loreau O, Balazy M. Cytochrome P450/NADPH-dependent formation of trans epoxides from trans-arachidonic acids. Bioorg Med Chem Lett. 2004;14:1019–1022. doi: 10.1016/j.bmcl.2003.11.054. [DOI] [PubMed] [Google Scholar]
- 31.Nor JE, et al. Thrombospondin-1 induces endothelial cell apoptosis and inhibits angiogenesis by activating the caspase death pathway. J Vasc Res. 2000;37:209–218. doi: 10.1159/000025733. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong LC, Bornstein P. Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol. 2003;22:63–71. doi: 10.1016/s0945-053x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 33.Guo N, Krutzsch HC, Inman JK, Roberts DD. Thrombospondin 1 and type I repeat peptides of thrombospondin 1 specifically induce apoptosis of endothelial cells. Cancer Res. 1997;57:1735–1742. [PubMed] [Google Scholar]
- 34.Dawson DW, et al. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimenez B, et al. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 36.Swerlick RA, Lee KH, Wick TM, Lawley TJ. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol. 1992;148:78–83. [PubMed] [Google Scholar]
- 37.Wada T, Penninger JM. Mitogen-activated protein kinases in apoptosis regulation. Oncogene. 2004;23:2838–2849. doi: 10.1038/sj.onc.1207556. [DOI] [PubMed] [Google Scholar]
- 38.Ishikawa Y, Kitamura M. Dual potential of extracellular signal-regulated kinase for the control of cell survival. Biochem Biophys Res Commun. 1999;264:696–701. doi: 10.1006/bbrc.1999.1542. [DOI] [PubMed] [Google Scholar]
- 39.Gauld SB, Blair D, Moss CA, Reid SD, Harnett MM. Differential roles for extracellularly regulated kinase-mitogen-activated protein kinase in B cell antigen receptor-induced apoptosis and CD40-mediated rescue of WEHI-231 immature B cells. J Immunol. 2002;168:3855–3864. doi: 10.4049/jimmunol.168.8.3855. [DOI] [PubMed] [Google Scholar]
- 40.Zghibeh CM, Raj Gopal V, Poff CD, Falck JR, Balazy M. Determination of trans-arachidonic acid isomers in human blood plasma. Anal Biochem. 2004;332:137–144. doi: 10.1016/j.ab.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Llorens S, Nava E. Cardiovascular diseases and the nitric oxide pathway. Curr Vasc Pharmacol. 2003;1:335–346. doi: 10.2174/1570161033476637. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 43.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 44.Tabuchi A, Oh E, Taoka A, Sakurai H, Tsuchiya T, Tsuda Rapid attenuation of AP-1 transcriptional factors associated with nitric oxide (NO)-mediated neuronal cell death. J Biol Chem. 1996;271:31061–7. doi: 10.1074/jbc.271.49.31061. [DOI] [PubMed] [Google Scholar]
- 45.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 46.Okuno M, Arimoto E, Nishizuka M, Nishihara T, Imagawa M. Isolation of up- or down-regulated genes in PPARgamma-expressing NIH-3T3 cells during differentiation into adipocytes. FEBS Lett. 2002;519:108–112. doi: 10.1016/s0014-5793(02)02720-5. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen JC, Naash MI, Anderson RE. The regional distribution of vitamins E and C in mature and premature human retinas. Invest Ophthalmol Vis Sci. 1988;29:22–26. [PubMed] [Google Scholar]
- 48.Flynn JT, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326:1050–1054. doi: 10.1056/NEJM199204163261603. [DOI] [PubMed] [Google Scholar]
- 49.Mann RM, Riva CE, Stone RA, Barnes GE, Cranstoun SD. Nitric oxide and choroidal blood flow regulation. Invest Ophthalmol Vis Sci. 1995;36:925–930. [PubMed] [Google Scholar]
- 50.Lahaie I, et al. A novel mechanism for vasoconstrictor action of 8-isoprostaglandin F2 alpha on retinal vessels. Am J Physiol. 1998;274:R1406–R1416. doi: 10.1152/ajpregu.1998.274.5.R1406. [DOI] [PubMed] [Google Scholar]