ABSTRACT
In many bacteria, the FtsH protease and its modulators, HflK and HflC, form a large protein complex that contributes to both membrane protein quality control and regulation of the cellular response to environmental stress. Both activities are crucial to the Lyme disease pathogen Borrelia burgdorferi, which depends on membrane functions, such as motility, protein transport, and cell signaling, to respond to rapid changes in its environment. Using an inducible system, we demonstrate that FtsH production is essential for both mouse and tick infectivity and for in vitro growth of B. burgdorferi. FtsH depletion in B. burgdorferi cells resulted in membrane deformation and cell death. Overproduction of the protease did not have any detectable adverse effects on B. burgdorferi growth in vitro, suggesting that excess FtsH does not proteolytically overwhelm its substrates. In contrast, we did not observe any phenotype for cells lacking the protease modulators HflK and HflC (ΔHflK/C), although we examined morphology, growth rate, growth under stress conditions, and the complete mouse-tick infectious cycle. Our results demonstrate that FtsH provides an essential function in the life cycle of the obligate pathogen B. burgdorferi but that HflK and HflC do not detectably affect FtsH function.
IMPORTANCE
Lyme disease is caused by Borrelia burgdorferi, which is maintained in nature in an infectious cycle alternating between small mammals and Ixodes ticks. B. burgdorferi produces specific membrane proteins to successfully infect and persist in these diverse organisms. We hypothesized that B. burgdorferi has a specific mechanism to ensure that membrane proteins are properly folded and biologically active when needed and removed if improperly folded or dysfunctional. Our experiments demonstrate that FtsH, a protease that fulfills this role in other microorganisms, is essential to B. burgdorferi viability. Cells depleted of FtsH do not survive in laboratory culture medium and cannot colonize mice or ticks, revealing an absolute requirement for this protease. However, the loss of two potential modulators of FtsH activity, HflK and HflC, does not detectably affect B. burgdorferi physiology. Our results provide the groundwork for the identification of FtsH substrates that are critical for the bacterium’s viability.
INTRODUCTION
The bacterial cytoplasmic membrane and constituent proteins provide a variety of essential functions for the prokaryotic cell, including the selective uptake of nutrients and ions, protein transport, cell signaling, and the export of waste products. To maintain the efficient functioning of the cytoplasmic membrane proteins, the bacterial cell employs chaperones and proteases. Chaperones escort proteins toward their final destinations and aid in proper folding. However, if the protein is misfolded, mistranslated, or damaged, chaperones present the aberrant proteins to proteases for degradation. Without this membrane quality control, abnormal proteins may aggregate and perturb membrane integrity, interfere with normal protein function, or jam the transport machinery of the cytoplasmic membrane.
In eubacteria, mitochondria, and chloroplasts, the protease FtsH fulfills this role and also regulates other proteins by programmed degradation. FtsH, an ATP-dependent protease anchored to the cytoplasmic membrane of bacteria (1, 2), can dislocate proteins from the membrane, unfold them in an ATP-dependent manner, and processively degrade the proteins (reviewed in reference 3). FtsH is essential to the viability of Escherichia coli, Bradyrhizobium japonicum, and Helicobacter pylori (4–6), while in other bacteria, FtsH depletion reduces cell viability under stress conditions or in the stationary phase but is dispensable during growth under normal, physiological conditions (7–9). In addition to membrane protein quality control, FtsH mediates a variety of other cellular functions by controlling degradation of specific substrates. These functions have been more fully characterized in E. coli, where some cytoplasmic proteins are also substrates for FtsH degradation, including the heat shock response regulator σ32 (also degraded by other proteases) (10) and λcII, a lambda phage regulator contributing to the decision between a lysogenic and a lytic life cycle (11). LpxC, an enzyme necessary for lipid A biosynthesis, is proteolytically controlled by FtsH. Depletion of cellular FtsH levels creates an imbalance between the synthesis of lipopolysaccharides and phospholipids and results in cell death, therefore making FtsH essential to E. coli (4). Although the full range of FtsH substrates remains unknown, several membrane protein targets of FtsH have been identified and include SecY, a subunit of the protein translocation machinery (12), KdtA, a transferase involved in oligosaccharide biosynthesis (13), and YccA, a membrane protein of unknown function (14).
Although the regulation and substrate range of FtsH have not been fully elucidated, even in E. coli, two modulators of the protease have been identified. The cytoplasmic membrane proteins HflK and HflC (here, HflK/C) form a high-molecular-weight complex with the protease, referred to as the FtsH holoenzyme (12, 15). Several reports indicate that HflK/C acts as a negative regulator of membrane substrates, selectively allowing specific membrane proteins access to the active site chamber of FtsH (11, 12, 14). Although these results are complex and not completely understood, evidence suggests that entry of cytoplasmic substrates into the FtsH protease may occur through a separate process (14). Unlike FtsH, HflK and HflC are not essential to E. coli and can be inactivated, with the main observable phenotype being a high frequency of lysogenization by phage λ (12).
All three proteins FtsH, HflK, and HflC, have chromosomally encoded homologs in Borrelia burgdorferi, the Lyme disease spirochete (16). B. burgdorferi is an obligate zoonotic parasite with limited metabolic capabilities which cycles between Ixodes ticks and vertebrate hosts. To survive in such disparate host environments, the spirochete differentially synthesizes various membrane proteins for sensing external stimuli, nutrient acquisition, immune evasion, and as adhesins. In addition, B. burgdorferi produces and maintains periplasmic flagella for motility, which are required for tick transmission and to disseminate and persist in the vertebrate host (17, 18). All of these functions are either embedded in or exported across the cytoplasmic membrane; therefore, membrane quality control is likely to be crucial to B. burgdorferi survival. Although the mechanism(s) by which B. burgdorferi maintains the proper functioning of cytoplasmic membrane proteins has not been examined, a library of transposon mutants identified insertions in the hflK and hflC homologs (bb0203 and bb0204, respectively) that resulted in reduced murine infectivity (19). Interestingly, only one transposon insertion at the terminus of the ftsH homolog (bb0789) was isolated, and the authors postulated that the dearth of insertions in and around the bb0789 locus indicated that the encoded FtsH homolog confers an essential function to B. burgdorferi. Recently, Drecktrah and colleagues observed that ftsH expression in B. burgdorferi was controlled by B. burgdorderi Rel (RelBbu) in response to nutrient starvation, whereas hflK and hflC transcript levels were not significantly affected (20).
To investigate the contributions of the FtsH, HflK, and HflC homologs to the life cycle of B. burgdorferi, we constructed mutants of all three genes and characterized the strains both in vitro and in vivo. Surprisingly, our results did not identify any phenotype for the ΔHflK/C double mutant, although we examined morphology, growth rate, growth under stress conditions, and the complete mouse-tick infectious cycle. In contrast, however, FtsH was shown to be essential to B. burgdorferi survival, both in vitro and in vivo. Using an inducible system to control ftsH expression, B. burgdorferi cells depleted of FtsH exhibited arrested cell growth and morphological defects during in vitro cultivation. Mouse-tick infection studies with the mutant strain demonstrated that FtsH is also required for survival in both the murine host and the tick vector. These studies indicate that the B. burgdorferi FtsH homolog provides an essential function both in vitro and in vivo, independently of the homologs of the HflK/C modulators.
RESULTS
BB0789, BB0203, and BB0204 are B. burgdorferi homologs of FtsH, HflK, and HflC, respectively.
B. burgdorferi BB0789, BB0203, and BB0204 are annotated as homologs of E. coli FtsH, HflK, and HflC in the NCBI database (16). BB0789 shares 50% identity with E. coli FtsH by amino acid sequence alignment but exhibits the highest identity within functional domains: ATPase domain (ATP binding Walker A and B motifs and the second region of homology [SRH]) and the Zn2+ protease active-site motif (3, 21) (see Fig. S1 in the supplemental material). BB0203 and BB0204 are 52% and 50% similar to E. coli HflK and HflC, respectively, with both proteins having 28% identity to their counterparts. The NCBI conserved domain search identifies BB0203 and BB0204 as members of the SPFH (stomatin, prohibitin, flotillin, and HflK/C) superfamily. BB0789, BB0203, and BB0204 are highly conserved in the B. burgdorferi sensu lato complex that causes human infection, with 95% to 98% sequence identity.
In vitro phenotypes of the HflK/C mutant are similar to that of the WT.
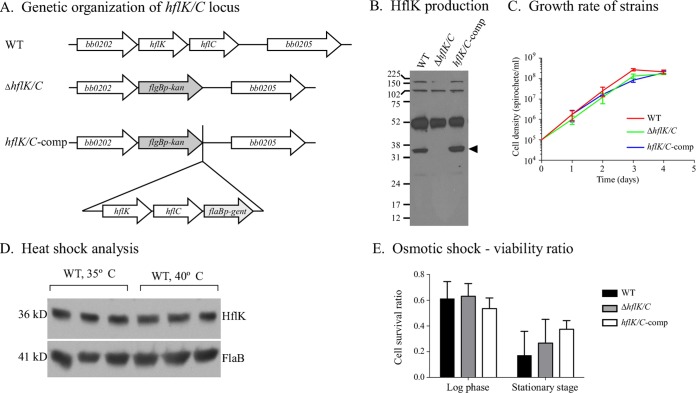
We hypothesized that FtsH function may be essential to B. burgdorferi, based on the lack of transposon insertions observed in a comprehensive signature-tagged mutagenesis library (19). Therefore, we initially focused on the modulators of FtsH substrate specificity, HflK and HflC, homologs of which are widespread among spirochetes. Using an allelic exchange vector, we generated a deletion mutant that lacked both hflK and hflC (Fig. 1A). The ΔhflK/C mutant was complemented (hflK/C-comp), and the genetic structures of both strains were confirmed by Southern blot and immunoblot analyses (Fig. 1B) (data not shown). Surprisingly, and despite extensive in vitro characterization, no distinguishing phenotypes were observed for the ΔhflK/C strain compared to the wild-type (WT) or complemented strains. The deletion mutant’s growth rate, protein profile (determined by Coomassie-stained SDS-PAGE, and immunoblots reacted with infected mouse sera), plating efficiency, length of time for colony formation on solid medium, and morphology (as assessed by electron microscopy) were similar to those of the WT (Fig. 1C) (data not shown).
FIG 1 .
Phenotypic characterization of the ΔhflK/C mutant. (A) Genetic organization of the WT, mutant, and complemented strains at the hflK and hflC loci. (B) Immunoblot analysis of HflK synthesis by different B. burgdorferi strains. Antiserum raised against B. burgdorferi HflK reacts with a protein of the appropriate size (36 kDa [arrowhead]) from whole-cell lysates of WT and complemented strains, but not with a lysate of the ΔhflK/C mutant. (C) In vitro growth rate of the ΔhflK/C mutant compared to the WT and hflK/C-comp strains. The mean and standard deviation (SD) are displayed. (D) Immunoblot analysis of cell lysates from WT B. burgdorferi exposed to a 40°C heat shock treatment for 1 h or maintained at the standard growth temperature (35°C). FlaB levels are shown to demonstrate equivalent protein loads. (E) Cell viability of B. burgdorferi strains subjected to 1 N NaCl osmotic stress compared to untreated spirochetes. After treatment, cells were plated in solid BSK medium, and the viability ratio was determined by counting CFU of treated compared to untreated spirochetes. SD bars are shown; no significant difference in cell viability was detected among B. burgdorferi strains at either the log phase (one-way analysis of variance [ANOVA], P = 0.2850) or stationary phase (one-way ANOVA, P = 0.1478).
In a further attempt to characterize the contribution of HflK/C to B. burgdorferi physiology, we assessed the response of the mutant, complemented, and WT strains to the environmental stresses of heat shock and osmotic shock. Previous studies demonstrated that B. burgdorferi responds to heat shock by differential production of specific proteins when the cultivation temperature is shifted from 35°C to 39 or 40°C (22–24). However, when we subjected the WT strain to either a 1- or 4-h incubation at 40°C, HflK levels remained unchanged compared to the protein levels from cells cultured at 35°C (Fig. 1D), nor did we see any difference in the relative ability of the mutant to survive a 1-h temperature shift, as assessed by plating for viable spirochetes (data not shown). Finally, we examined the response of the ΔhflK/C mutant relative to WT and hflK/C-comp strains to osmotic stress by exposing cells in different growth phases to 1 N NaCl and then determining cell viability (Fig. 1E). The number of viable spirochetes decreased after salt treatment for each strain at both the log and stationary phases. However, no significant differences in cell viability were detected among B. burgdorferi strains at either log phase or stationary phase.
The ΔhflK/C mutant persists throughout the B. burgdorferi infectious cycle.
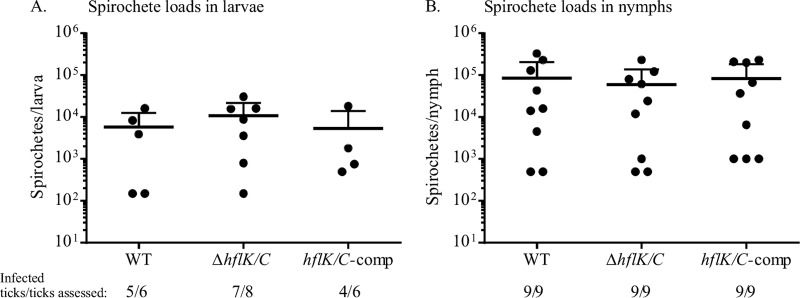
Although the ΔhflK/C mutant lacked a distinguishing phenotype in vitro, HflK and HflC function might potentially be important in vivo. Therefore, we assessed the ability of the mutant, complemented, and WT strains to complete the mouse-tick infectious cycle. However, no defect was observed in the ability to infect mice, either by needle inoculation or via tick bite (Table 1). Further, all three strains infected larval ticks and persisted through the molt at equivalent levels when Ixodes scapularis larvae were fed on infected mice, as determined by crushing fed ticks, plating, and counting CFU (Fig. 2). This result was independently confirmed by artificial infection of I. scapularis with all three strains and assessment of spirochete loads at both the larval and nymphal stages (data not shown) (25).
TABLE 1 .
Mouse infectivity of the WT, ΔhflK/C mutant, and complemented strains via needle inoculation and tick bite
| B. burgdorferi strain | Infection routea | No. of mice with reisolation from tissue/total assessedb |
No. infected/total | ||
|---|---|---|---|---|---|
| Ear | Bladder | Joint | |||
| WT | Needle injection | 14/14 | 14/14 | 14/14 | 14/14 |
| Tick bite | 2/3 | 3/3 | 3/3 | 3/3 | |
| ΔhflK/C mutant | Needle injection | 13/16 | 13/16 | 13/16 | 13/16c |
| Tick bite | 1/3 | 3/3 | 3/3 | 3/3 | |
| hflK/C-comp strain | Needle injection | 9/11 | 9/11 | 9/11 | 9/11 |
| Tick bite | 3/3 | 3/3 | 3/3 | 3/3 | |
The inocula for needle infection were 4 × 103 spirochetes injected intraperitoneally and 1 × 103 spirochetes injected subcutaneously. Cohorts of 5 to 10 infected nymphal ticks were fed on individual mice to assess transmission.
Data are the results from 3 to 4 independent experiments.
Mouse infection ratios were not significantly different between groups infected with the ΔhflK/C and WT strains (unpaired t test with Welch’s correction test, P = 0.1846).
FIG 2 .
The ΔhflK/C strain colonizes ticks as efficiently as WT B. burgdorferi. Fed larval I. scapularis ticks (A) and nymphs (B) were equally well colonized by all three B. burgdorferi strains. Larval ticks were allowed to feed on infected mice, and 8 to 10 days postfeeding, spirochete density was determined by macerating and plating individual ticks and enumerating CFU. Some fed larvae were allowed to molt to the nymphal stage and fed on naive mice, and nymphs were mechanically disrupted 10 days postfeeding and plated to determine spirochete CFU. Each point represents an individual tick, and the mean and upper standard deviation bars are shown (lower SD bars fall below the x axis). No significant difference among strains was detected using the Kruskal-Wallis test. The ratios below the graphs denote the number of ticks that had acquired B. burgdorferi relative to the number of ticks assessed for each strain.
Subtle defects that might lower the competitive fitness of a strain may not be detected in the gross laboratory assessment of the mouse-tick infectious cycle described above. Two methods of assessing fitness differences between strains were also performed. First, the doses of in vitro-grown spirochetes required to infect 50% of inoculated mice (ID50) were determined for the ΔhflK/C mutant (3.16 × 102 spirochetes) and for the WT (2.29 × 102 spirochetes) (see Table S2 in the supplemental material). These values are not significantly different from each other and agreed with the previously determined ID50 for WT B. burgdorferi strain A3 (26). Finally, mutant and WT strains were coinjected into mice at a 1:1 ratio to determine if the WT had a competitive advantage over the mutant. In three independent trials, each composed of 5 mice, neither strain consistently outperformed the other, as determined by sensitivity to the appropriate antibiotic (see Table S3 in the supplemental material). Further, larval I. scapularis ticks were artificially infected by immersion in Barbour-Stoenner-Kelly II (BSKII) medium containing both strains at approximately equivalent densities to determine if the ΔhflK/C mutant had a reduced-fitness phenotype in the vector. Both strains were present in fed larvae and survived through the molt and subsequent nymph feeding at approximately equal levels, spirochete numbers were quantified by plating crushed ticks on BSKII plates, and the data were analyzed with the Mann-Whitney test (see Fig. S2 in the supplemental material). The WT and ΔhflK/C mutant were transmitted equally from the infected nymphs to naive mice, as assessed by isolation from mouse tissues (data not shown). These results indicate that inactivation of bb0203 (hflK) and bb0204 (hflC) does not detectably decrease the fitness of B. burgdorferi during tick colonization, persistence through the nymphal stage, or transmission to the murine host.
Generation and characterization of an inducible ftsH mutant.
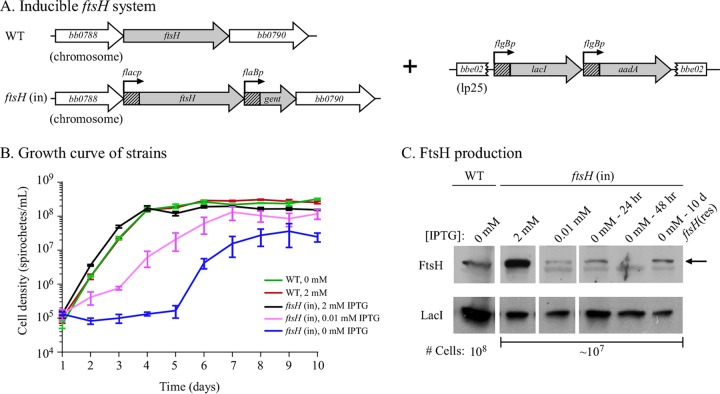
We attempted to delete bb0789 (the ftsH homolog) from the WT strain, B31-S9, in 6 independent transformations, but were unable to obtain a deletion mutant, supporting the hypothesis that FtsH function is essential to B. burgdorferi. Therefore, we generated an inducible ftsH mutant using the lac promoter/repressor system developed for B. burgdorferi (Fig. 3A) (27). Following the example of Gilbert et al., the lacI gene and a streptomycin resistance cassette were inserted into the bbe02 locus of the A3-68 WT strain, and the resulting strain was designated B31-68-LS (27). An allelic exchange construct that fused the inducible promoter, flacp, to ftsH and was linked to a gentamicin resistance marker was used to replace the endogenous ftsH open reading frame, and the resulting strain was designated the ftsH(in) strain. The structure of the inducible ftsH locus was confirmed by PCR (data not shown), and cell growth was shown to be dependent upon induction (Fig. 3B). Immunoblots confirmed LacI production and isopropyl-β-d-thiogalactopyranoside (IPTG)-controlled synthesis of FtsH (Fig. 3C).
FIG 3 .
FtsH is required for B. burgdorferi cell growth. (A) Genetic organization of an ftsH-inducible strain and the corresponding WT strain. The lacI repressor was inserted into the bbe02 locus on linear plasmid lp25 along with the streptomycin-resistance gene (aadA). The IPTG-inducible promoter (flacp) was derived from the B. burgdorferi flgB promoter with the 20-nucleotide lac operator sequence inserted (27). flaBp, B. burgdorferi flaB promoter. (B) Growth curve of the B. burgdorferi WT (B31-68-LS) and ftsH(in) strains. The IPTG-inducible ftsH(in) strain demonstrated a growth-dependent response to the concentration of IPTG in the medium. Cell numbers were monitored daily by dark-field microscopy using a Petroff-Hauser counting chamber. (C) FtsH protein levels reflect the IPTG concentration in the ftsH(in) strain. An arrow indicates the FtsH protein signal as determined by immunoblot analysis. The IPTG concentration is shown above the immunoblots along with the time post-IPTG depletion. LacI levels are shown to demonstrate relative protein loads. The number of cells loaded in each lane is indicated below the corresponding immunoblots. Note that the immunoblot corresponding to the WT strain contained approximately 10-fold more cell lysate relative to the other immunoblots. The faint band below the FtsH protein is due to nonspecific binding of the antibody to an unrelated protein, as it is present at the same level at all IPTG concentrations.
The growth rate of the parental B31-68-LS strain was not affected by the addition of 2 mM IPTG (Fig. 3B). However, the growth rate of the ftsH(in) strain was dependent on the concentration of IPTG added to the BSKII medium, growing normally at concentrations ranging from 0.1 mM up to 10 mM (Fig. 3B) (data not shown), but displaying a lower growth rate at or below IPTG concentrations of 0.01 mM. Immunoblot detection of FtsH showed that the ftsH(in) strain grown at concentrations of 1 mM IPTG and above produced significantly more FtsH than the parental B31-68-LS (WT) strain (Fig. 3C), but excess FtsH did not seem to adversely affect growth rate. When IPTG was depleted from the ftsH(in) culture, cell growth was arrested. Between 48 and 72 h post-IPTG depletion, FtsH levels were undetectable (Fig. 3C), spirochetes became nonmotile, and large membrane distortions developed (Fig. 4). By 72 h postdepletion, >99% of the cells were no longer viable. Strikingly, motile cells were again detected in these cultures around day 6 post-IPTG depletion, and a near-normal growth rate resumed (Fig. 3B). When examined at 10 days post-IPTG depletion, FtsH was again detectable (Fig. 3C), despite the absence of any inducing chemical. We designated this uncloned outgrowth genotype ftsH(res), for restored production of FtsH. These results were confirmed in three independent experiments.
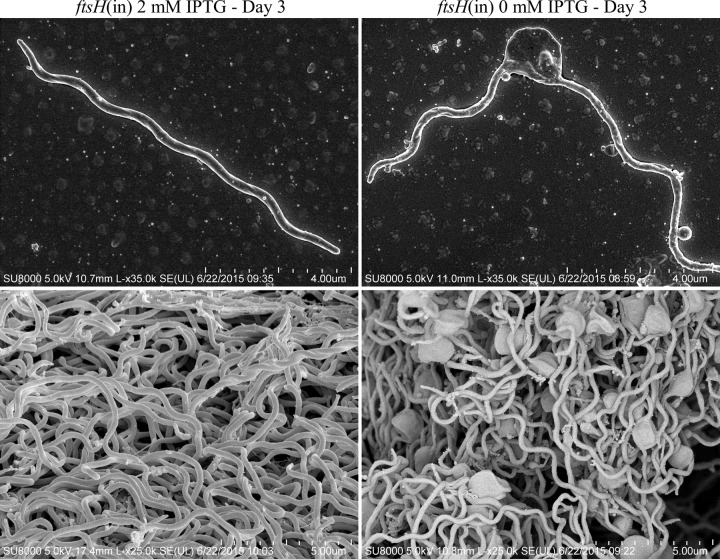
FIG 4 .
Morphology of FtsH-depleted B. burgdorferi cells compared to FtsH+ cells. Shown are representative scanning electron micrographs of the B. burgdorferi ftsH(in) strain grown for 3 days either in the presence of 2 mM IPTG and producing FtsH (left panel) or in the absence of IPTG, resulting in FtsH-depleted cells (right panel). Large membrane blebs are evident in FtsH-depleted cells.
Resumption of FtsH production might theoretically result from a suppressor mutation that eliminates LacI activity or LacI protein or from a mutation in the 20-nucleotide lac operator sequence inserted into the normally constitutive flgB promoter (27). A deletion or a base mutation in the short operator sequence might decrease the affinity of the LacI repressor for the altered sequence, freeing the promoter to allow transcription and translation to occur, and thereby restoring FtsH production. We tested these hypotheses by examining LacI protein levels from cell lysates obtained at days 2 and 10 post-IPTG depletion. Immunoblots that reacted with an anti-LacI antibody detected constitutive levels of the repressor at both time points, indicating that the suppressor mutation had not reduced or eliminated LacI levels. Sequencing lacI from 7 clones isolated from the ftsH(res) outgrowth strain did not detect any nucleotide changes from the original lacI gene used to transform B. burgdorferi, providing further evidence that the nature of the suppressor mutation was not related to LacI activity or production. Therefore, we determined the lac operator sequence from 9 clones isolated from the ftsH(res) culture. All clones contained a single transversion that altered a G to a T in the lac operator sequence (Fig. 5), potentially decreasing the binding affinity of the LacI repressor for the altered sequence.
FIG 5 .
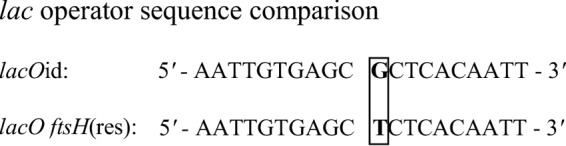
A mutation in the lac operator sequence may permit resumption of FtsH production. The ideal lac operator sequence (lacOid) used for construction of the ftsH(in) strain is symmetrical (49). A transversion mutation (boxed) in the lac operator isolated from ftsH(res) might allow production of FtsH to resume, independent of any inducer, as demonstrated in Fig. 3B and C.
The requirement for FtsH function in vitro indicated that an essential substrate(s) is processed during growth in BSKII medium. B. burgdorferi cells lacking FtsH display membrane defects after 48 h and are not viable after 72 h, potentially identifying a window in which FtsH substrates might accumulate and be visible on protein gels. Therefore, we compared WT cell lysate to that of the ftsH(in) strain grown in the presence of 1 mM IPTG (overproduction of FtsH) and to that of the ftsH(in) strain incubated for 48 h in the absence of inducer (FtsH depleted). However, no discrete differences in protein profiles among samples were observed by either Coomassie-stained or silver-stained gels (data not shown).
FtsH is required for infection of both mice and ticks.
Although we demonstrated that FtsH is required for in vitro survival of B. burgdorferi, it is possible that other proteins may be able to compensate for the loss of this protease during infection. To assess the requirement for FtsH in vivo, we inoculated mice with the ftsH(in) strain cultured in vitro with 1 mM IPTG, to ensure FtsH production and cell viability of the inoculum. The ftsH(in) strain was unable to establish an infection in mice, nor did the strain survive long enough to elicit an immune response, although all mice inoculated with WT B. burgdorferi became infected (Table 2). We also tested the ftsH(res) outgrowth strain, which has IPTG-independent expression of FtsH. Interestingly, this strain successfully established infection in 4 of 5 mice and was reisolated from all tissues examined from the 4 infected mice.
TABLE 2 .
Infectivity of the WT, ftsH(in), and ftsH(res) strains in mice
| B. burgdorferi straina | No. of mice with reisolation from tissue/total assessedb |
No. infected/total | ||
|---|---|---|---|---|
| Ear | Bladder | Joint | ||
| A3-68-LS | 5/5 | 5/5 | 5/5 | 5/5 |
| ftsH(in) | 0/5 | 0/5 | 0/5 | 0/5 |
| ftsH(res)b | 5/5 | 5/5 | 5/5 | 5/5 |
The inocula for needle infection were 8 × 103 spirochetes injected intraperitoneally and 2 × 103 spirochetes injected subcutaneously.
Day 10 outgrowth was isolated from the ftsH(in) strain cultured in the absence of IPTG and designated the ftsH(res) strain. A spontaneous mutation in the lac operator sequence presumably allowed for resumption of FtsH production and cell growth.
Since the ftsH(in) strain was not able to infect mice, we assessed the strain’s ability to infect and persist in Ixodes ticks by artificially infecting larvae by immersion in media containing either the WT or ftsH(in) strain. The immersion inocula were plated, and the WT and ftsH(in) strains were shown to be at similar concentrations: 5.6 × 107 and 5.0 × 107 spirochetes/ml, respectively. Artificially infected larvae were subsequently fed to repletion on naive mice, and spirochete burdens were again assessed directly by mechanical disruption of the ticks in BSK medium and plating in solid medium to enumerate viable colony-forming units. Eight of seventeen ticks (47%) exposed to WT B. burgdorferi were infected, and the spirochete burden/infected larvae was 24,600 ± 20,359 (mean ± standard deviation [SD]; n = 6). In contrast, none of 17 larvae exposed to the ftsH(in) strain contained viable spirochetes after the blood meal. An additional 10 ticks exposed to the ftsH(in) strain were assessed for infection after the molt to the nymphal stage, and again, no viable spirochetes were detected. Overall, the data indicate that FtsH provides an essential function necessary for B. burgdorferi viability throughout the mouse-tick infectious cycle.
DISCUSSION
B. burgdorferi, an obligate parasite, transitions between arthropod vectors and mammalian hosts, environmental conditions that are substantially different from each other. For example, nutrients, cell density, pH, and temperature all shift between hosts, and many of these cues result in production of different proteins (28–32). To be a successful pathogen, B. burgdorferi must respond to these rapid environmental changes by controlling both protein turnover and proper functioning of membrane processes, such as protein and nutrient transport, environmental sensing/signaling, and flagellar motor function. In other bacteria, the FtsH protease contributes to these crucial cellular functions (reviewed in reference 33).
A homolog of FtsH has been identified in B. burgdorferi (16) (see Fig. S1 in the supplemental material), but has not been previously studied in this spirochete. Construction and use of an inducible FtsH strain demonstrated that FtsH is essential to B. burgdorferi survival during in vitro cultivation. When cellular levels of FtsH were depleted, occurring between 24 and 48 h after removal of inducer (Fig. 3C), growth was arrested, and within 72 h, membrane deformations were clearly visible (Fig. 4). Although substrates of the B. burgdorferi FtsH have not been identified, the morphology of FtsH-negative cells suggests that a substrate of this protease contributes to membrane architecture. The substrate(s) that requires FtsH processing for B. burgdorferi viability presents an intriguing area of research for future investigation.
Surprisingly, viable cells were again detected on day 6 post-IPTG depletion. These cells were producing FtsH, when assessed by immunoblotting on day 10 (Fig. 3C), despite the absence of any inducer. Investigating the nature of the presumptive suppressor mutation in the ftsH(res) outgrowth strain, we identified a single-base mutation (a transversion of G to T) in the lac operator sequence (Fig. 5). In studies of the wild-type Lac repressor/operator sequence, this single-base mutation at the central nucleotide reduced the LacI binding affinity for the operator by 99% (34). However, the operator sequence constructed by Gilbert et al. and used in this study contained the “ideal” lac operator sequence, which lacks the central nucleotide and is perfectly symmetrical (27). We were unable to find any studies that examined repressor binding affinities for mutations in the ideal lac operator, but the transversion identified here would reduce the number of bases conferring symmetry. This result reinforces the essential nature of the B. burgdorferi FtsH by demonstrating that only cells with a secondary mutation (i.e., allowing FtsH production) are viable.
In another assessment of the requirement for FtsH, mice injected with the ftsH(in) strain did not become infected or seroconvert, whereas both the WT and the ftsH(res) strain in the outgrowth culture were infectious in mice (Table 2). The combined in vitro, murine, and tick infectivity data demonstrate that FtsH is essential to B. burgdorferi survival both in liquid culture and throughout the infectious cycle. Further, strict regulation of FtsH production does not seem to be required, at least under the conditions examined in this study, which seems surprising for a protein with an essential role in cell survival. Production of FtsH in the outgrowth culture was enough to restore infectivity, despite the fact that ftsH was no longer under the control of its native promoter. Equally surprising was the finding that overproduction of FtsH by increasing IPTG levels above 0.1 mM did not have any detectable adverse effects on B. burgdorferi growth in vitro, suggesting that excess FtsH does not proteolytically overwhelm its substrates.
In contrast to the FtsH results, we did not detect any phenotype for cells lacking HflK and HflC. In E. coli, these proteins act to modulate FtsH substrate recognition (11, 12, 14), and homologs of these accessory proteins are widespread among spirochetes, although not ubiquitous in prokaryotes. Recently, Toledo et al. demonstrated that FtsH, HflK, and HflC are present in membrane lipid rafts of B. burgdorferi (35), consistent with previous findings in E. coli, where these three proteins form a large, multimeric inner membrane complex (12, 15). Potentially, HflK and HflC regulate the accessibility of minor membrane substrates to the FtsH protease, and the processing of these substrates is not significant enough to present a phenotype in the laboratory setting but in nature would provide a selective advantage. Our ID50 results and mouse-tick competition studies indicate this is an unlikely scenario, but we are unable to eliminate this possibility. Alternatively, the B. burgdorferi HflK and HflC proteins may have evolved a function different from that of the E. coli homologs and perhaps separate from FtsH function, and we have not yet identified the conditions under which these proteins provide a beneficial function in B. burgdorferi.
Further investigation to identify the substrates of FtsH should provide insight into the essential nature of this protease and may indicate specific targets that inhibit B. burgdorferi viability. Additional characterization of FtsH-HflK-HflC function will undoubtedly be useful in identifying interesting mechanisms of this spirochete’s physiology and obligate pathogenic life cycle.
MATERIALS AND METHODS
Ethics statement.
All animal work was performed according to the guidelines of the National Institutes of Health, Public Health Service Policy on Humane Care and Use of Laboratory Animals (36), and the United States Institute of Laboratory Animal Resources, National Research Council, Guide for the Care and Use of Laboratory Animals (37). Protocols were approved by the Rocky Mountain Laboratories, NIAID, NIH Animal Care and Use Committee. The Rocky Mountain Laboratories are accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All efforts to minimize animal suffering were made.
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table S1 in the supplemental material. B. burgdorferi strains were grown in liquid Barbour-Stoenner-Kelly II (BSKII) medium (38, 39) supplemented with 6% rabbit serum (PelFreez Biologicals, Rogers, AZ) and appropriate antibiotics (kanamycin, 200 µg/ml; streptomycin, 50 µg/ml; gentamicin, 40 µg/ml). Cloning vectors were propagated using E. coli strain TOP10 (Invitrogen, Carlsbad, CA) or DH5α (New England Biolabs, Ipswich, MA).
Sequence analysis.
MacVector version 14.0.3 (MacVector, Inc., Apex, NC) was used to align amino acid sequences with ClustalW. The conserved domains of BB0789, BB0203 and BB0204 were searched and analyzed in the CDD database (CDD v3.12), a conserved domain database for the functional annotation of proteins (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Generation of BB0203 and BB0789 antisera.
Protein BB0203 lacking the predicted transmembrane helix domain (matching residues 1 to 35 of BB0203 protein) and a 16-amino-acid peptide matching residues 317 to 332 of BB0789 were commercially synthesized (GenScript, Piscataway, NJ). The 16-amino-acid peptide region is relatively well conserved among eubacteria and was originally described by Tomoyasu et al. (1). BB0203-specific and BB0789-specific antisera were generated by immunizing 1-year-old female New Zealand White rabbits with the corresponding protein in the Rocky Mountain Laboratories Animal Unit.
Construction of mutant strains.
All B. burgdorferi transformations were performed similarly: 10 µg of plasmid DNA was electroporated into wild-type strain B31-S9 (or specified strains). All B. burgdorferi transformants described were confirmed by PCR and Southern blot analysis, and plasmid content was determined as previously described (40, 41).
All primers used in this study are listed in Table S4 in the supplemental material. The allelic exchange construct for generation of the ΔhflK/C mutant (Fig. 1A) was constructed by PCR amplification of a 561-bp upstream region of bb0203 (hflK) and a 619-bp downstream region of bb0204 (hflC) from B31-S9 genomic DNA. The DNA fragments were cloned flanking the kanamycin resistance cassette driven by the B. burgdorferi flgB promoter (42) in pCR-XL-TOPO (Invitrogen). The resulting vector, designated pTAKO.bb0203/0204, was confirmed by sequencing and digestion with the appropriate restriction enzymes.
The mutant ΔhflK/C strain was complemented by inserting hflK and hflC into the native chromosomal locus adjacent to the deletion mutation (Fig. 1A). In brief, a DNA fragment containing hflK and hflC with 232 bp of the putative native promoter region was PCR amplified and sequenced to confirm nucleotide fidelity. This fragment was cloned into the allelic exchange vector (described above) with the gentamicin resistance cassette driven by the flaB promoter (43), creating vector pTAComple.bb0203/0204, and inserted after the kanamycin resistance cassette.
We attempted to isolate a deletion mutant of ftsH using an approach similar to that described above for the ΔhflK/C mutant. However, after 6 independent electroporations, we were unable to obtain a deletion mutant of ftsH, although this approach worked successfully for obtaining the HflK/C mutant. Therefore, we focused on constructing an FtsH-inducible strain.
The B. burgdorferi IPTG-inducible promoter system constructed by Gilbert et al. (27) was used to generate an inducible ftsH (bb0789) mutant. Constructs pTAflacp and pBBE02::lacI-Strepr were generously provided by Dan Drecktrah and Scott Samuels (see Table S1 in the supplemental material). Plasmid pBBE02::lacI-Strepr was used to construct a LacI-producing strain in an infectious background by transformation into B. burgdorferi clone B31 A3-68; the resulting strain was designated B31-68-LS (see Table S1). LacI production in B31-68-LS was confirmed by immunoblot analysis with an antibody raised against LacI (Rockland Immunochemicals, Pottstown, PA). The vector for generating an inducible bb0789 mutant was constructed by amplifying 646 nucleotides of the 3′ region of bb0788 from wild-type genomic DNA and cloning it upstream of the inducible promoter in plasmid pTAflacp. The bb0789 coding region was amplified and cloned downstream of the flac promoter to generate plasmid pTAbb0788-flacp-bb0789. The downstream flanking region (bb0790) and selectable marker were assembled in pCR-XL-TOPO. First, the flaBp-gentamicin resistance cassette was amplified and cloned into pCR-XL-TOPO. Subsequently, 672 nucleotides of the 5′ region of bb0790 was inserted downstream of the gentamicin marker to produce plasmid pTAflaBp-gent-bb0790. The bb0788-flacp-bb0789 fragment from pTAbb0788-flacp-bb0789 was subcloned into plasmid pTAflaBp-gent-bb0790 to generate an allelic exchange vector, pTAindu.bb0789. B31-68-LS was electroporated with pTAindu.bb0789 and selected on solid medium for gentamicin resistance in the presence of 2 mM IPTG to generate the inducible ftsH(in) strain (Fig. 3A). Transformants were confirmed by amplifying the gentamicin resistance cassette and the flacp promoter upstream of bb0789.
The IPTG-dependent growth of the ftsH(in) strain was assessed by culture in the presence and absence of IPTG. Spirochetes grown in medium supplemented with 2 mM IPTG and appropriate antibiotics were harvested and washed twice in BSK-H medium (Sigma-Aldrich, Atlanta, GA). Cells were resuspended in fresh BSKII medium to a density of 1 × 105 spirochetes/ml and supplemented with IPTG to final concentrations of 0.01, 0.1, 2, 5, or 10 mM or without IPTG. Growth of the parental strain B31-68-LS was also assessed with either 2 mM IPTG or without IPTG. Cultures were grown in triplicate for statistical accuracy, and the entire growth analysis was repeated independently. Cell densities were monitored every 24 h by dark-field microscopy using a Petroff-Hausser chamber.
To confirm IPTG-dependent regulation of BB0789 in the ftsH(in) strain, cell lysates from the growth assays were assessed by immunoblot analysis using an antibody raised against an FtsH peptide (described above). Spirochetes inoculated with 0.01, 0.1, 2, or 10 mM IPTG were harvested when cell densities reached approximately 1 × 108 spirochetes/ml. Cell lysates were prepared for immunoblot analysis using rabbit anti-BB0789 antiserum (1:1,000 dilution) and anti-LacI (rabbit) antibody (1:5,000 dilution).
Experimental mouse-tick infection studies.
Mouse infection studies were performed with 6- to 8-week-old female RML mice, an outbred strain of Swiss-Webster mice reared at the Rocky Mountain Laboratories breeding facility. Mice were inoculated intraperitoneally (4 × 103 spirochetes) and subcutaneously (1 × 103 spirochetes). The number of injected spirochetes was confirmed by plating an aliquot of the inoculum and determining CFU. Approximately 20 B. burgdorferi colonies per strain were screened by PCR to confirm the presence of plasmids lp25, lp28-1, and lp36, which are somewhat unstable in vitro but required for B. burgdorferi infectivity (41, 44). Mouse infection was assessed at 3 weeks postinjection by mouse seroconversion to B. burgdorferi antigens and isolation of spirochetes from mouse tissues (ear, bladder, and ankle joint) in liquid culture.
Approximately 100 to 200 naive I. scapularis larvae were fed to repletion on each infected mouse. Acquisition of B. burgdorferi by larval ticks was assessed 8 to 10 days postfeeding, and spirochete load was enumerated by mechanical disruption and plating. Basically, individual ticks were placed in a 1.5-ml Eppendorf tube containing 0.5 ml BSKII medium and crushed with a sterile, disposable pestle. After disruption of the tick, an additional 0.5 ml of medium was added, and aliquots were plated in solid BSK medium with appropriate antibiotics. Where relevant, the remaining I. scapularis larvae were allowed to molt to nymphs, and cohorts of 5 to 10 nymphs were fed on individual naive mice to assess B. burgdorferi transmission. Fed nymphs were mechanically disrupted 10 days postfeeding and plated in solid BSK medium to determine spirochete loads.
The relative fitness of B. burgdorferi strains was determined in three independent mouse coinfection studies and also within ticks by artificial infection. The inoculum per mouse was an equal mixture of two strains (i.e., the ΔhflK/C mutant to WT, or the complemented strain to WT) with 5 × 103 or 1 × 104 spirochetes. An aliquot of the inoculum was plated with or without antibiotic selection to determine the actual ratio of strains. Mouse infection was determined by seroreactivity and confirmed by spirochete isolation from mouse tissues (ear, bladder, and ankle joint) at 4, 8, or 10 weeks postinoculation. The ratio of the two strains in mouse tissues was determined by plating spirochetes in solid BSK medium (with or without antibiotic selection) and enumerating CFU and/or by PCR screening of the B. burgdorferi colonies from plates lacking antibiotics.
Artificial tick coinfection was previously described (25, 26). Briefly, about 200 I. scapularis larvae were artificially infected by immersion in a B. burgdorferi culture. To determine the relative fitnesses of two strains, equal numbers of ΔhflK/C and wild-type cells or the complemented strain and wild type at a combined density of ~1 × 108 spirochetes/ml were mixed together and used for immersion of ticks. The ratio of each strain in the mixture was determined by plating in solid medium, as described above. Each cohort of artificially infected larvae was fed to repletion on naive RML mice and allowed to molt to nymphs. The nymphs were subsequently fed to repletion on naive mice. The ratio of B. burgdorferi strains within ticks was determined by mechanically disrupting the ticks and plating with or without antibiotic selection.
Determination of ID50.
The ID50s for B31-S9 and the ΔhflK/C strain were assessed by inoculating mice with 10-fold serial dilutions from 105 to 102 spirochetes for each strain. Six mice were inoculated per dose. The actual number of injected viable spirochetes was determined by plating a portion of the inoculum and enumerating CFU. Mouse infection was assessed by B. burgdorferi isolation from mouse tissues at 4 weeks postinoculation. The ID50 value for each strain was calculated according to the method of Reed and Muench (45).
In vitro phenotype analysis of the ΔhflK/C mutant.
To assess any in vitro phenotypes of ΔhflK/C strain, the growth rate, morphology, and protein profile of the mutant strain were compared to those of the wild type, B31-S9, and the complemented strain. The growth rate was determined by inoculating triplicate 5-ml BSKII cultures with 1 × 105 spirochetes/ml and incubating the cultures at 35°C. Cell densities were monitored every 24 h by dark-field microscopy using a Petroff-Hausser chamber. Morphological characteristics of B. burgdorferi strains were observed by scanning electron microscopy, as previously described (46). The protein profiles and antigenic profiles of all three strains were determined by SDS-PAGE and immunoblot analysis using pooled sera from B. burgdorferi-infected mice as previously described (26, 47).
Osmotic shock and heat stress assays were performed similarly to compare the response of the ΔhflK/C strain to that of the WT and complemented strains. These three strains were individually inoculated in triplicate at an initial concentration of 1 × 105 spirochetes/ml, and each was subdivided into 2 portions when cultures reached a cell density of approximately 5 × 107 spirochetes/ml (mid-log phase) or 2 × 108 spirochetes/ml (stationary phase). For the osmotic shock assay, one set of cultures was treated with 1 N NaCl for 10 min for mid-log-phase cultures or 40 min for stationary-phase cultures, similar to the protocol of Elias et al. (48). Control cultures were supplemented with an equivalent volume of BSK medium. After treatment, spirochetes were immediately plated in solid BSK medium, and CFU were subsequently counted to assess cell viability under salt stress by calculating the ratio of CFU with salt treatment relative to that without treatment. The heat shock assays were set up as described above, except that one set of B. burgdorferi cultures was incubated for 1 h at 40°C, a temperature demonstrated to induce a heat shock response in B. burgdorferi (22–24), and the other was kept at 35°C for 1 h as a control. After treatment, equivalent numbers of spirochetes were harvested, and cell lysates were prepared for immunoblot analysis using rabbit anti-BB0203 antiserum (1:2,000 dilution). The experiment was repeated with the heat shock treatment extended to 4 h.
SUPPLEMENTAL MATERIAL
Sequence alignment of E. coli and B. burgdorferi FtsH homologs. Homologs share 50% identity, and identical residues are indicated in boldface type and shaded. Red boxes mark conserved AAA family motifs relative to the E. coli FtsH sequence (21). Walker A and B, ATP binding and hydrolysis motifs; Pore, substrate entry pore; SRH, second region of homology; Arg-finger, arginine finger; Zn2+ AS, zinc protease active-site motif; Edge, substrate-binding edge strand. The FtsH homologs are E. coli strain K-12 FtsH (CDJ73685.1) and B. burgdorferi strain B31 BB0789 (NP_212923.1). Download
Tick coinfection results. Naive larvae were artificially coinfected by immersion in a culture containing the ΔhflK/C and WT strains (left panel) or the hflK/C-comp and WT strains (right panel). Ticks were mechanically disrupted in an Eppendorf tube with a disposable pestle and individually plated. Each symbol represents the ratio of mutant or complemented strain to WT strain in an individual tick. The dotted lines indicate the ratio of mutant or complemented strains to the WT strain in the immersion culture. Values above the line indicate a predominance of the mutant or complemented strains, while values below the line indicate a predominance of the WT strain. Bars represent the median and interquartile range of the values. One data point for the unfed nymphs in the complement/WT graph is not shown, as the value, 121, was far outside the range of this figure. No significant difference was observed between groups, as determined by the Mann-Whitney test. Download
Bacterial strains and plasmids used in this study.
Determination of ID50 for the WT and ΔhflK/C strains.
Mouse coinfection studies. Mice were coinfected with approximately equal numbers of the WT strain and either the ΔhflK/C mutant or complemented strain. Mouse infection was initially assessed at about 1 week postinoculation by ear-punch culture and subsequently confirmed by spirochete isolation from mouse tissues (ear, bladder, and ankle joint) at 4, 8, or 10 weeks postinoculation. In three independent experiments, no strain consistently predominated, indicating that no strain had a competitive advantage.
Primers used in this study.
ACKNOWLEDGMENTS
We gratefully thank Teru Ogura for supplying antisera, Anita Mora for graphical expertise, and Paul Beare, Kendal Cooper, Kit Tilly, and Bharti Bhatia for thoughtful comments on the manuscript.
This research was supported by the Intramural Research Program of the NIAID, NIH.
Footnotes
Citation Chu C-Y, Stewart PE, Bestor A, Hansen B, Lin T, Gao L, Norris SJ, Rosa PA. 2016. Function of the Borrelia burgdorferi FtsH homolog is essential for viability both in vitro and in vivo and independent of HflK/C. mBio 7(2):e00404-16. doi:10.1128/mBio.00404-16.
REFERENCES
- 1.Tomoyasu T, Yamanaka K, Murata K, Suzaki T, Bouloc P, Kato A, Niki H, Hiraga S, Ogura T. 1993. Topology and subcellular localization of FtsH protein in Escherichia coli. J Bacteriol 175:1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomoyasu T, Yuki T, Morimura S, Mori H, Yamanaka K, Niki H, Hiraga S, Ogura T. 1993. The Escherichia coli FtsH protein is a prokaryotic member of a protein family of putative ATPases involved in membrane functions, cell cycle control, and gene expression. J Bacteriol 175:1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito K, Akiyama Y. 2005. Cellular functions, mechanism of action, and regulation of FtsH protease. Annu Rev Microbiol 59:211–231. doi: 10.1146/annurev.micro.59.030804.121316. [DOI] [PubMed] [Google Scholar]
- 4.Ogura T, Inoue K, Tatsuta T, Suzaki T, Karata K, Young K, Su LH, Fierke CA, Jackman JE, Raetz CR, Coleman J, Tomoyasu T, Matsuzawa H. 1999. Balanced biosynthesis of major membrane components through regulated degradation of the committed enzyme of lipid A biosynthesis by the AAA protease FtsH (HflB) in Escherichia coli. Mol Microbiol 31:833–844. doi: 10.1046/j.1365-2958.1999.01221.x. [DOI] [PubMed] [Google Scholar]
- 5.Narberhaus F, Urech C, Hennecke H. 1999. Characterization of the Bradyrhizobium japonicum ftsH gene and its product. J Bacteriol 181:7394–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge Z, Taylor DE. 1996. Sequencing, expression, and genetic characterization of the Helicobacter pylori ftsH gene encoding a protein homologous to members of a novel putative ATPase family. J Bacteriol 178:6151–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer B, Rummel G, Aldridge P, Jenal U. 2002. The FtsH protease is involved in development, stress response and heat shock control in Caulobacter crescentus. Mol Microbiol 44:461–478. doi: 10.1046/j.1365-2958.2002.02887.x. [DOI] [PubMed] [Google Scholar]
- 8.Deuerling E, Mogk A, Richter C, Purucker M, Schumann W. 1997. The ftsH gene of Bacillus subtilis is involved in major cellular processes such as sporulation, stress adaptation and secretion. Mol Microbiol 23:921–933. doi: 10.1046/j.1365-2958.1997.2721636.x. [DOI] [PubMed] [Google Scholar]
- 9.Fiocco D, Collins M, Muscariello L, Hols P, Kleerebezem M, Msadek T, Spano G. 2009. The Lactobacillus plantarum ftsH gene is a novel member of the CtsR stress response regulon. J Bacteriol 191:1688–1694. doi: 10.1128/JB.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rutman AJ, Oppenheim AB, Yura T, Yamanaka K, Niki H, et al.. 1995. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat-shock transcription factor sigma 32. EMBO J 14:2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kihara A, Akiyama Y, Ito K. 1997. Host regulation of lysogenic decision in bacteriophage lambda: transmembrane modulation of FtsH (HflB), the cII degrading protease, by HflKC (HflA). Proc Natl Acad Sci U S A 94:5544–5549. doi: 10.1073/pnas.94.11.5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kihara A, Akiyama Y, Ito K. 1996. A protease complex in the Escherichia coli plasma membrane: HflKC (HflA) forms a complex with FtsH (HflB), regulating its proteolytic activity against SecY. EMBO J 15:6122–6131. [PMC free article] [PubMed] [Google Scholar]
- 13.Katz C, Ron EZ. 2008. Dual role of FtsH in regulating lipopolysaccharide biosynthesis in Escherichia coli. J Bacteriol 190:7117–7122. doi: 10.1128/JB.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kihara A, Akiyama Y, Ito K. 1998. Different pathways for protein degradation by the FtsH/HflKC membrane-embedded protease complex: an implication from the interference by a mutant form of a new substrate protein, YccA. J Mol Biol 279:175–188. doi: 10.1006/jmbi.1998.1781. [DOI] [PubMed] [Google Scholar]
- 15.Saikawa N, Akiyama Y, Ito K. 2004. FtsH exists as an exceptionally large complex containing HflKC in the plasma membrane of Escherichia coli. J Struct Biol 146:123–129. doi: 10.1016/j.jsb.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb J-F, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidmann J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fujii C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 17.Malawista SE, de Boisfleury Chevance A. 2008. Clocking the Lyme spirochete. PLoS One 3:e1633. doi: 10.1371/journal.pone.0001633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sultan SZ, Manne A, Stewart PE, Bestor A, Rosa PA, Charon NW, Motaleb MA. 2013. Motility is crucial for the infectious life cycle of Borrelia burgdorferi. Infect Immun 81:2012–2021. doi: 10.1128/IAI.01228-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, Chaconas G, Philipp MT, Norris SJ. 2012. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One 7:e47532. doi: 10.1371/journal.pone.0047532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drecktrah D, Lybecker M, Popitsch N, Rescheneder P, Hall LS, Samuels DS. 2015. The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLoS Pathog 11:e1005160. doi: 10.1371/journal.ppat.1005160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langklotz S, Baumann U, Narberhaus F. 2012. Structure and function of the bacterial AAA protease FtsH. Biochim Biophys Acta 1823:40–48. doi: 10.1016/j.bbamcr.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Carreiro MM, Laux DC, Nelson DR. 1990. Characterization of the heat shock response and identification of heat shock protein antigens of Borrelia burgdorferi. Infect Immun 58:2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cluss RG, Boothby JT. 1990. Thermoregulation of protein synthesis in Borrelia burgdorferi. Infect Immun 58:1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cluss RG, Goel AS, Rehm HL, Schoenecker JG, Boothby JT. 1996. Coordinate synthesis and turnover of heat shock proteins in Borrelia burgdorferi: degradation of DnaK during recovery from heat shock. Infect Immun 64:1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Policastro PF, Schwan TG. 2003. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J Med Entomol 40:364–370. [DOI] [PubMed] [Google Scholar]
- 26.Bestor A, Rego RO, Tilly K, Rosa PA. 2012. Competitive advantage of Borrelia burgdorferi with outer surface protein BBA03 during tick-mediated infection of the mammalian host. Infect Immun 80:3501–3511. doi: 10.1128/IAI.00521-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert MA, Morton EA, Bundle SF, Samuels DS. 2007. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol Microbiol 63:1259–1273. doi: 10.1111/j.1365-2958.2007.05593.x. [DOI] [PubMed] [Google Scholar]
- 28.Tokarz R, Anderton JM, Katona LI, Benach JL. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun 72:5419–5432. doi: 10.1128/IAI.72.9.5419-5432.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indest KJ, Ramamoorthy R, Solé M, Gilmore RD, Johnson BJ, Philipp MT. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun 65:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol 37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- 31.Carroll JA, Cordova RM, Garon CF. 2000. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect Immun 68:6677–6684. doi: 10.1128/IAI.68.12.6677-6684.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A 92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okuno T, Ogura T. 2013. FtsH protease-mediated regulation of various cellular functions, p 53–69. In Dougan DA. (ed), Regulated proteolysis in microorganisms, vol 66 Springer, Dordrecht, Netherlands. [DOI] [PubMed] [Google Scholar]
- 34.Betz JL, Sasmor HM, Buck F, Insley MY, Caruthers MH. 1986. Base substitution mutants of the lac operator: in vivo and in vitro affinities for lac repressor. Gene 50:123–132. doi: 10.1016/0378-1119(86)90317-3. [DOI] [PubMed] [Google Scholar]
- 35.Toledo A, Pérez A, Coleman JL, Benach JL. 2015. The lipid raft proteome of Borrelia burgdorferi. Proteomics 15:3662–3675 doi: 10.1002/pmic.201500093. [DOI] [PubMed] [Google Scholar]
- 36.National Institutes of Health 2002. Public Health Service policy on humane care and use of laboratory animals. Office of Laboratory Animal Welfare, National Institutes of Health, Bethesda, MD. [Google Scholar]
- 37.National Research Council 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 38.Barbour AG. 1984. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med 57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 39.Rosa P, Samuels DS, Hogan D, Stevenson B, Casjens S, Tilly K. 1996. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J Bacteriol 178:5946–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart PE, Bestor A, Cullen JN, Rosa PA. 2008. A tightly regulated surface protein of Borrelia burgdorferi is not essential to the mouse-tick infectious cycle. Infect Immun 76:1970–1978. doi: 10.1128/IAI.00714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci U S A 97:13865–13870. doi: 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bono JL, Elias AF, Kupko JJ III, Stevenson B, Tilly K, Rosa P. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J Bacteriol 182:2445–2452. doi: 10.1128/JB.182.9.2445-2452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elias AF, Bono JL, Kupko JJ, Stewart PE, Krum JG, Rosa PA. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J Mol Microbiol Biotechnol 6:29–40. [DOI] [PubMed] [Google Scholar]
- 44.Jewett MW, Lawrence K, Bestor AC, Tilly K, Grimm D, Shaw P, VanRaden M, Gherardini F, Rosa PA. 2007. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol Microbiol 64:1358–1374. doi: 10.1111/j.1365-2958.2007.05746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Hyg 27:493–497. [Google Scholar]
- 46.Fischer ER, Hansen BT, Nair V, Hoyt FH, Dorward DW. 2012. Scanning electron microscopy. Curr Protoc Microbiol Chapter 2:Unit 2B.2. doi: 10.1002/9780471729259.mc02b02s25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilly K, Casjens S, Stevenson B, Bono JL, Samuels DS, Hogan D, Rosa P. 1997. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol 25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- 48.Elias AF, Bono JL, Carroll JA, Stewart P, Tilly K, Rosa P. 2000. Altered stationary-phase response in a Borrelia burgdorferi rpoS mutant. J Bacteriol 182:2909–2918. doi: 10.1128/JB.182.10.2909-2918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simons A, Tils D, von Wilcken-Bergmann B, Müller-Hill B. 1984. Possible ideal lac operator: Escherichia coli lac operator-like sequences from eukaryotic genomes lack the central G × C pair. Proc Natl Acad Sci U S A 81:1624–1628. doi: 10.1073/pnas.81.6.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rego RO, Bestor A, Rosa PA. 2011. Defining the plasmid-borne restriction-modification systems of the Lyme disease spirochete Borrelia burgdorferi. J Bacteriol 193:1161–1171. doi: 10.1128/JB.01176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of E. coli and B. burgdorferi FtsH homologs. Homologs share 50% identity, and identical residues are indicated in boldface type and shaded. Red boxes mark conserved AAA family motifs relative to the E. coli FtsH sequence (21). Walker A and B, ATP binding and hydrolysis motifs; Pore, substrate entry pore; SRH, second region of homology; Arg-finger, arginine finger; Zn2+ AS, zinc protease active-site motif; Edge, substrate-binding edge strand. The FtsH homologs are E. coli strain K-12 FtsH (CDJ73685.1) and B. burgdorferi strain B31 BB0789 (NP_212923.1). Download
Tick coinfection results. Naive larvae were artificially coinfected by immersion in a culture containing the ΔhflK/C and WT strains (left panel) or the hflK/C-comp and WT strains (right panel). Ticks were mechanically disrupted in an Eppendorf tube with a disposable pestle and individually plated. Each symbol represents the ratio of mutant or complemented strain to WT strain in an individual tick. The dotted lines indicate the ratio of mutant or complemented strains to the WT strain in the immersion culture. Values above the line indicate a predominance of the mutant or complemented strains, while values below the line indicate a predominance of the WT strain. Bars represent the median and interquartile range of the values. One data point for the unfed nymphs in the complement/WT graph is not shown, as the value, 121, was far outside the range of this figure. No significant difference was observed between groups, as determined by the Mann-Whitney test. Download
Bacterial strains and plasmids used in this study.
Determination of ID50 for the WT and ΔhflK/C strains.
Mouse coinfection studies. Mice were coinfected with approximately equal numbers of the WT strain and either the ΔhflK/C mutant or complemented strain. Mouse infection was initially assessed at about 1 week postinoculation by ear-punch culture and subsequently confirmed by spirochete isolation from mouse tissues (ear, bladder, and ankle joint) at 4, 8, or 10 weeks postinoculation. In three independent experiments, no strain consistently predominated, indicating that no strain had a competitive advantage.
Primers used in this study.