Abstract
Within its mammalian host, Leishmania resides and replicates as an intracellular parasite. The direct activity of antileishmanials must therefore depend on intracellular drug transport, metabolism, and accumulation within the host cell. In this study, we explored the role of human macrophage transporters in the intracellular accumulation and antileishmanial activity of miltefosine (MLF), the only oral drug available for the treatment of visceral and cutaneous leishmaniasis (CL). Membrane transporter gene expression in primary human macrophages infected in vitro with Leishmania Viannia panamensis and exposed to MLF showed modulation of ABC and solute liquid carrier transporters gene transcripts. Among these, ABCA3, a lipid transporter, was significantly induced after exposure to MLF, and this induction was confirmed in primary macrophages from CL patients. Functional validation of MLF as a substrate for ABCA3 was performed by shRNA gene knockdown (KD) in THP-1 monocytes. Intracellular accumulation of radiolabeled MLF was significantly higher in ABCA3KD macrophages. ABCA3KD resulted in increased cytotoxicity induced by MLF exposure. ABCA3 gene expression inversely correlated with intracellular MLF content in primary macrophages from CL patients. ABCA3KD reduced parasite survival during macrophage infection with an L. V. panamensis strain exhibiting low in vitro susceptibility to MLF. Confocal microscopy showed ABCA3 to be located in the cell membrane of resting macrophages and in intracellular compartments in L. V. panamensis-infected cells. These results provide evidence of ABCA3 as an MLF efflux transporter in human macrophages and support its role in the direct antileishmanial effect of this alkylphosphocholine drug.
Keywords: ABC transporter, drug transport, host-pathogen interaction, Leishmania, macrophage, ABCA3, miltefosine, pharmacodynamics
Introduction
Leishmania parasites are the causative agent of cutaneous leishmaniasis (CL)2 and visceral leishmaniasis, neglected tropical diseases with a combined annual incidence of an estimated 1.5 million cases globally (1). Among the scarce chemotherapeutic options available, antimonial drugs remain the first line treatment in most affected countries. However, loss of susceptibility against pentavalent antimonials shown in Leishmania isolates from both visceral leishmaniasis and CL patients, increased rates of therapeutic failure, and high toxicity, threaten its usefulness (2). An alternative treatment option is the alkylphosphocholine miltefosine (MLF), the only oral drug available and registered for the treatment of CL and visceral leishmaniasis (3). The response to MLF treatment during L. Viannia infections apparently varies among patients infected with different L. Viannia species, with cure rates that range from 60% in regions of Guatemala where L. V. braziliensis and Leishmania mexicana are common (4), to 90% in Bolivia and Colombia in infections caused by L. V. braziliensis and L. V. panamensis, respectively (5, 6). Although it has been suggested that the therapeutic response to MLF is potentially linked to species-specific loss of susceptibility (7, 8), recent studies show a wide range of susceptibilities among clinical isolates of the same Leishmania species (9), and therapeutic failure has been reported in visceral leishmaniasis patients infected with drug-susceptible strains (10). We have recently shown that underexposure was associated with MLF treatment failure (11). Together, these findings indicate that parasite factors, other than classical drug resistance, and host factors are critical to the efficacy of MLF treatment.
During human infection, Leishmania preferentially resides and replicates within macrophages. Thus, the direct activity of antileishmanial drugs is dependent upon effective drug delivery to host cells and to the intracellular compartments (phagolysosomes) inhabited by the parasite. However, which host factors contribute to the therapeutic response and how remain poorly understood. Our group and others have shown that transport and accumulation of drugs within human macrophages affect the antileishmanial activity of first line antimonials (12, 13). The mechanisms regulating MLF trafficking within host macrophages and its role in drug exposure and direct drug activity against intracellular Leishmania remain unknown.
We have recently observed that intracellular MLF concentrations in peripheral blood mononuclear cells isolated from miltefosine-treated CL patients followed a similar kinetic profile as plasma MLF concentrations (14). A trend of higher end of treatment intracellular concentrations versus plasma concentrations was observed, suggesting intracellular MLF accumulation in human leukocytes (14). Because MLF is an amphipathic phospholipid molecule, it is expected to cross passively over lipid membranes of the cell (15, 16). However, an additional carrier-mediated transport mechanism dependent on temperature and ATP has also been shown to contribute to MLF accumulation in human cells (15). Overexpression of the ATP binding cassette (ABC) transporter MDR1 in cancer cell lines results in reduced sensitivity to MLF (17). ABC transporters LMDR1/LABCB4 (18, 19), LiABCG4 (20), and LiABCG6 (21) have also been shown to mediate MLF transport in Leishmania and have been implicated in MLF resistance. Thus, alterations in parasite and host cell drug transport could modify the antileishmanial effect of MLF. The purpose of this study was to identify the active carriers involved in transport and accumulation of miltefosine in human macrophages and to characterize their impact on intracellular drug-mediated parasite killing.
Experimental Procedures
Ethics Statement
This study was approved and monitored by the institutional review board for ethical conduct of research involving human subjects of the Centro Internacional de Entrenamiento e Investigaciones Médicas in accordance with national (resolution 008430, República de Colombia, Ministry of Health, 1993) and international (Declaration of Helsinki and amendments, World Medical Association, Fortaleza, Brazil, October 2013) guidelines. All individuals voluntarily participated in the study, and written informed consent was obtained from each participant.
Study Design and Study Subjects
This study was designed to identify MLF membrane transporters in human macrophages and to evaluate their effect in the antileishmanial activity of MLF, aiming to define host cell factors that alter drug exposure of intracellular Leishmania. To identify candidate molecules with putative MLF transport function, we profiled gene expression of membrane transporters modulated by Leishmania infection and exposure to MLF in primary macrophages from healthy individuals. Modulation of gene expression was confirmed in primary macrophages from CL patients. Functional validation was performed using shRNA gene knockdown in THP-1 monocytes. MLF transport function was assessed by measurement of intracellular drug concentrations, cytotoxicity, and intracellular parasite killing assays.
Adult patients (n = 19) with parasitologically confirmed diagnosis of active CL with a time of evolution <6 months, 18–65 years of age, and without apparent immune deficiencies (negative HIV test, no evidence of immunological disorder, nor treatment with medication having immunomodulating effects) participated in this study. Peripheral blood samples were obtained prior to initiation of antileishmanial treatment.
Reagents and Chemicals
MLF (Cayman Chemical Company, catalog no. 63280, lot no. 0410809-56) was dissolved in sterile DMSO following the manufacturer's instructions (50 mg/62,5 ml DMSO) and stored at −20 °C until use. When indicated, MLF solutions were also prepared in PBS and used immediately after preparation. Working solutions were prepared in RPMI culture medium from the DMSO or PBS-dissolved stocks. Phorbol 12-myristate 13-acetate (PMA) was obtained from Sigma-Aldrich.
Cell Culture and Differentiation
Primary Macrophages
Peripheral blood samples were taken from study participants, and mononuclear cells (PBMCs) were obtained by separation using a Ficoll-Hypaque (Sigma-Aldrich) gradient, following the manufacturer's instructions. Macrophages were differentiated from PBMCs by adherence to cell culture plasticware as previously described (12).
THP-1 Cell Line
The human pro-monocytic cell line THP-1 and derived cell lines were maintained at 1 × 106 cells/ml in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated FBS, 100 μg/ml streptomycin, and 100 units/ml penicillin at 37 °C and 5% CO2. Cultured cells were differentiated using 250 ng/ml PMA for 3 h and washed with PBS, and adherence was allowed for 24 h.
Parasites, Infection, and Intracellular Parasite Survival Assays
MLF-susceptible L. (Viannia) panamensis promastigotes (EC50 = 2.9 μm; 1.2 μg/ml (22)) stably transfected with the luciferase reporter gene (L.p-LUC001) and MLF nonsusceptible L. V. panamensis (L.p-LUC056; EC50 > 32 μm; >13.0 μg/ml, (22)) were kept at 25 °C in RPMI supplemented with 10% heat-inactivated FBS and 5 mg/ml hemin. L.p-LUC001 was maintained with additional 120 μg/ml Geneticin and L.p-LUC056 cultured in the presence of 60 μm MLF and 160 μg/ml Geneticin. Macrophages were infected stationary phase promastigotes opsonized with human AB+ serum at a 10:1 Leishmania-macrophage ratio for 2 h, washed twice with PBS, and incubated for 24 h at 34 °C 5% CO2. After infection was established, the cells were exposed for 24 h to MLF (4–16 μm) or left untreated for additional 24 h. Intracellular parasite survival was measured by luciferase activity (23).
Drug Transporter Quantitative PCR Arrays and Gene Expression Profiling
Screening of drug transporter gene expression was conducted as previously described (12). The drug transporter Quantitative reverse transcriptase PCR array used was PAHS-070Z (SABiosciences-Qiagen). Selection of candidate genes was based on up- or down-regulation (±1.5-fold) following Leishmania infection and exposure to MLF at 16 μm. Corroboration of expression of selected genes was conducted by quantitative RT-PCR in macrophages from CL patients (n = 19) using TaqMan® gene expression assays (Applied Biosystems): abcb1 (P-glycoprotein, Hs01070641_g1), abca3 (Hs00975530_m1), and gapdh (Hs99999905_m1). Reactions were run on a Bio-Rad CFX-96 detection platform. Ct values were normalized to gapdh, and gene expression was calculated by ΔΔCt method and expressed as Log2 fold change.
shRNA-mediated Transporter Gene Silencing in Host THP-1 Cells
A lentivirus-based system was used for shRNA-mediated gene silencing in THP-1 monocytes as previously described (24). Four independent sets of lentiviral particles for shRNA gene knockdown of ABCA3 were purchased from Sigma-Aldrich (SHCLNV-NM_001089: TRCN0000303547, TRCN0000315649, TRCN0000315713, and TRCN0000315714) THP-1 monocytes were transduced at an average multiplicity of infection of five viral particles to one monocyte in medium containing 10 μg/ml Polybrene. Transduced cells were selected and maintained under puromycin pressure (5 μg/ml) for a minimum of 5 days. pLKO.1 (Addgene) empty vector transduced cells were used as negative control. Gene knockdown was assessed by quantitative RT-PCR prior to every experimental procedure and considered acceptable when knockdown was >50%, compared with empty vector transfected THP-1 cells. Gene knockdown was also confirmed at the protein level by flow cytometry. Briefly, 1 × 106 monocytes were collected and washed with cold PBS and fixed with 0.5% formaldehyde for 30 min at 4 °C. The cells were permeabilized with 0.2% PBS-Tween 20 for 15 min at 37 °C, washed, and incubated with anti-human ABCA3 (Santa Cruz Biotechnology sc-134560) at a 1:50 ratio in FACS buffer containing 50% human AB+ serum for 1 h at 4 °C. The cells were then washed and incubated for 30 min with FITC-labeled anti-rabbit antibody (Jackson ImmunoResearch). Staining was measured on a BD-Accuri C6 cytometer. A total of 50.000 events were acquired and analyzed in FlowJo Vx software (Tree Star).
Quantification of Intracellular MLF in THP-1 Cells
PMA-differentiated empty vector control and ABCA3 knockdown (ABCA3KD) THP-1 cells were infected with L. V. panamensis (or left uninfected) and exposed for 24 h to [14C]MLF (kindly donated by Paladin Labs Inc.). Afterward, macrophages were washed three times with cold PBS-BSA (1%) to remove surface bound [14C]MLF. The cells were collected and centrifuged at 800 × g for 5 min, and the remaining pellet was resuspended in 50 μl of PBS. Intracellular MLF was quantified by scintillation counting measured in a Chameleon multiplate reader (Hidex) and documented as cpm. The number of cpm was converted to concentration and quantified using a linear calibration curve with a range of 0.25–128 μm MLF.
Quantification of Intracellular MLF in Primary Macrophages
Primary macrophages from CL patients were exposed in vitro to MLF (16 μm and 32 μm) for 24 h, washed with PBS-BSA (1%), and resuspended as described above. Intracellular MLF was quantified using a recently developed and validated LC-MS/MS method (14). Briefly, macrophages were lysed with 62.5% methanol. The homogenized solution was evaporated and resuspended in plasma, after which MLF was quantified on a calibration curve prepared in blank human K-EDTA plasma. This concentration was then back-calculated to total amount of miltefosine in the cell pellet.
Cytotoxicity Assays
PMA-differentiated ABCA3KD and empty vector control cells, and primary human macrophages were exposed to a dose range of MLF for 24 h. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (ATCC).
Phagocytosis Assays
THP-1 cell lines were cultured and differentiated in 12-well plates at 0.35 × 106 cells/well in complete RPMI. The cells were incubated with 1 μl of stock solution of 1.0-micron fluorescent latex beads (Fluoresbrite Carboxilate YG 1.00 μm, catalog no. 15702, Polysciences, Inc.) for 2, 24, and 48 h. Adherent cells were detached with trypsin-EDTA, washed with PBS, and collected for FACS. A total of 20,000 events were acquired and analyzed.
Confocal Microscopy
PMA-differentiated THP-1 cells and primary human macrophages were plated on glass slides in 24-well plates, infected with L. V. panamensis transfected with green fluorescent protein and then exposed to 16 μm MLF or left untreated. The cells were washed, fixed with 4% formaldehyde, permeabilized with PBS containing 0.05% Nonidet P-40 (Igepal) and 1% BSA for 5 min, and blocked with 5% skim milk in PBS. Subsequently the cells were incubated with anti ABCA3 antibody for 1 h in a humid chamber, washed twice with PBS, and incubated with Alexa Fluor 568 donkey anti-rabbit IgG (Molecular Probes, Invitrogen) for 1 h. The slides were mounted and analyzed on a Zeiss Observer Z1 microscope and images captured using Zen 2009 software.
Statistical Analysis
D'Agostino and Pearson's omnibus test (25) was used to test for departures of quantitative data from normal distributions. Differences in gene expression were tested with one-way analysis of variance and Tukey's multiple comparisons test. Differences in variance for the remaining experiments were analyzed with unpaired t test. Statistical dependence of variables was determined by Spearman's rank correlation. A significance level of p < 0.05 was used for all statistical tests. Statistical analysis was performed using GraphPad Prism software (version 5).
Results
Exposure to Miltefosine Modulates Expression of Membrane Transporters in Human Macrophages
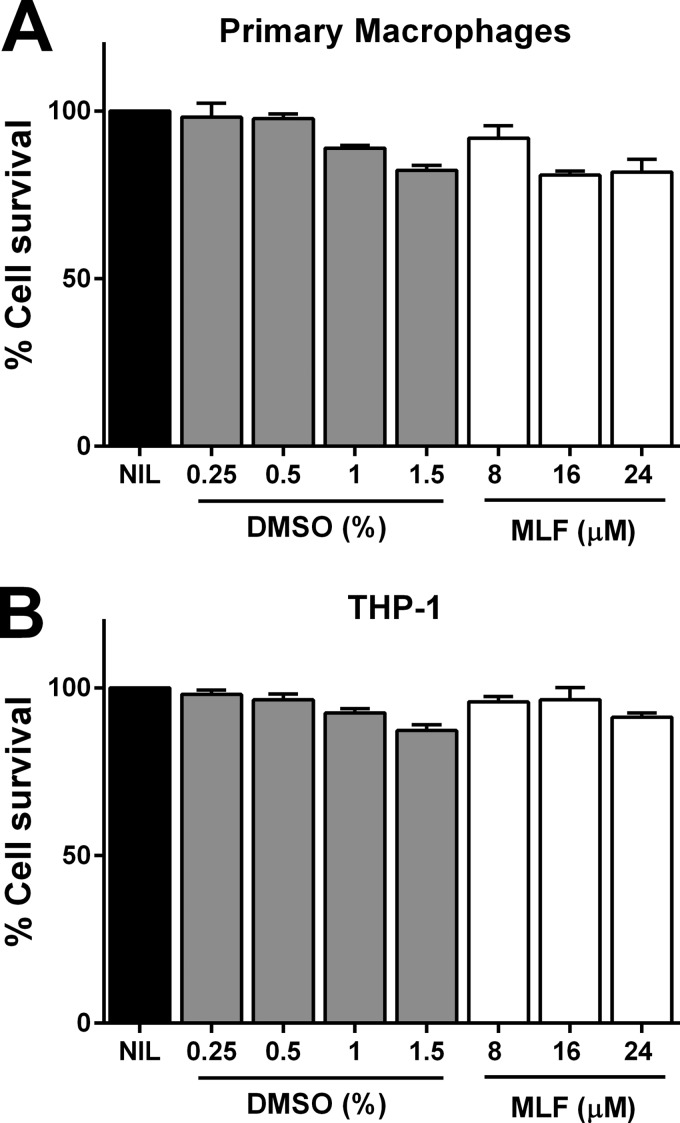
To identify candidate proteins with putative MLF transport function in human macrophages, we evaluated the effect of MLF exposure on the modulation of expression of transporter genes in L. V. panamensis-infected primary human macrophages from healthy donors (n = 3). Miltefosine concentrations used for all experiments were not toxic for either primary macrophages or THP-1 cells (Fig. 1). Expression of 84 transporter genes including members of the ABC transporters, solute liquid carriers, and aquaporins, was assessed by quantitative RT-PCR. As shown in Table 1, both L. V. panamensis infection and exposure to MLF modulate the expression of membrane transporters in human macrophages. Four of nine ABC transporters modulated by exposure to MLF (ABCA1, ABCA3, ABCB1, and ABCD1) have been shown to transport lipids or lipid-related compounds (26). Among these, expression of ABCA3 and ABCB1 was significantly higher in infected macrophages exposed to MLF (Table 1). The ratio of ABCA3 expression between infected and infected and drug-exposed macrophages was the highest (Table 1), followed by that of ABCB1. ABCA3 and ABCB1 expression was significantly induced by exposure to MLF in primary PBMC-derived macrophages from CL patients (n = 19), whereas ABCA3 expression was down-regulated by L. V. panamensis infection (Fig. 2, A and B). ABCA3 was also induced upon exposure to MLF dissolved in PBS, substantiating the drug mediated modulation of host cells gene expression (Fig. 2C).
FIGURE 1.
DMSO and MLF cytotoxicity. PBMC-derived human macrophages from healthy donors (n = 2) (A) and THP-1 cells (B were exposed to increasing doses of DMSO and MLF dissolved in DMSO for 24 h. Cell viability was evaluated by reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and measured by absorbance at 570 nm. The data are shown as percentages of cell survival compared with untreated controls and represented as means ± S.E. of measurements performed in triplicate. NIL, untreated control.
TABLE 1.
Macrophage transporter genes modulated by exposure to miltefosine and L. V. panamensis infection
| Functional gene grouping | Symbol | GenBankTM accession no. | Up-down regulation (comparing to control group)a |
p valued | Ratio (infected + MLF/infected)e | |
|---|---|---|---|---|---|---|
| Infectedb | Infected + MLFc | |||||
| ABC Transporters | ABCA1 | NM_005502 | 1.58 ± 0.22 | 1.95 ± 0.38 | 0.223 | 1.23 |
| ABCA3 | NM_001089 | 0.71 ± 0.30 | 1.90 ± 0.56 | 0.031 | 2.67 | |
| ABCB1 | NM_000927 | 0.56 ± 0.22 | 1.05 ± 0.17 | 0.038 | 1.87 | |
| ABCB3 | NM_000544 | 1.83 ± 1.15 | 1.77 ± 0.83 | 0.947 | 0.97 | |
| ABCB5 | NM_178559 | 1.19 ± 0.32 | 1.57 ± 0.75 | 0.466 | 1.32 | |
| ABCC5 | NM_005688 | 1.17 ± 0.28 | 1.55 ± 0.73 | 0.444 | 1.33 | |
| ABCC10 | NM_033450 | 1.48 ± 0.47 | 1.54 ± 0.69 | 0.912 | 1.04 | |
| ABCD1 | NM_000033 | 1.24 ± 0.33 | 1.58 ± 0.51 | 0.385 | 1.28 | |
| ABCG2 | NM_004827 | 1.30 ± 1.16 | 1.60 ± 1.82 | 0.822 | 1.23 | |
| Aquaporins | AQP9 | NM_020980 | 0.96 ± 0.45 | 0.29 ± 0.21 | 0.079 | 0.30 |
| SLC Transporters | SLC3A1 | NM_000341 | 1.17 ± 0.42 | 3.64 ± 2.21 | 0.130 | 3.11 |
| SLC7A11 | NM_014331 | 1.20 ± 0.25 | 3.59 ± 3.18 | 0.264 | 3.00 | |
| SLC16A3 | NM_004207 | 2.89 ± 2.24 | 2.43 ± 2.36 | 0.817 | 0.84 | |
| SLCO4A1 | NM_016354 | 0.91 ± 0.45 | 0.47 ± 0.27 | 0.219 | 0.52 | |
| SLC28A3 | NM_022127 | 1.11 ± 0.59 | 0.40 ± 0.31 | 0.137 | 0.36 | |
| SLCO3A1 | NM_013272 | 0.93 ± 0.18 | 1.55 ± 0.59 | 0.155 | 1.67 | |
a Expression data are shown as relative quantitations based on gene expression levels compared to uninfected and untreated macrophages from the same donor. Shown are genes that were up- or down-regulated beyond a threshold of ±1.5-fold compared to control cells. The data are shown as mean values ± S.D. (n = 3).
b Primary human macrophages infected with L. V. panamensis for 48 h.
c Primary human macrophages infected with L. V. panamensis for 24 h followed by exposure to MLF 16 μm for 24 h.
d Two-tailed unpaired t test of the comparison between gene expression of infected and infected and drug treated macrophages.
e Gene expression ratio between infected and drug-exposed cells over infected macrophages.
FIGURE 2.
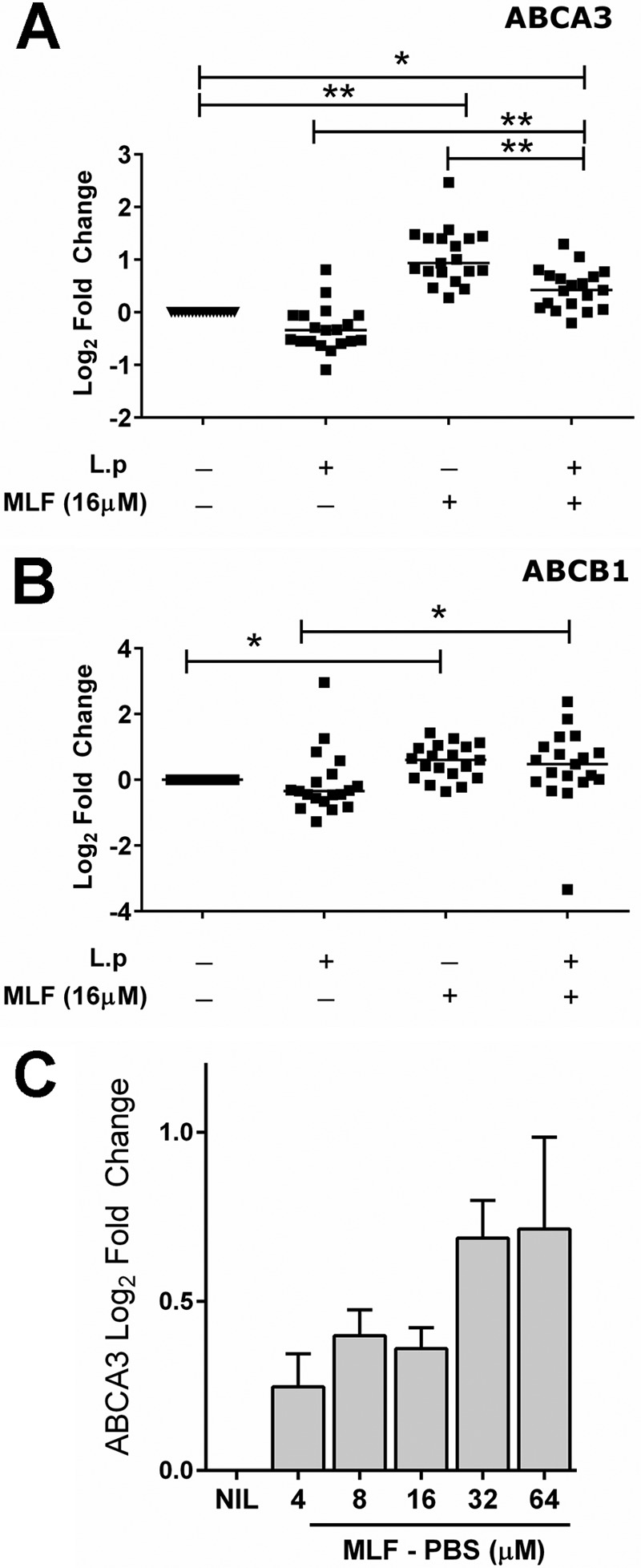
Expression of macrophage ABCA3 and ABCB1 is induced by exposure to miltefosine. ABCA3 (A) and ABCB1 (B) gene expression was evaluated in macrophages from CL patients (n = 19) infected in vitro with L. V. panamensis (L.p) for 24 h and treated with 16 μm miltefosine dissolved in DMSO (MLF) for subsequent 24 h or left untreated. Expression of ABCA3 was also measured in uninfected THP-1 cells exposed to increasing concentrations of MLF dissolved in PBS (C). Statistical differences were determined by one-way analysis of variance followed by Tukey's multiple comparisons test. Mean values are shown. NIL, untreated control.
ABCA3 Is a Miltefosine Transporter in Human Macrophages
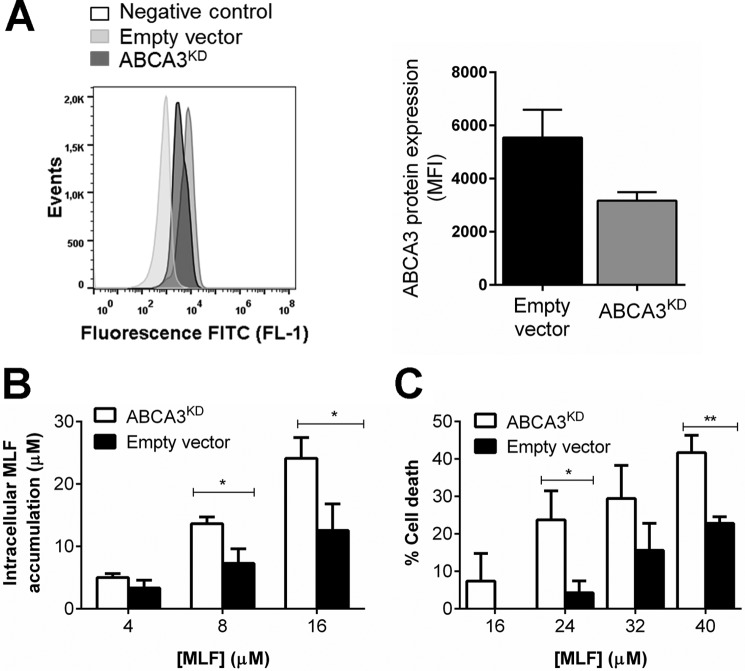
In mammalian cells, ABCA3 functions as a lysosomal and lysosome-related organelle lipid transporter (27, 28). Given that miltefosine is a phosphocholine analogue, we sought to explore the involvement of ABCA3 in intracellular MLF trafficking in human macrophages. We constructed an ABCA3 knockdown THP-1 cell line (ABCA3KD) using shRNA. More than 50% ABCA3 gene knockdown was achieved with TCR clone TCRN0000315713. Average gene knockdown evaluated in six independent biological replicas was 58 ± 7%, and this was confirmed at the protein level by flow cytometry (Fig. 3A); further experiments were conducted with this transduced cell line. Measurement of intracellular [14C]MLF showed a dose-dependent drug accumulation within THP-1 cells (Fig. 3B). Higher accumulation of MLF was observed in ABCA3KD cells compared with empty vector transfected controls. This difference was significant at drug concentrations of 8 and 16 μm. In line with higher intracellular MLF accumulation, cytotoxicity assays showed significantly higher MLF-related cytotoxicity in ABCA3KD cells (Fig. 3C).
FIGURE 3.
ABCA3 knockdown augments miltefosine accumulation and cytotoxicity in THP-1 cells. A, ABCA3 protein expression was evaluated by flow cytometry in empty vector controls and ABCA3KD cells. The data are shown as median fluorescence intensity (MFI). A, a representative image of the shift in fluorescence intensity among cell lines in at least three biological replicas is shown. Intracellular MLF accumulation (B) and cytotoxicity (C) in control empty vector transduced THP-1 monocytes and ABCA3KD cells after 24 h of exposure to increasing MLF doses. The graphs represent mean values ± S.E. of five independent experiments. Statistical differences were estimated by unpaired t tests, and significance was established when p < 0.05. MFI, mean fluorescence intensity.
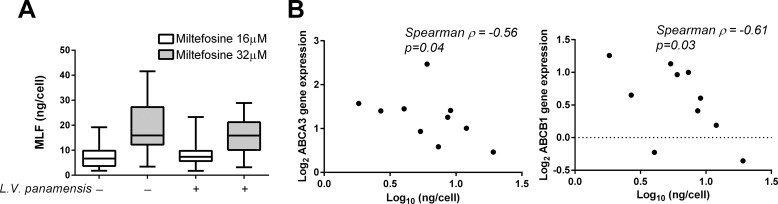
Intracellular MLF concentrations were also measured in PBMC-derived macrophages from CL patients (n = 10). Macrophages were exposed in vitro to MLF (16 and 32 μm) for 24 h. Intracellular drug accumulation was dose-dependent (Fig. 4A) and unaffected by L. V. panamensis infection. MLF content measured in uninfected cells inversely correlated to drug induced expression of ABCA3 and ABCB1 (Fig. 4B). However, no correlation was observed between gene expression and intracellular MLF in L. V. panamensis-infected cells (p = 0.26 and p = 0.1 for ABCA3 and ABCB1, respectively).
FIGURE 4.
ABCA3 gene expression inversely correlates with intracellular miltefosine accumulation. A, intracellular MLF was quantified in L. V. panamensis-infected or uninfected PBMCs-derived macrophages from CL patients (n = 10) exposed ex vivo to MLF for 24 h. B, correlation analysis of intracellular MLF and ABCA3 and ABCB1 gene expression. Statistical correlation was assessed by the Spearman correlation test, and significance was established when p < 0.05.
Macrophage ABCA3 Gene Knockdown Promotes MLF-mediated Intracellular Killing of Nonsusceptible L. V. panamensis
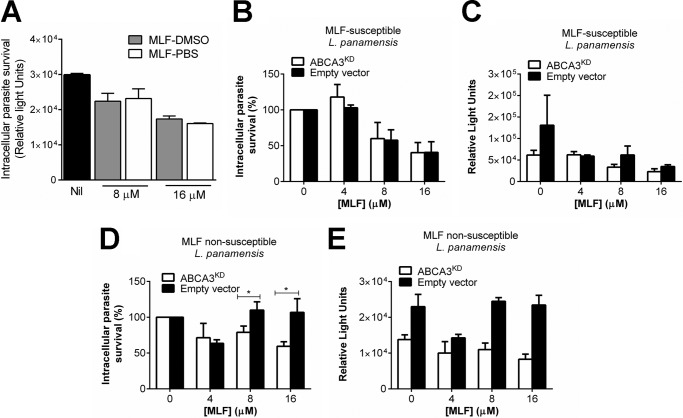
The effect of host cell ABCA3 expression on MLF-mediated parasite killing was evaluated. Parasite killing was comparable when cells were exposed to MLF preparations in DMSO and PBS (Fig. 5A). ABCA3KD and empty vector control PMA-differentiated THP-1 cells were infected with MLF-susceptible and nonsusceptible L. V. panamensis. MLF-mediated parasite killing was dose-dependent. No difference in intracellular parasite killing of the MLF-susceptible line was observed among KD and control cells (Fig. 5, B and C). However, MLF-non susceptible Leishmania was significantly killed in ABCA3KD cells, whereas no drug-mediated killing was observed in empty vector controls (Fig. 5, D and E).
FIGURE 5.
Intracellular survival of L. V. panamensis in ABCA3 knockdown cells. A, parasite survival in THP-1 wild type cells exposed for 24 h to increasing miltefosine preparations dissolved in either DMSO or PBS. Nil, untreated control. B–E, Leishmania survival in empty vector controls and ABCA3KD cells infected for 24 h with MLF-susceptible (B and C) and non-MLF-susceptible (D and E) L. V. panamensis and exposed to increasing concentrations of MLF dissolved in DMSO for additional 24h. The results are shown as percentages of survival over infected and untreated controls (B and D) and as absolute luciferase counts in relative light units (A, C, and E). The data are presented as mean values ± S.E. of experiments done in triplicate. Statistical significance was considered when p < 0.05 applying unpaired t test.
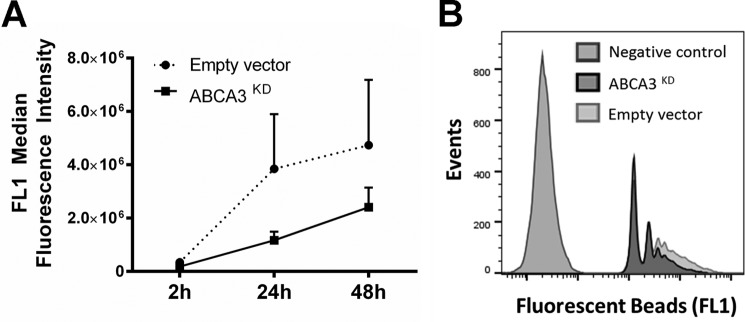
The average parasite burden in untreated ABCA3KD cells after infection with either MLF-susceptible or nonsusceptible parasites was 2-fold lower than control cells (Fig. 5, C and E), suggesting an additional effect of this membrane transporter in parasite uptake, intracellular survival, or replication. We evaluated the phagocytosis capacity of ABCA3KD cells using fluorescent latex beads. As shown in Fig. 6, ABCA3KD cells showed a reduced capacity to uptake latex beads reflected in lower fluorescence intensity, and this phenomenon was sustained in time (up to 48 h). These data support an additional influence of ABCA3 in macrophage phagocytosis.
FIGURE 6.
ABCA3 knockdown alters phagocytosis. Empty vector control and ABCA3KD cells were exposed to fluorescence-labeled latex beads, and uptake was measured by flow cytometry. Shown are results of time course experiments of three biological replicas each conducted in duplicate (A) and a representative image of the shift in fluorescence intensity in control and ABCA3KD cells (B).
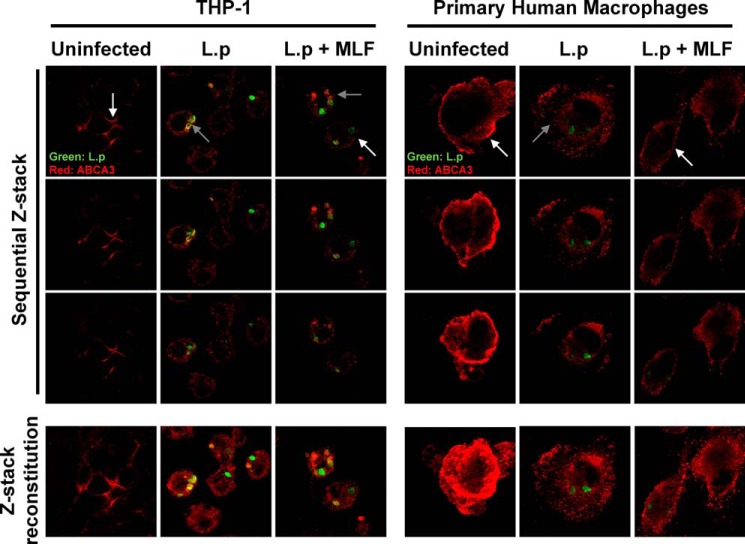
Intracellular Localization of ABCA3 in Leishmania-infected Macrophages
THP-1 cells and primary human macrophages were infected with GFP-expressing L. V. panamensis and exposed to 16 μm MLF. In uninfected and unexposed cells, the ABCA3 transporter was found primarily at the cell membrane (Fig. 7). Upon L. V. panamensis infection, ABCA3 localization was mainly intracellular in a vesicular pattern staining and in the proximity of Leishmania containing phagosomes in the THP-1 line. Exposure to MLF partially restored the cell membrane localization of ABCA3.
FIGURE 7.
Subcellular localization of ABCA3 in macrophages. Localization of host cell ABCA3 in THP-1 (left panel) and primary macrophages (right panel). The cells were left uninfected, infected with GFP-transfected L. V. panamensis (L.p) for 24 h, or infected and exposed to MLF for additional 24 h. ABCA3 is shown in red, and intracellular Leishmania in green. White and gray arrows depict the membrane or intracellular localization of ABCA3, respectively. Shown are sequential Z stack images and the Z stack reconstitution of sequential images taken at a Z scaling value of 0.6–0.9 μm.
Discussion
The effect of antimicrobials that target intracellular pathogens is, among others, determined by the capacity of drugs to be internalized into host cells and to traffic to the intracellular compartment in which pathogens reside. Our group and others have shown that killing of intracellular Leishmania by in vitro exposure to antimonial drugs is affected by the nature of the host cell (29) and by differential expression and function of ABC transporters (12, 13). In this study, we explored the role of human macrophages in the transport, accumulation, and exposure of intracellular Leishmania to MLF, the only oral drug available for the treatment of visceral and cutaneous leishmaniasis. Our results demonstrate the participation of ABCA3 in the transport of MLF across human macrophage membranes.
Gene expression profiles have been one of the most powerful tools to understand the mechanisms of action of bioactive compounds, the cellular response to drugs, and the identification of therapeutic targets and mechanisms of resistance (30, 31). Profiling the expression of membrane transporters modulated by L. V. panamensis infection and exposure to MLF suggested a possible contribution of ABCA1, ABCA3, and ABCD1 lipid transporters in MLF trafficking within human macrophages and supported the previously recognized role of ABCB1 in MLF efflux (17). Although two solute liquid carriers, SLC7A11 and SLC3A1, were also induced by exposure to miltefosine, higher interindividual variability was observed and thus not selected for further functional validation.
ABCA3 gene knockdown in THP-1 cells showed that MLF is a substrate of this membrane transporter. ABCA3 is a cholesterol and phosphatidylcholine influx transporter (27, 32, 33) found in the membranes of lysosomes and lamellar bodies, and its function has been primarily related to surfactant lipid metabolism and lamellar body biogenesis (28, 32, 34). Mutations in this transporter have been associated with surfactant dysfunction and respiratory diseases, especially in children (35) and recently its expression in lung cells has been reported to modulate susceptibility to cisplatin and paclitaxel (36). Increased ABCA3 expression in mononuclear cells during acute myeloid leukemia has been shown to mediate chemoresistance by intravesicular drug sequestration (33). The predominant plasma membrane localization of ABCA3 in macrophages, coupled to increased drug accumulation in the ABCA3KD line and the inverse correlation between ABCA3 gene expression and intracellular MLF accumulation, suggests a function of ABCA3 as an efflux transporter in human macrophages. Following L. V. panamensis infection, ABCA3 was relocalized to intracellular compartments, potentially lysosomes as previously described (33). Whether recruitment of this transporter to vesicular membranes promotes its delivery to the phagosomal membrane remains to be validated.
Unexpectedly, ABCA3 knockdown did not result in a significant difference in drug-mediated parasite killing of an MLF-susceptible L. V. panamensis strain. After 24 h of exposure to extracellular MLF concentrations as low as 4 μm, average intracellular concentrations were 3.3 and 5 μm in empty vector control and ABCA3KD THP-1 cells, respectively. This, together with the low EC50 of the MLF-susceptible strain (2.9 μm; 1.2 μg/ml (22)), suggests that the intracellular drug concentrations achieved in either control or ABCA3 KD macrophages are sufficient to kill MLF-susceptible parasites. In contrast, the nonsusceptible L. V. panamensis strain used in this study has been shown to have an EC50 value of >32 μm (22). The observed ∼40% reduction in parasite load in ABCA3KD cell in the presence of 16 μm extracellular MLF is consistent with intracellular MLF concentrations that are toxic even to nonsusceptible strains achieved in ABCA3KD cells. This indicates that increased drug accumulation within host macrophages could be exploited as a mechanism to partially revert nonsusceptibility phenotypes.
The role of ABCA3 during infection may not be restricted to MLF transport. ABCA3 knockdown decreased Leishmania uptake, potentially linked to reduced phagocytosis in ABCA3KD cells. Interestingly, Ced-7, a homologue of ABCA3 in Caenorhabditis elegans, functions in the engulfment of cell corpses during programmed cell death (37). Alterations in lipid transport could affect cell membrane composition, indirectly impacting on cell membrane-dependent processes such as endocytosis and phagocytosis (26). Thus, reduced parasite burden in ABCA3KD macrophages could be mediated by an additive effect of decreased Leishmania internalization and increased intracellular MLF accumulation and drug-mediated parasite killing.
Identification of a role for ABCA3 in reducing the antileishmanial activity of miltefosine raises the possibility of inhibiting ABCA3 to improve treatment success. Inhibition of ABCA3 expression has been reported to occur upon exposure of acute myeloid leukemia cells to the nonsteroidal anti-inflammatory drug indomethacin (38). The immunomodulatory properties of indomethacin make it unsuitable as component of Leishmania therapy, but a better understanding of how it affects ABCA3 expression may enable discovery of other, more suitable repressors.
We have previously reported an inverse correlation between Leishmania ABCA3 gene expression in amastigotes and MLF susceptibility, supporting the possibility that higher expression of vesicular ABCA3 in the parasite could promote susceptibility through increased drug accumulation (22). Although not functionally validated, this hypothesis would suggest that ABCA3 inhibitors could induce drug resistance in the parasite. However, the amino acid sequences of Leishmania ABCA3 (retrieved from either L.V. braziliensis, Leishmania major, or Leishmania infantum genomes) and mammalian ABCA3 share only 30% sequence identity; this divergence supports targeted intervention of host ABCA3. Conceivably, pharmacological modulation of host ABCA3 and potentially other ABC lipid transporters could potentiate the antileishmanial effect of miltefosine for the treatment of infections caused by Leishmania strains of diverse drug susceptibilities.
Here we report the expression of ABCA3 in human macrophages, its role in the transport of miltefosine, and its modulation during exposure to miltefosine. Our results provide evidence in support of the central role of host cell drug transport and accumulation in the direct activity of drugs that target intracellular pathogens. Characterization of the molecular mechanisms that mediate host cell drug influx, efflux, and trafficking between intracellular compartments increases our understanding of the pharmacokinetic-pharmacodynamic relationships of drugs active against intracellular pathogens. More importantly, it allows for identification of novel host-directed therapeutic strategies to optimize intracellular exposure and thus efficacy of both available and future antileishmanial drugs.
Author Contributions
L. C. T. D. performed, analyzed, and interpreted the data shown in Figs. 3, 5, and 7. A. N. performed and analyzed the experiments shown in Figs. 2, 4, and 6 and in Table 1. D. J. G. conceived and analyzed experiments using ABCA3 knockdown cells. A. K. performed and analyzed intracellular MLF quantitation assays. T. P. C. D. analyzed and interpreted data on intracellular drug transport and measurement of drug concentrations. D. A. V. performed and analyzed the experiments shown in Figs. 1–3, 5, and 7. M. A. G. conceived and coordinated the study and assembled the paper. All authors actively participated in writing and revising all versions of the paper, reviewed the results, and approved the final version of the manuscript.
Acknowledgments
We gratefully acknowledge the patients and volunteers who participated in this study and the members of the Clinical Unit of the Centro Internacional de Entrenamiento e Investigaciones Médicas in Cali and Tumaco for recruitment of participants and follow-up. We thank Paladin Labs Inc. for donation 14C-radiolabeled miltefosine and BD Biosciences for donation of the Accuri C6 platform.
This work was supported, in part, by COLCIENCIAS Grants 250-2010, Code 2229-519-28930, and 738-2009, Code 2229-49326161, the UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases Re-entry Grant B00032, and NIAID, National Institutes of Health Grant R01AI104823. The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- CL
- cutaneous leishmaniasis
- MLF
- miltefosine
- KD
- knockdown
- ABC
- ATP binding cassette
- PMA
- phorbol 12-myristate 13-acetate
- PBMC
- peripheral blood mononuclear cell.
References
- 1. Alvar J., Vélez I. D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M., and WHO Leishmaniasis Control Team (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vanaerschot M., Dumetz F., Roy S., Ponte-Sucre A., Arevalo J., and Dujardin J. C. (2014) Treatment failure in leishmaniasis: drug-resistance or another (epi-) phenotype? Expert Rev. Anti. Infect. Ther. 12, 937–946 [DOI] [PubMed] [Google Scholar]
- 3. Dorlo T. P., Balasegaram M., Beijnen J. H., and de Vries P. J. (2012) Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 67, 2576–2597 [DOI] [PubMed] [Google Scholar]
- 4. Soto J., Arana B. A., Toledo J., Rizzo N., Vega J. C., Diaz A., Luz M., Gutierrez P., Arboleda M., Berman J. D., Junge K., Engel J., and Sindermann H. (2004) Miltefosine for new world cutaneous leishmaniasis. Clin. Infect. Dis. 38, 1266–1272 [DOI] [PubMed] [Google Scholar]
- 5. Soto J., Rea J., Balderrama M., Toledo J., Soto P., Valda L., and Berman J. D. (2008) Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 78, 210–211 [PubMed] [Google Scholar]
- 6. Rubiano L. C., Miranda M. C., Muvdi Arenas S., Montero L. M., Rodríguez-Barraquer I., Garcerant D., Prager M., Osorio L., Rojas M. X., Pérez M., Nicholls R. S., and Gore Saravia N. (2012) Noninferiority of miltefosine versus meglumine antimoniate for cutaneous leishmaniasis in children. J. Infect. Dis. 205, 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Escobar P., Matu S., Marques C., and Croft S. L. (2002) Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Tropica 81, 151–157 [DOI] [PubMed] [Google Scholar]
- 8. Yardley V., Croft S. L., De Doncker S., Dujardin J. C., Koirala S., Rijal S., Miranda C., Llanos-Cuentas A., and Chappuis F. (2005) The sensitivity of clinical isolates of Leishmania from Peru and Nepal to miltefosine. Am. J. Trop. Med. Hyg. 73, 272–275 [PubMed] [Google Scholar]
- 9. Fernández O. L., Diaz-Toro Y., Ovalle C., Valderrama L., Muvdi S., Rodríguez I., Gomez M. A., and Saravia N. G. (2014) Miltefosine and antimonial drug susceptibility of Leishmania Viannia species and populations in regions of high transmission in Colombia. PLoS Negl. Trop. Dis. 8, e2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rijal S., Ostyn B., Uranw S., Rai K., Bhattarai N. R., Dorlo T. P., Beijnen J. H., Vanaerschot M., Decuypere S., Dhakal S. S., Das M. L., Karki P., Singh R., Boelaert M., and Dujardin J. C. (2013) Increasing failure of miltefosine in the treatment of Kala-azar in Nepal and the potential role of parasite drug resistance, reinfection, or noncompliance. Clin. Infect. Dis. 56, 1530–1538 [DOI] [PubMed] [Google Scholar]
- 11. Dorlo T. P., Rijal S., Ostyn B., de Vries P. J., Singh R., Bhattarai N., Uranw S., Dujardin J. C., Boelaert M., Beijnen J. H., and Huitema A. D. (2014) Failure of miltefosine in visceral leishmaniasis is associated with low drug exposure. J. Infect. Dis. 210, 146–153 [DOI] [PubMed] [Google Scholar]
- 12. Gómez M. A., Navas A., Márquez R., Rojas L. J., Vargas D. A., Blanco V. M., Koren R., Zilberstein D., and Saravia N. G. (2014) Leishmania panamensis infection and antimonial drugs modulate expression of macrophage drug transporters and metabolizing enzymes: impact on intracellular parasite survival. J. Antimicrob. Chemother. 69, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mookerjee Basu J., Mookerjee A., Banerjee R., Saha M., Singh S., Naskar K., Tripathy G., Sinha P. K., Pandey K., Sundar S., Bimal S., Das P. K., Choudhuri S. K., and Roy S. (2008) Inhibition of ABC transporters abolishes antimony resistance in Leishmania infection. Antimicrob. Agents Chemother. 52, 1080–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kip A. E., Rosing H., Hillebrand M. J., Castro M. M., Gomez M. A., Schellens J. H., Beijnen J. H., and Dorlo T. P. (2015) Quantification of miltefosine in peripheral blood mononuclear cells by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 998–999, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ménez C., Buyse M., Farinotti R., and Barratt G. (2007) Inward translocation of the phospholipid analogue miltefosine across Caco-2 cell membranes exhibits characteristics of a carrier-mediated process. Lipids 42, 229–240 [DOI] [PubMed] [Google Scholar]
- 16. Ménez C., Buyse M., Dugave C., Farinotti R., and Barratt G. (2007) Intestinal absorption of miltefosine: contribution of passive paracellular transport. Pharm. Res. 24, 546–554 [DOI] [PubMed] [Google Scholar]
- 17. Rybczynska M., Liu R., Lu P., Sharom F. J., Steinfels E., Di Pietro A., Spitaler M., Grunicke H., and Hofmann J. (2001) MDR1 causes resistance to the antitumour drug miltefosine. Br. J. Cancer 84, 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pérez-Victoria J. M., Pérez-Victoria F. J., Parodi-Talice A., Jiménez I. A., Ravelo A. G., Castanys S., and Gamarro F. (2001) Alkyl-lysophospholipid resistance in multidrug-resistant Leishmania tropica and chemosensitization by a novel P-glycoprotein-like transporter modulator. Antimicrob. Agents Chemother. 45, 2468–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pérez-Victoria J. M., Di Pietro A., Barron D., Ravelo A. G., Castanys S., and Gamarro F. (2002) Multidrug resistance phenotype mediated by the P-glycoprotein-like transporter in Leishmania: a search for reversal agents. Curr. Drug Targets 3, 311–333 [DOI] [PubMed] [Google Scholar]
- 20. Castanys-Muñoz E., Alder-Baerens N., Pomorski T., Gamarro F., and Castanys S. (2007) A novel ATP-binding cassette transporter from Leishmania is involved in transport of phosphatidylcholine analogues and resistance to alkyl-phospholipids. Mol. Microbiol. 64, 1141–1153 [DOI] [PubMed] [Google Scholar]
- 21. Castanys-Muñoz E., Pérez-Victoria J. M., Gamarro F., and Castanys S. (2008) Characterization of an ABCG-like transporter from the protozoan parasite Leishmania with a role in drug resistance and transbilayer lipid movement. Antimicrob. Agents Chemother. 52, 3573–3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Obonaga R., Fernández O. L., Valderrama L., Rubiano L. C., Castro Mdel M., Barrera M. C., Gomez M. A., and Gore Saravia N. (2014) Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus Viannia species. Antimicrob. Agents Chemother. 58, 144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roy G., Dumas C., Sereno D., Wu Y., Singh A. K., Tremblay M. J., Ouellette M., Olivier M., and Papadopoulou B. (2000) Episomal and stable expression of the luciferase reporter gene for quantifying Leishmania spp. infections in macrophages and in animal models. Mol. Biochem. Parasitol. 110, 195–206 [DOI] [PubMed] [Google Scholar]
- 24. Zhou H., DeLoid G., Browning E., Gregory D. J., Tan F., Bedugnis A. S., Imrich A., Koziel H., Kramnik I., Lu Q., and Kobzik L. (2012) Genome-wide RNAi screen in IFN-γ-treated human macrophages identifies genes mediating resistance to the intracellular pathogen Francisella tularensis. PLoS One 7, e31752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. D'Agostino R., and Pearson E. S. (1973) Tests for departure from normality: empirical results for the distributions of b2 and b1. Biometrika 60, 613–622 [Google Scholar]
- 26. Tarling E. J., de Aguiar Vallim T. Q., and Edwards P. A. (2013) Role of ABC transporters in lipid transport and human disease. Trends Endocrinol. Metab. 24, 342–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsumura Y., Sakai H., Sasaki M., Ban N., and Inagaki N. (2007) ABCA3-mediated choline-phospholipids uptake into intracellular vesicles in A549 cells. FEBS Lett. 581, 3139–3144 [DOI] [PubMed] [Google Scholar]
- 28. Cheong N., Madesh M., Gonzales L. W., Zhao M., Yu K., Ballard P. L., and Shuman H. (2006) Functional and trafficking defects in ATP binding cassette A3 mutants associated with respiratory distress syndrome. J. Biol. Chem. 281, 9791–9800 [DOI] [PubMed] [Google Scholar]
- 29. Seifert K., Escobar P., and Croft S. L. (2010) In vitro activity of anti-leishmanial drugs against Leishmania donovani is host cell dependent. J. Antimicrob. Chemother. 65, 508–511 [DOI] [PubMed] [Google Scholar]
- 30. Cohen A. L., Soldi R., Zhang H., Gustafson A. M., Wilcox R., Welm B. E., Chang J. T., Johnson E., Spira A., Jeffrey S. S., and Bild A. H. (2011) A pharmacogenomic method for individualized prediction of drug sensitivity. Mol. Syst. Biol. 7, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bosch T. M. (2008) Pharmacogenomics of drug-metabolizing enzymes and drug transporters in chemotherapy. Methods Mol. Biol. 448, 63–76 [DOI] [PubMed] [Google Scholar]
- 32. Cheong N., Zhang H., Madesh M., Zhao M., Yu K., Dodia C., Fisher A. B., Savani R. C., and Shuman H. (2007) ABCA3 is critical for lamellar body biogenesis in vivo. J. Biol. Chem. 282, 23811–23817 [DOI] [PubMed] [Google Scholar]
- 33. Chapuy B., Koch R., Radunski U., Corsham S., Cheong N., Inagaki N., Ban N., Wenzel D., Reinhardt D., Zapf A., Schweyer S., Kosari F., Klapper W., Truemper L., and Wulf G. G. (2008) Intracellular ABC transporter A3 confers multidrug resistance in leukemia cells by lysosomal drug sequestration. Leukemia 22, 1576–1586 [DOI] [PubMed] [Google Scholar]
- 34. Ban N., Matsumura Y., Sakai H., Takanezawa Y., Sasaki M., Arai H., and Inagaki N. (2007) ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J. Biol. Chem. 282, 9628–9634 [DOI] [PubMed] [Google Scholar]
- 35. Agrawal A., Hamvas A., Cole F. S., Wambach J. A., Wegner D., Coghill C., Harrison K., and Nogee L. M. (2012) An intronic ABCA3 mutation that is responsible for respiratory disease. Pediat. Res. 71, 633–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Overbeck T. R., Hupfeld T., Krause D., Waldmann-Beushausen R., Chapuy B., Güldenzoph B., Aung T., Inagaki N., Schöndube F. A., Danner B. C., Truemper L., and Wulf G. G. (2013) Intracellular ATP-binding cassette transporter A3 is expressed in lung cancer cells and modulates susceptibility to cisplatin and paclitaxel. Oncology 84, 362–370 [DOI] [PubMed] [Google Scholar]
- 37. Wu Y. C., and Horvitz H. R. (1998) The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 93, 951–960 [DOI] [PubMed] [Google Scholar]
- 38. Song J. H., Kim S. H., Kim H. J., Hwang S. Y., and Kim T. S. (2008) Alleviation of the drug-resistant phenotype in idarubicin and cytosine arabinoside double-resistant acute myeloid leukemia cells by indomethacin. Int. J. Oncol. 32, 931–936 [PubMed] [Google Scholar]