Abstract
Microneme secretion is essential for motility, invasion, and egress in apicomplexan parasites. Although previous studies indicate that Ca2+ and cGMP control microneme secretion, little is known about how these pathways are naturally activated. Here we have developed genetically encoded indicators for Ca2+ and microneme secretion to better define the signaling pathways that regulate these processes in Toxoplasma gondii. We found that microneme secretion was triggered in vitro by exposure to a single host protein, serum albumin. The natural agonist serum albumin induced microneme secretion in a protein kinase G-dependent manner that correlated with increased cGMP levels. Surprisingly, serum albumin acted independently of elevated Ca2+ and yet it was augmented by artificial agonists that raise Ca2+, such as ethanol. Furthermore, although ethanol elevated intracellular Ca2+, it alone was unable to trigger secretion without the presence of serum or serum albumin. This dichotomy was recapitulated by zaprinast, a phosphodiesterase inhibitor that elevated cGMP and separately increased Ca2+ in a protein kinase G-independent manner leading to microneme secretion. Taken together, these findings reveal that microneme secretion is centrally controlled by protein kinase G and that this pathway is further augmented by elevation of intracellular Ca2+.
Keywords: calcium, calcium imaging, calcium intracellular release, cyclic GMP (cGMP), protein kinase G (PKG), protein secretion, second messenger
Introduction
Toxoplasma gondii is an important opportunistic pathogen and model organism for studying the biology of members of the phylum Apicomplexa (1). Micronemes are specialized secretory vesicles present in all motile stages of apicomplexan parasites (reviewed in Ref. 2). The majority of internal microneme (MIC)3 proteins (i.e. cargo) consist of adhesive proteins that translocate to the surface of the parasite following the regulated fusion of the organelle with the apical plasma membrane. Although some MIC proteins are released as soluble proteins, a number contain transmembrane domains that are thought to span the parasite plasma membrane and participate in substrate-based gliding motility (3).
In Toxoplasma and other apicomplexans, microneme secretion occurs constitutively at low levels but is up-regulated in response to elevated intracellular calcium (Ca2+) (reviewed in 4). In studies first performed in Toxoplasma, equalizing Ca2+ gradients across membranes with A23187, or liberating Ca2+ from intracellular stores with ethanol, stimulated microneme secretion, whereas chelating intracellular Ca2+ with BAPTA-AM blocked it (5, 6). Ethanol is thought to act by activating phospholipase C and inducing production of inositol trisphosphate (IP3) production followed by release of intracellular Ca2+ (7). A variety of alcohols in addition to acetaldehyde show this activity in T. gondii, although we have chosen to use ethanol here as it is the most potent secretagogue (7). The downstream effectors that regulate Ca2+-dependent microneme secretion include the kinases TgCDPK1 (8) and TgCDPK3 (9–11) as well as other downstream Ca2+ effectors including TgPRP1 (12), TgDJ-1 (13), and TgDOC2.1 (14). Genetic or chemical ablation of TgCDPK1 activity blocks microneme release and inhibits motility, invasion, and egress (8). TgCDPK3 also contributes to microneme secretion in some conditions and is important for regulating egress (9–11). Notably, activation of Toxoplasma cyclic GMP-dependent protein kinase (TgPKG), which is also required for invasion (15) and egress, can compensate for the role of TgCDPK3 (9). Consistent with this finding, cyclic GMP (cGMP) has emerged as a second signaling molecule that stimulates microneme secretion. Indirect evidence for this pathway is provided by inhibitors of cGMP-specific phosphodiesterases (PDE), such as zaprinast and BIPPO, which stimulate microneme secretion and egress in Toxoplasma (9, 16), and Plasmodium falciparum merozoites (17). More directly, chemical-genetic studies showed that inhibition of PKG blocks microneme secretion in Eimeria tenella sporozoites (15), Toxoplasma tachyzoites (15), and P. falciparum merozoites (17). These studies relied on a specific inhibitor called Compound 1 that inhibits the wild-type enzyme, which has a Thr gatekeeper, whereas mutation of this residue to Met/Gln results in resistance (18). Together, it is thought that cGMP-mediated PKG activation and Ca2+-mediated CDPK activation control microneme secretion. There also may be significant cross-talk between these two signaling pathways because PKG has been shown to regulate calcium signaling by increasing phosphoinositol metabolism during gliding motility in Plasmodium berghei ookinetes, activation of P. berghei gametocytes, and egress of P. falciparum merozoites (19). Whether PKG has a similar function in other apicomplexans is currently not known.
Traditional methods to monitor calcium flux and secretion in T. gondii are cumbersome. Western blotting has been the primary means to detect microneme proteins such as MIC2 in cell-free excreted/secreted antigen (ESA) (5). Additionally, previous studies of microneme secretion in Toxoplasma were performed in the presence of bovine serum (5–8, 20–22), which has been shown to stimulate sporozoite microneme secretion in the related apicomplexan E. tenella (23). Although it is generally accepted that elevated Ca2+ is critical for microneme secretion, monitoring intracellular calcium is technically challenging (reviewed in Ref. 24). Therefore, new and improved tools are needed for detecting microneme secretion and second messengers in apicomplexan parasites.
Here we have developed and adapted genetically encoded indicators to monitor microneme secretion and Ca2+ in Toxoplasma. Using these new and improved tools, we have assessed the sufficiency of agonists and signaling pathways that lead to microneme secretion. Our findings reveal a complex interplay between activation of PKG and elevated Ca2+ in regulating microneme secretion in Toxoplasma.
Experimental Procedures
Chemicals and Reagents
Non-denatured ethanol (100%) was purchased from Pharmco-Aaper. BAPTA-AM, A23187, and zaprinast were purchased from EMD Millipore and reconstituted in dimethyl sulfoxide (Sigma). The tri-substituted pyrrole known as Compound 1 (4-[2-(4-fluorophenyl)-5-(1-methylpiperidine-4-yl)-1H-pyrrol-3-yl]pyridine) was obtained from Merck under an academic MTA (9). HyCloneTM characterized fetal bovine serum (FBS) containing 1.8% (w/v) albumin was purchased from GE Healthcare Life Sciences. Serum filtrate was made by passing 1% FBS through a 30-kDa molecular weight cut off (MWCO) Amicon Ultra 0.5-ml filter (EMD Millipore). Bovine serum albumin (catalog number B4287), bovine serum γ-globulins, ovalbumin, 8-Br-cGMP, 8-Br-cAMP, 8-CPT-cGMP, 8-CPT-cAMP, H-89, pyrimethamine, 5-fluorodeoxyuridine, and other chemicals were purchased from Sigma.
Antibodies
MIC2 was detected with mouse monoclonal antibody 6D10 as described (25). Myc-tagged proteins were detected with mouse anti-C-myc (9E10) (Life Technologies, ThermoFisher Scientific). Rabbit anti-M2AP was kindly provided by Dr. Vern Carruthers (University of Michigan). In-house rabbit antisera were used to detect GRA2 (WU1228) (26) and ROP5 (M0556) (27). Rabbit anti-SAG1 was kindly provided by Dr. John Boothroyd (Stanford University). Normal mouse IgG (Santa Cruz Biotechnology) was used as an isotype control for anti-MIC2 and anti-C-myc where indicated. Alexa Fluor- and IR dye-conjugated goat secondary antibodies were obtained from Life Technologies/ThermoFisher Scientific and Li-COR, respectively. All antibodies were diluted immediately prior to use in PBS containing 5% FBS for immunofluoroscent assay or 5% nonfat milk with 0.1% Tween 20 for Western blotting.
Parasite Culture
Toxoplasma strain RH, RHΔhxgprtΔku80 (28), and transgenic derivatives were passaged in vitro as tachyzoites as described (8). Parasites were freshly released from human foreskin fibroblast cultures using a 22-guage needle and purified by filtration through 3-μm Whatman Nuclepore membranes (GE Healthcare Life Sciences) and resuspended in intracellular (IC) buffer for biological assays.
Plasmid Construction
All plasmids and primers used in this study are listed in supplemental Tables S2 and S3, respectively. Detailed plasmid construction information is listed in footnotes in supplemental Table S2. Briefly, pMIC2-GLuc-C-myc and ptub-GCaMP6f/sagCAT were generated by traditional restriction site cloning. The plasmids pUPRT::DHFR-MIC10-GLuc-C-myc, pUPRT::DHFR-MIC2-GLuc-C-myc, and pUPRT::DHFR-GCaMP6f were generated by Gibson assembly according to the manufacturer's instructions (New England Biolabs).
Generation of Transgenic Parasites
Freshly prepared Toxoplasma tachyzoites were transfected by electroporation, as described previously (29). Following all drug selections, stable clones were isolated by limiting dilution.
Generation of RH-MIC2-GLuc-C-myc
RH tachyzoiteswere co-transfected with 5 μg each of pMIC2-GLuc-C-myc and pBS-TUB1CatSAG1 (29) and selected with 20 μm chloramphenicol.
Generation of RH-MIC10-GLuc-C-myc
RHΔhxgprtΔku80 tachyzoites were co-transfected with 2 μg of pSAG1::CAS9-U6::sgUPRT (30) and 0.2 μg of PCR-amplified UPRT::DHFR-MIC10-GLuc-C-myc. Stable transfectants were selected with 3 μm pyrimethamine (PYR) and 10 μm fluorodeoxyuridine (FdUdr).
Generation of RH-PKGT/M-MIC2-GLuc-C-myc
RH-PKGT and RH-PKGM were generated as described (31). Freshly purified RH-PKGT and RH-PKGM parasites were co-transfected with 2 μg of pSAG1::CAS9-U6::sgUPRT and 0.2 μg of PCR-amplified UPRT::DHFR-MIC2-GLuc-C-myc. Stable transfectants were selected with 3 μm PYR and 10 μm FdUdr.
Generation of RH-GCaMP6f
RH parasites were transfected with 5 μg of ptub-GCaMP6(x)/sagCAT were selected with 20 μm chloramphenicol.
Generation of RH-PKGT/M-GCaMP6f
RH-PKGT and RH-PKGM were co-transfected with 2 μg of pSAG1::CAS9-U6::sgUPRT and 0.2 μg of PCR-amplified UPRT::DHFR-GCaMP6f. Stable transfectants were selected with 3 μm PYR and 10 μm FUDR.
Flow Cytometry
For flow cytometric analysis, purified extracellular RH or RH-MIC2-GLuc-C-myc parasites were fixed with 4% formaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 5% FBS and 5% normal goat serum. Parasites were then incubated with rabbit anti-SAG1 in combination with mouse anti-MIC2, mouse anti-myc, or normal mouse IgG and visualized with secondary goat anti-mouse IgG AF488 and goat anti-rabbit IgG AF647 antibodies. Cellular and fluorescence data were acquired using a FACSCanto flow cytometer (BD Biosciences). Histograms of the data were created using FlowJo version 10 (FLOWJO, LLC).
Indirect Immunofluorescence Assays
For subcellular localization of proteins by fluorescence microscopy, parasites grown in human foreskin fibroblast monolayers on glass coverslips were fixed, permeabilized, and blocked as described above for flow cytometry. The fixed and permeabilized monolayers were incubated with mouse anti-C-myc in combination with rabbit anti-M2AP, rabbit anti-GRA2, or rabbit anti-ROP5 antibodies and visualized with secondary goat anti-mouse IgG AF488 and goat anti-rabbit IgG AF647 antibodies. Nuclei were stained with Hoechst 33342 dye. Images were captured using a ×100 oil objective on an Axioskop 2 MOT Plus Wide Field Fluorescence Microscope (Carl Zeiss, Inc). Scale bars and linear adjustments were made to images using Axiovision LE64 software (Carl Zeiss, Inc.).
Stimulation of Microneme Secretion and Collection of ESA
For standard microneme secretion assays, freshly harvested parasites were purified, washed, and resuspended in IC buffer (142 mm KCl, 5 mm NaCl, 1 mm MgCl2, 5.6 mm d-glucose, 2 mm EGTA, 25 mm HEPES, pH 7.4) or extracellular (EC) buffer (5 mm KCl, 142 mm NaCl, 1 mm MgCl2, 1.8 mm CaCl2, 5.6 mm d-glucose, 25 mm HEPES, pH 7.4). Where indicated, inhibitors were added 10 min prior to the addition of agonists. Parasites were allowed to secrete for 10 min at 37 °C prior to collection of ESA.
SDS-PAGE and Western Blotting
ESA was mixed 1:5 with ×5 Laemmli buffer, boiled for 5 min, resolved on a 4–20% Tris glycine gel (Bio-Rad Laboratories, Inc.) by SDS-PAGE, and transferred to nitrocellulose membrane by standard tank electroblotting. Membranes were blocked with 5% nonfat milk, probed with a mixture of mouse anti-C-myc and rabbit anti-GRA2 antibodies, and visualized with goat anti-mouse IRdye 800CW and goat anti-rabbit 680RD secondary on a LI-COR Odyssey imaging system (LI-COR Biosciences). Linear adjustments were made to the TIF images to reduce membrane autofluorescence in Photoshop CS4 (Adobe Systems).
Gaussia Luciferase Assay
ESA was mixed with BioLux Gaussia Luciferase Assay Kit working reagent (New England Biolabs) and luminescence was detected using a Cytation 3 Cell Imaging Multimode Imager (BioTek Instruments, Inc.). Buffer control relative luminescence unit values were subtracted from their corresponding ESA relative luminescence unit values to correct for background autoluminescence.
Stimulation of Microneme Secretion and Collection of ESA for LC-MS/MS
In brief, Immulon 4 HBX extra-high binding 96-well plates (Thermo Scientific) were coated with 1% BSA in PBS or PBS alone at 4 °C overnight. The next day, the wells were washed with PBS to remove soluble BSA just prior to the secretion assay. Freshly harvested RH-MIC2-GLuc-C-myc parasites were purified, resuspended in IC buffer, and treated with four conditions in triplicate: mock, 50 μm BAPTA-AM, plate-bound BSA, and plate-bound BSA + 500 μm zaprinast. Parasites were allowed to secrete for 10 min at 37 °C prior to collection of ESA. The ESA replicates for each treatment were pooled and snap frozen for LC/MS-MS. Samples from two independent experiments were submitted and processed for LC/MS-MS on two separate occasions at the Danforth Plant Sciences Center, St. Louis, MO.
Identification of Toxoplasma-secreted Proteins in ESA by LC-MS/MS
ESA samples were reduced with 10 mm Tris-(2-carboxyethyl)phosphine and alkylated with 20 mm iodoacetamide before digestion overnight with 0.5 μg of trypsin. After desalting, the digest was then dried down and resuspended in 15 μl of 5% acetonitrile, 0.1% formic acid. Five microliters was run by LC-MS/MS on a NanoLC Ultra (Eksigent Technologies) coupled with an LTQ-Velos Pro Orbitrap (Thermo Scientific) using a 2-h gradient. Raw data were processed as described previously (32). For comparative semi-quantitative analysis, the spectrum count for each gene product detected by LC/MS-MS was normalized to the total number of spectra in the sample and represented as %Total to account for the variation in spectrum counts between samples and experiments. As a measure of enrichment for secreted proteins, Δ% Total was calculated for each gene by subtracting the negative control (BAPTA-AM) %totalBAPTA-AM value from the agonist %totalTreatment value. The Δ% total values for each treatment were averaged between the two experiments and ranked from high to low Δ% total values. In general, gene products with Δ% total >0 were enriched over the background negative control. Gene products with Δ% total </ = 0 were considered background excreted proteins.
Kinetic Measurements of Intracellular Ca2+
For time-resolved microscopy, purified RH-GCaMP6f parasites in IC buffer were added to glass-bottom culture dishes (MatTek) and warmed to 37 °C with a heated stage. Alternating phase and fluorescent images (3 s interval, 5 min duration) were collected on a Zeiss AxioObserver Z1 (Carl Zeiss, Inc.) equipped with an ORCA-ER digital camera (Hamamatsu Photonics) using Zen software (Carl Zeiss, Inc.). Images were exported from Zen and fluorescence intensities in the GFP channel were quantified in ImageJ for each time slice and F/F0 of single parasites with their mean was plotted with GraphPad Prism version 6 (GraphPad Software, Inc.). For time-resolved flow cytometry, parasites were pre-warmed to 37 °C in IC buffer and treated with inhibitors and agonists. Data acquisition of GCaMP6f fluorescence was collected in the FL-1 channel on a FACSCanto (BD Biosciences). Ca2+ responses were analyzed using the kinetic analysis module in FlowJo version 10. The raw data for percent responders and mean fluorescent intensity versus time was exported from FlowJo and graphed using GraphPad Prism version 6.
Cyclic GMP Determination by ELISA
Purified RH-MIC2-GLuc parasites were resuspended in IC buffer and treated with secretagogues for various times at 37 °C. The reactions were stopped by immediately moving the plate to ice, adding cold 0.2 n HCl and flash freezing with liquid nitrogen. Frozen lysates were assayed immediately or stored at −80 °C until needed. Cyclic GMP levels in Toxoplasma lysate were determined using a cGMP ELISA Detection Kit (GenScript) according to the manufacturer's instructions with a modification to account for the acidic pH of the lysates. Lysates were thawed on ice and clarified by centrifugation. Sample lysates were diluted 1:3 with neutralizing sample diluent (Buffer A + 35 mm KOH). The cGMP standards were serially diluted 1:3 in modified standard dilution buffer (4 parts Buffer A + 35 mm KOH, 1 part IC buffer (with or without 1% BSA), and 1 part 0.2 n HCl) to exactly match the samples. The remaining steps were performed according to the manufacturer's instructions (Genscript). Standard curves for cGMP were generated by fitting B/B0 versus log [cGMP], (pmol/ml) to sigmoidal, 4 parameter logistic function in GraphPad Prism version 6. The cGMP values for Toxoplasma lysates were interpolated from the standard curve and adjusted by the dilution factor. For each time point, cGMP concentrations were normalized to the mock treated control that remained steady throughout the assay.
Results
MIC2-GLuc-C-myc Is a Highly Sensitive Reporter of Microneme Secretion in T. gondii
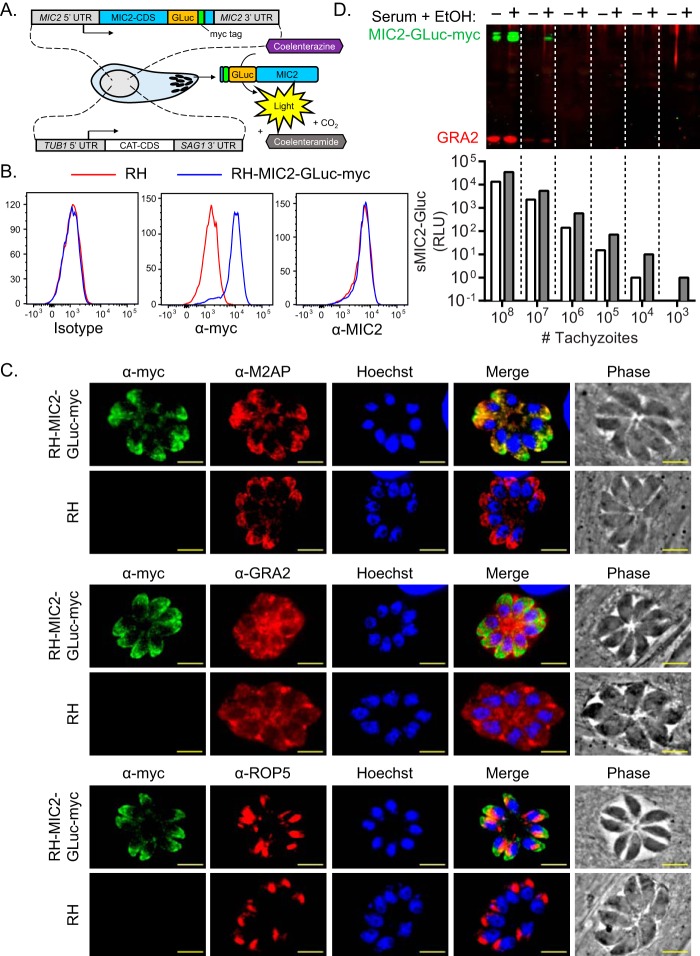
To provide a more efficient quantitative methods for monitoring microneme secretion, we constructed a novel reporter of microneme secretion by fusing Gaussia luciferase (GLuc) (33, 34) to C-myc-tagged MIC2 driven by its endogenous promoter. We co-transfected this fusion construct with a drug-selectable marker plasmid into Toxoplasma to generate RH-MIC2-GLuc-C-myc (Fig. 1A). To confirm the expression of the reporter, we performed flow cytometric analysis on fixed and permeabilized RH-MIC2-GLuc-C-myc parasites, compared with the parental control, stained with antibodies to detect MIC2 and C-myc tag. Fluorescent secondary antibodies revealed that MIC2-GLuc-C-myc staining was exclusive to the reporter strain and that both strains expressed equivalent levels of total MIC2 (Fig. 1B). To determine whether MIC2-GLuc-C-myc traffics correctly to micronemes, intracellular RH-MIC2-GLuc-C-myc and the parental control strain were fixed, permeabilized, and immunolabeled with anti-C-myc antibody in combination withantibodies directed toward micronemes (anti-M2AP), dense granules (anti-GRA2), and rhoptries (anti-ROP5). Indirect immunofluorescence microscopy confirmed that MIC2-GLuc-C-myc correctly localized to micronemes (Fig. 1C).
FIGURE 1.
Generation and validation of MIC2-GLuc-C-myc: a novel reporter of microneme secretion. A, schematic representation of MIC2-GLuc-C-myc fusion reporter that was introduced into the genome by cotransfection with a chloramphenicol selection cassette (CAT-CDS). Microneme secretion from this epitope-tagged line can be detected by traditional Western blotting or luciferase assay. B, comparison of total MIC2 and MIC2-GLuc-C-myc expression in wild-type (RH) and luciferase expressing (RH-MIC2-GLuc-C-myc) parasites. Extracellular tachyzoites were fixed, permeabilized, and immunolabeled with rabbit anti-SAG1, mouse anti-C-myc, or mouse anti-MIC2 followed by their respective Alexa Fluor-conjugated secondary antibodies and visualized by flow cytometry. Histograms were gated on SAG1+ parasites. C, subcellular localization of MIC2-GLuc-C-myc. Intracellular tachyzoites grown in human foreskin fibroblasts were fixed, permeabilized, and immunolabeled to detect MIC2-GLuc-C-myc (mouse anti-C-myc) and markers for micronemes (rabbit anti-M2AP, upper panel), dense granules (rabbit anti-GRA2, middle panel), rhoptries (rabbit anti-ROP5, lower panel) by indirect immunofluorescence microscopy. Scale bar = 5 μm. D, comparison of methods to detect microneme secretion. Serial dilutions of purified RH-MIC2-GLuc-C-myc tachyzoites were incubated in the presence or absence of 1% serum, 1% EtOH. After incubating for 10 min at 37 °C, ESA was collected and equivalent volumes were subjected in parallel to Western blotting (upper panel) and luciferase (lower panel) assays. For Western blotting, membranes were probed with mouse anti-c-Myc and rabbit anti-GRA2 antibodies and visualized using IR dye-conjugated secondary antibodies.
To directly compare Western blotting and luciferase-based detection methods of microneme secretion, serial dilutions of purified RH-MIC2-GLuc-C-myc tachyzoites were incubated in the presence or absence of 1% serum and 1% ethanol, a classical microneme secretagogue (6). Cell-free ESA was collected and subjected to Western blot and luciferase assays. By Western blot, the limit of detection for secreted MIC2-GLuc-C-myc was ∼108 parasites and ∼107 parasites for basal and stimulated secretion, respectively (Fig. 1D, upper panel). In stark contrast, MIC2-GLuc-C-myc could be detected with as few as 104 parasites for basal and 103 parasites for stimulated microneme secretion by luciferase assay (Fig. 1D, lower panel). This difference represents a 10,000-fold greater sensitivity in the luciferase assay over Western blotting for measuring microneme secretion. Taken together, the MIC2-GLuc-C-myc reporter allows for highly sensitive and rapid detection of microneme secretion in a high-throughput, multiwell luminescence-based readout. We used this improved technology to dissect signaling pathways that control microneme secretion in Toxoplasma.
Serum Albumin Is a Natural Agonist of Microneme Secretion in Toxoplasma
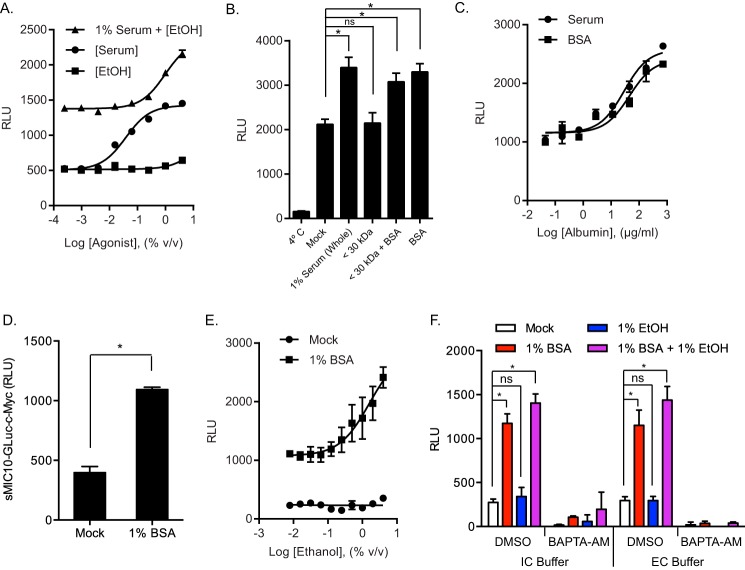
Ethanol has been described as a potent trigger of microneme secretion in Toxoplasma; however, these studies are generally performed in the presence of serum, which could also influence the outcome (6). To test the sufficiency of ethanol and serum in stimulating microneme secretion, RH-MIC2-GLuc-C-myc parasites were resuspended in IC buffer and stimulated with serial dilutions of ethanol alone, serum alone, and ethanol in the presence of 1% serum. After incubating for 10 min at 37 °C, cell-free ESA samples were collected and microneme secretion was assessed by measuring secreted MIC2-GLuc-C-myc in a luciferase assay. Serum alone efficiently stimulated microneme secretion in a dose-dependent manner with maximal response around 1% (v/v) (Fig. 2A). Although, on its own, ethanol could not stimulate microneme secretion at any concentration tested up to 4% (v/v), it enhanced serum-induced microneme secretion in a dose-dependent manner (Fig. 2 A). These findings reveal that ethanol is not a secretagogue but instead acts as an enhancer of serum-induced microneme secretion.
FIGURE 2.
Serum albumin stimulates microneme secretion and is enhanced by ethanol. A, MIC2-GLuc-C-myc secretion assay. Purified RH-MIC2-GLuc-C-myc parasites were stimulated with serial dilutions of ethanol alone, serum alone, or ethanol in the presence of 1% serum for 10 min at 37 °C. Release of MIC2-GLuc-C-myc in ESA was determined by luciferase assay. The graph indicates the average and S.D. of triplicate wells for each treatment dilution and is representative of two independent experiments with similar outcomes. B, MIC2-GLuc-C-myc secretion assay comparing whole serum to filtered serum. Purified RH-MIC2-GLuc-C-myc parasites were incubated for 10 min at 37 °C with buffer alone (mock), 1% serum (whole), 30-kDa MWCO filtered 1% serum (<30 kDa), 30-kDa MWCO filtered 1% serum + 0.18% bovine serum albumin (BSA) add back (<30 kDa + BSA), or 0.18% serum albumin alone (BSA). Release of MIC2-GLuc-C-myc in ESA was determined by luciferase assay. The graph indicates the average and S.D. of triplicate wells for each treatment and is representative of two independent experiments with similar outcomes. *, p ≤ 0.05 versus mock by one-way analysis of variance with Tukey's multiple comparison test. C, MIC2-GLuc-C-myc secretion assay. Purified RH-MIC2-GLuc-C-myc parasites were stimulated with serial dilutions of serum or equivalent amounts of serum albumin alone for 10 min at 37 °C. Release of MIC2-GLuc-C-myc in ESA was determined by luciferase assay. The graph indicates the average and S.D. of triplicate wells for each treatment dilution and is representative of two independent experiments with similar outcomes. D, MIC10-GLuc-C-myc secretion assay. Purified RH-MIC10-Gluc parasites were treated with or without 1% BSA for 10 min at 37 °C. ESA was collected and subjected to a luciferase assay. *, p ≤ 0.05, unpaired Student's t test. E, MIC2-GLuc-C-myc secretion assay. Purified RH-MIC2-GLuc-C-myc parasites were stimulated with serial dilutions of ethanol with or without 1% serum albumin for 10 min at 37 °C. Release of MIC2-GLuc-C-myc in ESA was determined by luciferase assay. The graph indicates the average and S.D. of triplicate wells for each treatment dilution and is representative of two independent experiments with similar outcomes. F, MIC2-GLuc-C-myc secretion assay comparing IC versus EC buffer in the presence or absence of BAPTA-AM. Purified RH-MIC2-GLuc-C-myc parasites resuspended in IC buffer or EC buffer were pretreated with BAPTA-AM or vehicle control and incubated for 10 min at 37 °C with buffer alone (mock), 1% BSA, 1% ethanol, or 1% BSA + 1% ethanol. Release of MIC2-GLuc-C-myc in ESA was determined by luciferase assay. The graph indicates the average and S.D. of two independent experiments consisting of triplicate wells for each treatment. *, p ≤ 0.05 versus mock by two-way analysis of variance with Tukey's multiple comparison test. All BAPTA-AM treatments were significantly lower compared with their corresponding dimethyl sulfoxide (DMSO) controls.
Because serum alone was sufficient to stimulate microneme secretion, we speculated that individual serum proteins were responsible for this activity. To test this hypothesis, we performed secretion assays with RH-MIC2-GLuc-C-myc parasites treated with buffer containing serum or serum passed through a 30-kDa MWCO filter (serum filtrate). We found that removing molecules greater than 30 kDa abolished the secretagogue activity of serum (Fig. 2B). Because serum albumin represents more than half of the total protein in serum (35) and is greater than 30 kDa, we tested whether adding back an equivalent concentration of purified serum albumin would restore secretagogue activity of serum. Indeed, serum albumin restored the activity of filtered serum, and was sufficient on its own to stimulate microneme secretion (Fig. 2B). Furthermore, serum albumin likely represents the major secretagogue found in serum because equivalent concentrations of serum albumin fully phenocopied the activity of serum in a dose-dependent manner (Fig. 2C). Conversely, the second most abundant protein class found in serum, γ-globulins, had no effect on microneme secretion (supplemental Fig. S1A), suggesting that the activity of serum albumin on secretion is specific and not a property of serum proteins in general. Serum albumin was also significantly better at stimulating microneme secretion than other albumins, like ovalbumin (supplemental Fig. S1A). Additionally, different preparations of serum albumin, refined using different chemical treatments, had equivalent secretagogue activity, regardless of purity, fractionation method, or the presence of fatty acids (supplemental Fig. S1B). To determine whether this effect was specific to MIC2, our surrogate for microneme secretion, we performed proteomic analysis of mock and stimulated ESA samples by LC/MS-MS. In addition to MIC2, we detected a large number of tryptic peptides from several microneme proteins that were enriched in serum albumin-stimulated ESA, e.g. MIC1, MIC4, MIC5, MIC6, and MIC10 (supplemental Table S1). The presence of soluble microneme proteins like MIC10 in serum albumin-stimulated ESA suggested that serum albumin was not simply enhancing surface shedding of MICs with transmembrane domains, like MIC2. To further validate the result obtained with MIC2, we generated Toxoplasma expressing MIC10-GLuc-C-myc and independently confirmed that serum albumin stimulates microneme secretion (Fig. 2D). This result indicates that the response is not limited to MIC2 and is likely true in general, as supported by MS data on the secreted fraction (supplemental Table S1). Furthermore, we saw no difference in surface levels of MIC2 after serum albumin treatment (data not shown). Collectively, these data identify serum albumin as a natural agonist that directly stimulates microneme secretion in Toxoplasma.
Given that ethanol enhances serum-induced microneme secretion, we tested whether this effect is also seen in serum albumin-induced secretion. RH-MIC2-GLuc-C-myc parasites were treated with serial dilutions of ethanol in the presence or absence of serum albumin and MIC2 levels were measured in ESA fractions. Similar to the effect on serum, ethanol enhanced serum albumin-induced secretion in a dose-dependent manner (Fig. 2E). The apparent synergy that exists between serum albumin and ethanol suggests that they could operate at discrete steps along a common signaling pathway. Because ethanol is thought to function by elevating levels of the second messenger Ca2+ (7), we examined the role of Ca2+ on serum albumin ± ethanol-induced microneme secretion. Using buffers that mimic IC and EC ionic concentrations for Ca2+, Mg2+, Na+, and Cl−, we found that basal and stimulated microneme secretion were equivalent in either buffer (Fig. 2F). Among the differences in these buffers, the intracellular buffer contains very low free calcium, mimicking the cytosol, whereas the extracellular buffer contains millimolar levels, similar to the extracellular milieu. The equal response in both buffers indicates that release of intracellular stores is sufficient to drive the response with little additional gain seen due to inclusion of calcium in the extracellular medium. Conversely, chelation of intracellular Ca2+ with BAPTA-AM inhibited both basal and stimulated microneme secretion regardless of buffer (Fig. 2F). Thus, intracellular Ca2+ is required for microneme secretion and this requirement cannot be bypassed by serum albumin, ethanol, or a combination of the two, as previously described (5).
Serum Albumin Stimulates Microneme Secretion without Elevating Intracellular Ca2+
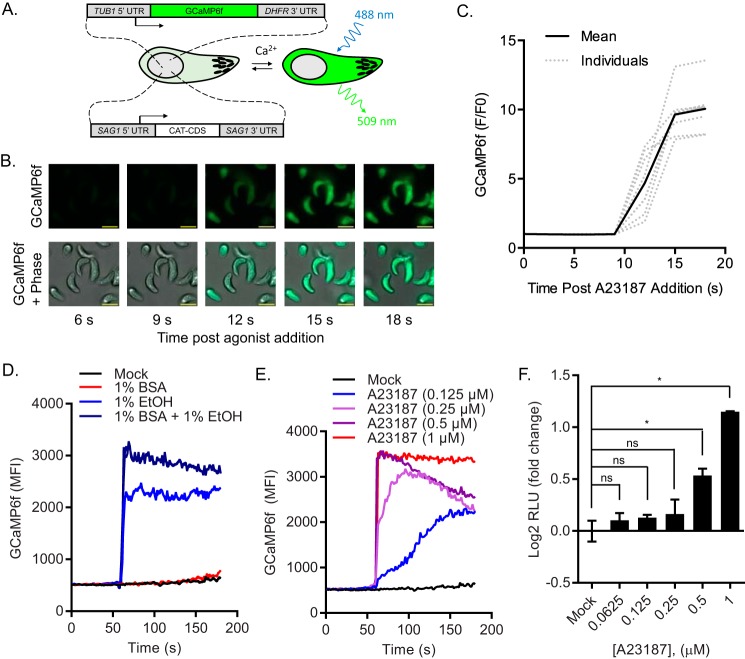
Given that intracellular Ca2+ was required for serum albumin-induced secretion (Fig. 2F), and that Ca2+ elevation stimulates microneme secretion (5–7, 20), we questioned whether serum albumin operates by raising intracellular Ca2+ in Toxoplasma. To monitor Ca2+ dynamics in vivo, we adapted the genetically encoded fluorescent Ca2+ indicator GCaMP6f (36), for expression in Toxoplasma (Fig. 3A). To assess the performance of GCaMP6f in Toxoplasma, we performed time-lapse video microscopy of A23187-treated RH-GCaMP6f parasites (Fig. 3B). We observed that GCaMP6f fluorescence increases rapidly, with a 10-fold dynamic range (Fmax/F0 = 10.05) when treated with A23187 (Fig. 3C), which collapses Ca2+ gradients across membranes by catalyzing exchange of calcium for protons. To determine whether serum albumin also elevates cytosolic Ca2+, we used time-resolved flow cytometric analysis of GCaMP6f fluorescence in live RH-GCaMP6f parasites. Paradoxically, serum albumin, which stimulates microneme secretion, did not elevate Ca2+ levels, whereas ethanol, which is not sufficient to stimulate microneme secretion, did (Fig. 3D). Equally surprising, serum albumin enhanced peak ethanol-induced Ca2+ levels by ∼35% (Fig. 3D). Taken together, it appears that serum albumin uses an alternate pathway to stimulate microneme secretion that does not require Ca2+ elevation and yet acts synergistically with signals that enhance intracellular Ca2+.
FIGURE 3.
Monitoring intracellular Ca2+ dynamics with GCaMP6f expressing Toxoplasma. A, schematic of transgenic RH-GCaMP6f parasites that express a fluorescent reporter that is sensitive to cytosolic Ca2+ levels. B, time-lapse microscopy of A23187-treated RH-GCaMP6f. RH-GCaMP6f parasites were imaged before and after addition of 2 μm A12387 using bright field phase microscopy and epifluorescence in the GFP channel. Times are indicated in seconds (s). Scale bar = 5 μm. C, quantification of data in B. Fluorescence intensity (F) in the GFP channel was quantified for each parasite in B using ImageJ at each time slice and compared with their initial fluorescence intensity (F0). F/F0 was plotted for each parasite and their mean. D and E, kinetic analysis of intracellular Ca2+ in RH-GCaMP6f parasites. Purified RH-GCaMP6f parasites were treated with secretagogues as indicated and monitored by flow cytometry. Fluorescent output in the FL-1 channel was collected at each second for before and after addition of secretagogues. Traces indicate the mean fluorescent intensities of GCaMP6f versus time and are representative of at least two independent experiments with similar outcomes. F, microneme secretion assay. Purified RH-MIC2-GLuc-C-myc parasites were treated with serial dilutions of A23187 for 10 min at 37 °C and relative MIC2-GLuc-C-myc levels in ESA were determined by luciferase assay. The graph indicates the average and S.D. of duplicate wells for each treatment dilution and is representative of two independent experiments with similar outcomes. *, p ≤ 0.05 versus mock by one-way analysis of variance with Tukey's multiple comparison test. ns, not significant.
To determine whether a critical threshold exists for Ca2+ to stimulate microneme secretion, we titrated intracellular Ca2+ levels with A23187 (Fig. 3E). We found that concentrations of A23187 that elevated Ca2+ to a similar degree as ethanol (∼4.4-fold) were also insufficient to stimulate microneme secretion (Fig. 3F). Conversely, concentrations of A23187 that elevated Ca2+ to a similar degree as ethanol + serum albumin (∼6-fold) or greater were sufficient to stimulate microneme secretion (Fig. 3F).
The Role of Cyclic GMP and PKG on Stimulated Microneme Secretion
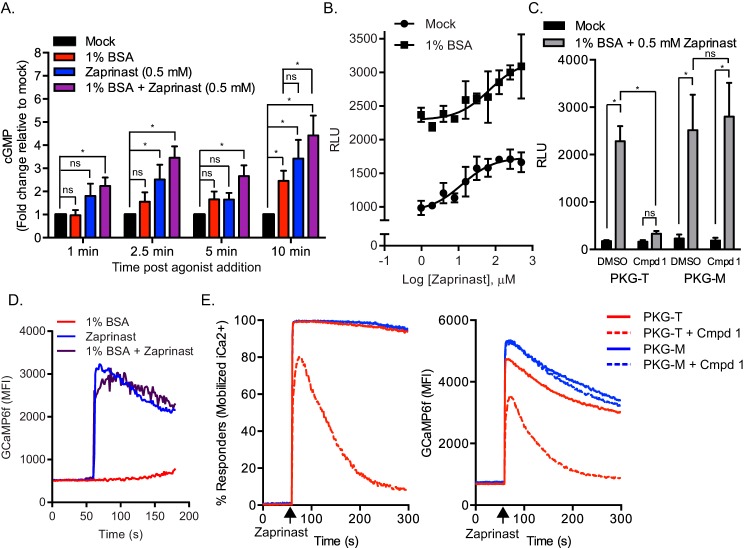
Previous studies have shown that PKG is required for Toxoplasma microneme secretion and microneme-dependent cellular processes (15, 18, 37, 38). Because PKG is activated by the second messenger cGMP, compounds that elevate cGMP levels should stimulate microneme secretion. Consistent with studies showing that zaprinast, an inhibitor of cGMP-PDEs, stimulates egress (9), it was later shown that zaprinast stimulates microneme secretion in Toxoplasma, presumably by elevating cGMP (16). Therefore it is possible that serum albumin stimulates microneme secretion by activating PKG via increasing cGMP levels. To determine whether serum albumin affects cGMP levels, RH-MIC2-GLuc-C-myc parasites were mock-treated or treated with serum albumin. In parallel, mock- or serum albumin-treated parasites were further treated with zaprinast as a positive control. Compared with mock treated controls, we found that serum albumin and zaprinast each triggered statistically significant increases in cGMP levels (Fig. 4A). Furthermore, the combination of serum albumin with zaprinast was superior to zaprinast alone at every time point measured (Fig. 4A).
FIGURE 4.
Role of cGMP pathway on stimulated microneme secretion. A, determination of cyclic GMP kinetics in Toxoplasma. Purified RH-MIC2-GLuc parasites were mock-treated or treated with secretagogues for different time intervals at 37 °C and lysates were prepared. Cyclic GMP levels were determined by a competition HRP-linked immunoassay. Relative cGMP levels for each time point were normalized to the mock treated control. The graph indicates the average and S.D. of four independent experiments consisting of duplicate wells for each treatment. *, p ≤ 0.05, two-way analysis of variance with Tukey's multiple comparison test. ns, not significant. B, MIC2-GLuc-C-myc secretion assay. Purified RH-MIC2-GLuc-C-myc parasites were stimulated with serial dilutions of zaprinast alone or in the presence of 1% BSA for 10 min at 37 °C. Relative MIC2-GLuc-C-myc levels in ESA were determined by luciferase assay. The graph indicates the average and S.D. of triplicate wells for each treatment dilution and is representative of two independent experiments. C, MIC2-GLuc-C-myc secretion assay. Transgenic parasites expressing PKG with gatekeeper T or M alleles and expressing the MIC2-GLuc-C-myc reporter were pretreated with 2 μm Compound 1 or vehicle control for 10 min followed by stimulation with the indicated secretagogues. The graph indicates the average and S.D. of duplicate wells for each treatment from four independent experiments with similar outcomes. *, p ≤ 0.05, two-way analysis of variance with Tukey's multiple comparison test. ns, not significant. D, kinetic analysis of intracellular Ca2+ in RH-GCaMP6f. Purified RH-GCaMP6f parasites were treated with secretagogues as indicated and monitored by flow cytometry. Fluorescent output in the FL-1 channel was collected at each second before and after addition of secretagogues. Traces indicate the mean fluorescent intensities of GCaMP6f versus time and are representative of two independent experiments with similar outcomes. E, role of PKG on zaprinast-induced Ca2+. RH-PKGT/M expressing strains expressing GCaMP6f were pre-treated for 10 min with 2 μm Compound 1 or vehicle control and Ca2+ levels were monitored by flow cytometry by acquiring data in the FL-1 channel before and after addition of 0.5 mm zaprinast. Traces from a representative experiment indicate the percent of parasites that mobilized Ca2+ in response to zaprinast (left graph) and the mean fluorescent intensities of GCaMP6f (right graph) versus time.
If microneme secretion is proportional to the amount of cGMP within the parasite, then the combination of serum albumin and zaprinast should stimulate microneme secretion more efficiently than either secretagogue alone. To test this hypothesis, we incubated RH-MIC2-GLuc-C-myc parasites with serial dilutions of zaprinast in the presence or absence of serum albumin. We confirmed that zaprinast alone stimulates microneme secretion in a dose-dependent manner (Fig. 4B). Interestingly, zaprinast also elevated serum albumin-induced secretion in a dose-dependent manner (Fig. 4B). This data suggests that serum albumin and zaprinast may cooperate to activate PKG by elevating cGMP.
Previous studies on albumin-induced sporozoite motility in Plasmodium speculated that albumin operates by elevating cAMP and activating cAMP-dependent protein kinase A (PKA) (39). However, we saw no difference in cAMP levels in albumin-stimulated T. gondii compared with mock-treated parasites (supplemental Fig. S2A) and were unable to stimulate microneme secretion using cell permeable cyclic nucleotide analogs (supplemental Fig. S2B), although these analogs have not yet been validated to activate PKA or PKG in T. gondii. Furthermore, the PKA inhibitor H89 did not block albumin-induced microneme secretion at concentrations that are expected to inhibit PKA specifically (supplemental Fig. S2C). Therefore albumin does not appear to stimulate microneme secretion through the cAMP pathway in T. gondii.
To investigate the role of PKG activity on serum albumin- and zaprinast-induced microneme secretion, we took advantage of an established chemical genetic system to selectively inhibit PKG with Compound 1 based a mutation at Thr-761 to Met that renders PKG resistant to the compound (18, 31, 38). Using CRISPR genome editing (30, 31), we inserted a DHFR-MIC2-GLuc-C-myc reporter construct into RH-PKGT and RH-PKGM strains (31) at the UPRT locus under double selection with PYR and FUDR. Single clones of RH-PKGT/M strains expressing MIC2-GLuc-C-myc were pre-treated with Compound 1 or vehicle control followed by incubation with or without serum albumin. In parallel, mock- or serum albumin-treated parasites were also administered zaprinast. Following stimulation, ESA was collected, and secreted MIC2-GLuc-C-myc was detected by luciferase assay. As expected, treatment with Compound 1 fully inhibited both serum albumin and zaprinast-induced microneme secretion in the Compound 1-sensitive background RH-PKGT (Fig. 4C). In contrast, RH-PKGM parasites secreted micronemes in the presence and absence of Compound 1 (Fig. 4C).
Zaprinast has been shown to elevate Ca2+ in P. falciparum schizonts (19), although the precise mechanism of this remains unknown. To determine whether this effect is also seen in Toxoplasma, we used time-resolved flow cytometric analysis of Ca2+ levels in live RH-GCaMP6f parasites. GCaMP6f fluorescence was measured before and after addition of serum albumin, zaprinast, or a combination of the two. Similar to Plasmodium, zaprinast was able to stimulate over a 6-fold increase in cytosolic Ca2+ in Toxoplasma (Fig. 4D). As seen above (Fig. 3C) treatment with serum albumin alone did not elevate Ca2+ (Fig. 4D). Additionally, no appreciable change in zaprinast-induced Ca2+ was detected by co-administration of serum albumin (Fig. 4D).
To determine whether PKG activity is required for zaprinast-induced Ca2+, we inserted a DHFR-pTUB1-GCaMP6f reporter construct into RH-PKGT and RH-PKGM strains at their UPRT loci under double selection with PYR and FUDR. Single clones of RH-PKGT/M strains expressing GCaMP6f were pre-treated with Compound 1 or vehicle control and Ca2+ levels were monitored by flow cytometry before or after addition of zaprinast. As expected, RH-PKGM parasites, which are resistant to Compound 1, treated with zaprinast showed elevated Ca2+ in the presence or absence of Compound 1 (Fig. 4E). The RH-PKGT background responded to zaprinast normally in the absence of Compound 1. Surprisingly, in the presence of Compound 1, the initial Ca2+ response induced by zaprinast was only partially inhibited by Compound 1 but diminished rapidly in the absence of PKG activity as shown by both the % of responding cells and mean fluorescent intensity (Fig. 4E). Therefore, zaprinast elevates both cGMP and Ca2+, whereas serum albumin only elevates cGMP. Despite these differences, both secretagogues required PKG activity to stimulate secretion.
Discussion
Apicomplexan parasite motility, invasion, and egress require secretion of microneme proteins (3, 40). Although prior studies have demonstrated that this pathway is regulated by intracellular Ca2+ (5, 7, 20, 41), limitations of gel-based assay for monitoring secretion have restricted understanding of the signaling pathways that govern this vital process. Here we describe two new quantitative readouts for intracellular Ca2+ and microneme secretion that can be used for real-time, sensitive, quantitative measurement of Ca2+ changes and secretion. Our findings demonstrate that serum albumin is a natural agonist that is both necessary and sufficient for microneme secretion, whereas also acting synergistically with artificial agonists such as ethanol and zaprinast, which raise Ca2+. Surprisingly, serum albumin enhances secretion without elevating Ca2+, indicating that it activates a pathway that operates at basal Ca2+ levels. Serum albumin treatment elevates cGMP, similar to the PDE inhibitor zaprinast, and both agonists are completely suppressed by selective inhibition of PKG with the inhibitor Compound 1. Collectively, these findings reveal that exposure to serum albumin triggers microneme secretion through a PKG-mediated pathway that is further augmented by elevated Ca2+.
We describe an improved system for detecting and quantitating microneme secretion in Toxoplasma that can easily be adapted for use in other apicomplexan parasites. By fusing Gaussia luciferase (GLuc) lacking a signal peptide to the microneme protein, TgMIC2, we are able to detect microneme secretion with 10,000-fold greater sensitivity via luciferase assay than by traditional Western blotting. This rapid microplate-based readout facilitates screening of multiple treatment conditions and technical replicates, greatly improving the throughput of the assay. In addition to the MIC2-GLuc reporter, we also generated a soluble MIC10-GLuc reporter. This reporter provides a readout for the soluble microneme proteins that do not require proteolytic shedding from the plasma membrane after vesicle fusion. Taken together, these MIC-GLuc assays support microneme secretion assays with improvements to sensitivity and throughput. Intracellular Ca2+ has also been difficult to monitor due to lack of efficient tools. To overcome the technical limitations of Ca2+-dye indicators, we and others (42) have adapted genetically encoded Ca2+ indicators for use in Toxoplasma. Specifically, we employed GCaMP6f, one of the fastest and brightest of such indicators described to date (36). By stably expressing GCaMP6f in Toxoplasma under the control of the constitutive TgTUB1 promoter, we were able to detect dynamic Ca2+ fluctuations in response to microneme secretagogues, in real time, at single-cell and population levels using fluorescence microscopy and flow cytometry.
In previous studies of Toxoplasma, microneme secretion assays have been performed in the presence of low levels of FBS, presumably to preserve parasite viability (5–8, 20–22). Because serum is made up numerous nutrients, metabolites, proteins, and lipids, we wondered whether secretagogues like ethanol were sufficient to stimulate secretion in the absence of serum. We were surprised to find that the ability of ethanol to stimulate microneme secretion depended on the presence of serum. Conversely, serum alone was sufficient to stimulate microneme secretion, compelling us to identify the factor in serum responsible for this activity. Filtration experiments with serum suggested that the active component was greater than 30 kDa. Serum albumin (66.5 kDa), the most abundant protein in serum, restored the secretagogue activity of filtered serum and was sufficient to stimulate microneme secretion on its own. Of note, the second most abundant class of serum proteins, γ-gobulins, had no effect on microneme secretion, ruling out a nonspecific mechanism of stimulation by all serum proteins. Because serum albumin is a known carrier protein, at present we cannot formally exclude the possibility that a secondary molecule contributes to the apparent secretagogue activity of this protein. However, serum albumin was an effective secretagogue regardless of the chemical process used to purify it, suggesting the effect is not simply due to a contaminant. Further studies will be required to elucidate whether serum albumin acts by engaging a specific receptor or by perturbing another surface membrane signaling pathway in T. gondii.
Serum and serum albumin have also been linked to motility and microneme secretion in other apicomplexan parasites. For example, serum albumin, but not other serum proteins, stimulates sporozoite motility in P. berghei and Plasmodium yoelii (39, 43), and both motility and microneme secretion in E. tenella sporozoites by an unknown mechanism (23, 44). Although previous studies in P. berghei suggest that PKA is involved in this pathway (39), we found no evidence for PKA involvement in T. gondii, where instead PKG seems to play a major role. Regardless of the signaling pathway that is engaged, it seems probable that when parasites egress from infected cells, their initial contact with serum is a trigger that primes micronemes for secretion, a process likely further enhanced by agonists that raise intracellular Ca2+. This effect is likely to be important for motility and cell invasion, although a different mechanism would likely be necessary to activate egress as intracellular parasites presumably are not exposed to serum.
Ca2+ is a well established second messenger that drives microneme secretion by activating CDPKs and other Ca2+-binding proteins presumed to be involved in vesicle trafficking and/or fusion (4). The primary source of Ca2+ is from intracellular stores because chelation of intracellular, but not extracellular, Ca2+ blocks microneme secretion (20). Pharmacological evidence exists for IP3-sensitive Ca2+ channels in Toxoplasma and secretagogues like ethanol are thought to operate by stimulating IP3 production (7, 41). Given that BAPTA-AM blocked serum albumin-induced secretion, we wondered whether serum albumin might also elevate Ca2+, and if the combined effect of serum and ethanol were due to a heightened or sustained response. In GCaMP6f-expressing parasites, we confirmed previous reports that ethanol elevates cytosolic Ca2+ (6). Given that ethanol was insufficient to stimulate microneme secretion, we hypothesized that cytosolic Ca2+ must surpass a threshold to stimulate microneme secretion. In support of this hypothesis, we were able to titrate cytosolic Ca2+ levels with A23187 and observed that stimulated microneme secretion correlated with ≥5-fold increased cytosolic Ca2+ as measured by GCaMP6f fluorescence. Interestingly, the combination of serum albumin and ethanol elevated Ca2+ to higher levels than ethanol alone, which may explain why ethanol requires serum or serum albumin for secretion. More importantly, we found that serum albumin alone did not affect cytosolic Ca2+ levels suggesting that serum albumin stimulates microneme secretion by triggering an alternative pathway, although basal calcium levels are still required for this process.
Several studies indicate that signaling through the cGMP pathway controls motility related phenotypes in apicomplexan parasites (reviewed in Ref. 45). In Toxoplasma tachyzoites, specific chemical inhibition of PKG ablates Toxoplasma growth (18) due to inhibition of microneme secretion, motility, invasion, and egress (9, 15). Because PKG activity is tightly regulated by cGMP levels, it has been presumed that elevation of cGMP leads to microneme secretion and other motility related phenotypes by activating PKG. In support of this notion, chemical inhibition of the PDE(s) that degrade cGMP has been shown to elevate cGMP and trigger microneme secretion and egress in P. falciparum schizonts (17). However, natural agonists of cGMP and microneme secretion have not been identified, nor has elevated cGMP actually been detected in parasites under these conditions. Here we demonstrated that serum albumin elevates cGMP levels in Toxoplasma. This effect likely explains its ability to drive microneme secretion as it was fully inhibited in a specific manner by Compound 1. Inhibition of the PDE(s) that degrade cGMP with zaprinast further elevated serum albumin-induced cGMP levels. Interestingly, zaprinast was sufficient to stimulate microneme secretion on its own and also enhanced serum albumin-induced secretion, both of which were fully dependent on PKG activity.
Zaprinast treatment also elevated cytosolic Ca2+ in Toxoplasma similar to P. falciparum schizonts (19). In Toxoplasma, we found that the initial release of Ca2+ from intracellular stores by zaprinast was largely independent of PKG activity but this increase in Ca2+ was relatively short-lived suggesting that it is normally taken up or exported from the cell. Consistent with this model, when PKG was not inhibited, zaprinast treatment led to a sustained elevation in Ca2+. Together these findings suggest that zaprinast acts to elevate Ca2+ by both independent (rapid onset) and PKG-dependent (prolonged duration) pathways. It is possible that the rapid PKG-independent pathway is due to release of Ca2+ from cyclic nucleotide-dependent calcium-release channels, or an off-target activity of the compound. In contrast, the PKG-dependent, prolonged duration of elevated Ca2+ is reminiscent of the finding in P. berghei ookinetes and gametocytes, as well as P. falciparum schizonts, that PKG regulates phosphoinositide metabolism, leading to IP3-dependent Ca2+ increases (19). Therefore in Toxoplasma, PKG may sustain cytosolic free Ca2+ by maintaining IP3 levels high via stimulating the production of and/or hydrolysis of PIP2. Alternatively, PKG may sustain cytosolic Ca2+ by impeding the transport of Ca2+ back to intracellular stores. Consistent with this model, it has been shown in P. berghei ookinetes (19) and P. falciparum schizonts (46) that sarcoplasmic/endoplasmic reticulum Ca2+-ATPase 6 (ATP6), an ATPase that transfers cytosolic Ca2+ to the lumen of the ER, was differentially phosphorylated when PKG was inhibited. Given that albumin also enhanced the ethanol-induced Ca2+ response, yet did not trigger Ca2+ on its own, PKG likely operates downstream of the release of stored Ca2+, in addition to augmenting calcium release.
The current model for microneme secretion suggests that PKG together with CDPKs are required for efficient secretion. Support for this model is provided by inhibition of PKG with Compound 1 (15, 17), and selective inhibition of CDPK1 with pyrazolopyrimidine inhibitors (8, 47). Both classes of compounds offer promise for development of alternative therapeutics, given the essential nature of these kinases and the potent and specific inhibition offered by these respective chemical scaffolds (48, 49). Evidence that PKG may enhance Ca2+ release via increased production of IP3 links these two pathways (19). Further support for this model is provided by zaprinast, which is thought to elevate cGMP and stimulate PKG; although zaprinast also elevates Ca2+ making it difficult to discern the relative contribution of each pathway to microneme secretion. Our findings separate the roles of PKG and Ca2+ in this process for the first time and allow us to refine this model into a temporal sequence. We found that serum albumin triggers elevations in cGMP levels and activate PKG-dependent microneme secretion at basal cytosolic Ca2+ levels. Furthermore, ethanol, which raises Ca2+, failed to stimulate microneme secretion in the absence of serum albumin. However, in the presence of serum, ethanol led to greater Ca2+ increases and enhanced secretion, demonstrating that these two pathways are synergistic. These findings suggest that albumin-induced activation of PKG primes the parasite to become more sensitive to elevated Ca2+, and this may, in part, be due to sustained Ca2+ levels, possibly through altered inositol phosphate metabolism or sarcoplasmic/endoplasmic reticulum Ca2+-ATPase inhibition. Finally, the fact that calcium ionophores cannot bypass the requirement for PKG (15) suggests that PKG controls a terminal step in microneme secretion, such as vesicle docking or fusion, whereas CDPKs may control earlier steps in vesicle trafficking or maturation (50).
We have generated improved genetically encoded reporters to investigate microneme secretion and Ca2+ signaling in Toxoplasma. We found that microneme secretion can be triggered in vitro by using a single host protein, serum albumin. This represents a more natural way to stimulate microneme secretion because Toxoplasma likely encounters serum albumin when it naturally egresses from host cells in vivo. Furthermore, serum albumin-induced microneme secretion was fully dependent on PKG activity and correlated with increased cGMP levels. Surprisingly, albumin did not act by raising intracellular Ca2+, although it was augmented by artificial agonists that did so. Collectively our findings indicate that microneme secretion relies on two independent pathways, one operating through PKG and a second pathway that enhances secretion through elevated Ca2+. Further studies on additional natural triggers will help elucidate how these two pathways are regulated during motility, invasion, and egress.
Author Contributions
K. M. B. designed and conducted the experiments, analyzed the data, generated figures, and wrote the manuscript. S. L. designed the MIC2 secretion reporter, and provided key biological reagents and advice. L. D. S. supervised the project and contributed to writing the manuscript.
Supplementary Material
Acknowledgments
We thank Sophie Alvarez (Danforth Plant Sciences Center) who performed the LC/MS-MS analysis and Jennifer Barks for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grant AI034036 (to L. D. S.) and American Heart Association Postdoctoral Fellowship 15POST22130001 (to K. M. B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors declare that they have no conflict of interest with the contents of this article.

This article contains supplemental Figs. S1 and S2 and Tables S1–S3.
- MIC
- microneme protein
- GRA
- dense granule protein
- ROP
- rhoptry protein
- IP3
- inositol 1,4,5-trisphosphate
- cGMP
- cyclic guanosine monophosphate
- CDPK
- calcium-dependent protein kinase
- PKG
- cGMP-dependent protein kinase
- PDE
- cyclic nucleotide phosphodiesterase
- MWCO
- molecular weight cutoff
- IC
- intracellular buffer
- EC
- extracellular buffer
- PYR
- pyrimethamine
- FdUdr
- 5-fluorodeoxyuridine
- GECI
- genetically encoded calcium indicator
- GLuc
- Gaussia luciferase
- BAPTA-AM
- 2′,7′-bis(carboxyethyl)-S-(and 6)carboxyfluorescein acetoxy methylester.
References
- 1. Dubey J. P. (2010) Toxoplasmosis of animals and humans, CRC Press, Boca Raton, FL [Google Scholar]
- 2. Carruthers V. B., and Tomley F. M. (2008) Microneme proteins in apicomplexans. Subcell. Biochem. 47, 33–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sibley L. D. (2010) How apicomplexan parasites move in and out of cells. Curr. Opin. Biotechnol. 21, 592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lourido S., and Moreno S. N. (2015) The calcium signaling toolkit of the Apicomplexan parasites Toxoplasma gondii and Plasmodium spp. Cell Calcium 57, 186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carruthers V. B., and Sibley L. D. (1999) Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol. Microbiol. 31, 421–428 [DOI] [PubMed] [Google Scholar]
- 6. Carruthers V. B., Moreno S. N., and Sibley L. D. (1999) Ethanol and acetaldehyde elevate intracellular [Ca2+] calcium and stimulate microneme discharge in Toxoplasma gondii. Biochem. J. 342, 379–386 [PMC free article] [PubMed] [Google Scholar]
- 7. Lovett J. L., Marchesini N., Moreno S. N., and Sibley L. D. (2002) Toxoplasma gondii microneme secretion involves intracellular Ca2+ release from IP3/ryanodine sensitive stores. J. Biol. Chem. 277, 25870–25876 [DOI] [PubMed] [Google Scholar]
- 8. Lourido S., Shuman J., Zhang C., Shokat K. M., Hui R., and Sibley L. D. (2010) Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465, 359–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lourido S., Tang K., and Sibley L. D. (2012) Distinct signalling pathways control Toxoplasma egress and host-cell invasion. EMBO J. 31, 4524–4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garrison E., Treeck M., Ehret E., Butz H., Garbuz T., Oswald B. P., Settles M., Boothroyd J., and Arrizabalaga G. (2012) A forward genetic screen reveals that calcium-dependent protein kinase 3 regulates egress in Toxoplasma. PLoS Pathog. 8, e1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCoy J. M., Whitehead L., van Dooren G. G., and Tonkin C. J. (2012) TgCDPK3 regulates calcium-dependent egress of Toxoplasma gondii from host cells. PLoS Pathog. 8, e1003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matthiesen S. H., Shenoy S. M., Kim K., Singer R. H., and Satir B. H. (2003) Role of the parafusin orthologue, PRP1, in microneme exocytosis and cell invasion in Toxoplasma gondii. Cell Microbiol. 5, 613–624 [DOI] [PubMed] [Google Scholar]
- 13. Hall C. I., Reese M. L., Weerapana E., Child M. A., Bowyer P. W., Albrow V. E., Haraldsen J. D., Phillips M. R., Sandoval E. D., Ward G. E., Cravatt B. F., Boothroyd J. C., and Bogyo M. (2011) Chemical genetic screen identifies Toxoplasma DJ-1 as a regulator of parasite secretion, attachment, and invasion. Proc. Natl. Acad. Sci. U.S.A. 108, 10568–10573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Farrell A., Thirugnanam S., Lorestani A., Dvorin J. D., Eidell K. P., Ferguson D. J., Anderson-White B. R., Duraisingh M. T., Marth G. T., and Gubbels M. J. (2012) A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science 335, 218–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiersma H. I., Galuska S. E., Tomley F. M., Sibley L. D., Liberator P. A., and Donald R. G. (2004) A role for coccidian cGMP-dependent protein kinase in motility and invasion. Int. J. Parasitol. 34, 369–380 [DOI] [PubMed] [Google Scholar]
- 16. Howard B. L., Harvey K. L., Stewart R. J., Azevedo M. F., Crabb B. S., Jennings I. G., Sanders P. R., Manallack D. T., Thompson P. E., Tonkin C. J., and Gilson P. R. (2015) Identification of potent phosphodiesterase inhibitors that demonstrate cyclic nucleotide-dependent functions in apicomplexan parasites. ACS Chem. Biol. 10, 1145–1154 [DOI] [PubMed] [Google Scholar]
- 17. Collins C. R., Hackett F., Strath M., Penzo M., Withers-Martinez C., Baker D. A., and Blackman M. J. (2013) Malaria parasite cGMP-dependent protein kinase regulates blood stage merozoite secretory organelle discharge and egress. PLoS Pathog. 9, e1003344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donald R. G., Allocco J., Singh S. B., Nare B., Salowe S. P., Wiltsie J., and Liberator P. A. (2002) Toxoplasma gondii cyclic GMP-dependent kinase: chemotherapeutic targeting of an essential parasite protein kinase. Eukaryot. Cell 1, 317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brochet M., Collins M. O., Smith T. K., Thompson E., Sebastian S., Volkmann K., Schwach F., Chappell L., Gomes A. R., Berriman M., Rayner J. C., Baker D. A., Choudhary J., and Billker O. (2014) Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca2+ signals at key decision points in the life cycle of malaria parasites. PLos Biol. 12, e1001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carruthers V. B., Giddings O. K., and Sibley L. D. (1999) Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell Microbiol. 1, 225–235 [DOI] [PubMed] [Google Scholar]
- 21. Hoff E. F., Cook S. H., Sherman G. D., Harper J. M., Ferguson D. J., Dubremetz J. F., and Carruthers V. B. (2001) Toxoplasma gondii: molecular cloning and characterization of a novel 18-kDa secretory antigen, TgMIC10. Exp. Parasitol. 97, 77–88 [DOI] [PubMed] [Google Scholar]
- 22. Huynh M. H., Rabenau K. E., Harper J. M., Beatty W. L., Sibley L. D., and Carruthers V. B. (2003) Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. EMBO J. 22, 2082–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bumstead J., and Tomley F. (2000) Induction of secretion and surface capping of microneme proteins in Eimeria tenella. Mol. Biochem. Parasitol. 110, 311–321 [DOI] [PubMed] [Google Scholar]
- 24. Takahashi A., Camacho P., Lechleiter J. D., and Herman B. (1999) Measurement of intracellular calcium. Physiol. Rev. 79, 1089–1125 [DOI] [PubMed] [Google Scholar]
- 25. Wan K. L., Carruthers V. B., Sibley L. D., and Ajioka J. W. (1997) Molecular characterisation of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol. Biochem. Parasitol. 84, 203–214 [DOI] [PubMed] [Google Scholar]
- 26. Charron A. J., and Sibley L. D. (2002) Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 115, 3049–3059 [DOI] [PubMed] [Google Scholar]
- 27. Behnke M. S., Khan A., Wootton J. C., Dubey J. P., Tang K., and Sibley L. D. (2011) Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseuodokinases. Proc. Natl. Acad. Sci. U.S.A. 108, 9631–9636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huynh M. H., and Carruthers V. B. (2009) Tagging of endogenous genes in a Toxoplasma gondii strain lacking Ku80. Eukaryot. Cell 8, 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soldati D., and Boothroyd J. C. (1993) Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science 260, 349–352 [DOI] [PubMed] [Google Scholar]
- 30. Shen B., Brown K. M., Lee T. D., and Sibley L. D. (2014) Efficient gene disruption in diverse strains of Toxoplasma gondii using CRISPR/CAS9. mBio 5, e01114–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sidik S. M., Hackett C. G., Tran F., Westwood N. J., and Lourido S. (2014) Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PloS One 9, e100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Etheridge R. D., Alaganan A., Tang K., Lou H. J., Turk B. E., and Sibley L. D. (2014) ROP18 and ROP17 kinase complexes synergize to control acute virulence of Toxoplasma in the mouse. Cell Host Microbe 15, 537–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verhaegent M., and Christopoulos T. K. (2002) Recombinant Gaussia luciferase: overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal. Chem. 74, 4378–4385 [DOI] [PubMed] [Google Scholar]
- 34. Tannous B. A., Kim D. E., Fernandez J. L., Weissleder R., and Breakefield X. O. (2005) Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 11, 435–443 [DOI] [PubMed] [Google Scholar]
- 35. Anderson N. L., and Anderson N. G. (2002) The human plasma proteome: history, character, and diagnostic prospects. Mol. Cell Proteomics 1, 845–867 [DOI] [PubMed] [Google Scholar]
- 36. Chen T. W., Wardill T. J., Sun Y., Pulver S. R., Renninger S. L., Baohan A., Schreiter E. R., Kerr R. A., Orger M. B., Jayaraman V., Looger L. L., Svoboda K., and Kim D. S. (2013) Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Donald R. G., and Liberator P. A. (2002) Molecular characterization of a coccidian parasite cGMP dependent protein kinase. Mol. Biochem. Parasitol. 120, 165–175 [DOI] [PubMed] [Google Scholar]
- 38. Donald R. G., Zhong T., Wiersma H., Nare B., Yao D., Lee A., Allocco J., and Liberator P. A. (2006) Anticoccidial kinase inhibitors: identification of protein kinase targets secondary to cGMP-dependent protein kinase. Mol. Biochem. Parasitol. 149, 86–98 [DOI] [PubMed] [Google Scholar]
- 39. Kebaier C., and Vanderberg J. P. (2010) Initiation of Plasmodium sporozoite motility by albumin is associated with induction of intracellular signalling. Int. J. Parasitol. 40, 25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kappe S., Bruderer T., Gantt S., Fujioka H., Nussenzweig V., and Ménard R. (1999) Conservation of a gliding motility and cell invasion machinery in apicomplexan parasites. J. Cell Biol. 147, 937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lovett J. L., and Sibley L. D. (2003) Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J. Cell Sci. 116, 3009–3016 [DOI] [PubMed] [Google Scholar]
- 42. Borges-Pereira L., Budu A., McKnight C. A., Moore C. A., Vella S. A., Hortua Triana M. A., Liu J., Garcia C. R., Pace D. A., and Moreno S. N. (2015) Calcium signaling throughout the Toxoplasma gondii lytic cycle: a study using genetically encoded calcium indicators. J. Biol. Chem. 291, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vanderberg J. P. (1974) Studies on the motility of Plasmodium sporozoites. J. Protozool. 21, 527–537 [DOI] [PubMed] [Google Scholar]
- 44. Upton S. J., and Tilley M. (1992) Effect of select media supplements on motility and development of Eimeria nieschulzi in vitro. J. Parasitol. 78, 329–333 [PubMed] [Google Scholar]
- 45. Gould M. K., and de Koning H. P. (2011) Cyclic-nucleotide signalling in protozoa. FEMS Microbiol. Rev. 35, 515–541 [DOI] [PubMed] [Google Scholar]
- 46. Alam M. M., Solyakov L., Bottrill A. R., Flueck C., Siddiqui F. A., Singh S., Mistry S., Viskaduraki M., Lee K., Hopp C. S., Chitnis C. E., Doerig C., Moon R. W., Green J. L., et al. (2015) Phosphoproteomics reveals malaria parasite Protein Kinase G as a signalling hub regulating egress and invasion. Nat. Commun. 6, 7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sugi T., Kato K., Kobayashi K., Watanabe S., Kurokawa H., Gong H., Pandey K., Takemae H., and Akashi H. (2010) Use of the kinase inhibitor analog 1-NM-PP1 reveals a role for Toxoplasma gondii CDPK1 in the invasion step. Eukaryot. Cell 9, 667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hui R., El Bakkouri M., and Sibley L. D. (2015) Designing selective inhibitors for calcium-dependent protein kinases in apicomplexans. Trends Pharmacol. Sci. 36, 452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lucet I. S., Tobin A., Drewry D., Wilks A. F., and Doerig C. (2012) Plasmodium kinases as targets for new-generation antimalarials. Future Med. Chem. 4, 2295–2310 [DOI] [PubMed] [Google Scholar]
- 50. Lourido S., Jeschke G. R., Turk B. E., and Sibley L. D. (2013) Exploiting the unique ATP-binding pocket of Toxoplasma calcium-dependent protein kinase 1 to identify its substrates. ACS Chem. Biol. 8, 1155–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.