Abstract
Cardiac stem cell therapy has shown very promising potential to repair the infarcted heart but is severely limited by the poor survival of donor cells. Nitric oxide (NO) has demonstrated cytoprotective properties in various cells, but its benefits are unknown specifically for human cardiac stem cells (hCSCs). Therefore, we investigated whether pretreatment of hCSCs with a widely used NO donor, diethylenetriamine nitric oxide adduct (DETA-NO), promotes cell survival. Results from lactate dehydrogenase release assays showed a dose- and time-dependent attenuation of cell death induced by oxidative stress after DETA-NO preconditioning; this cytoprotective effect was abolished by the NO scavenger. Concomitant up-regulation of several cell signaling molecules after DETA-NO preconditioning was observed by Western blotting, including elevated phosphorylation of NRF2, NFκB, STAT3, ERK, and AKT, as well as increased protein expression of HO-1 and COX2. Furthermore, pharmaceutical inhibition of ERK, STAT3, and NFκB activities significantly diminished NO-induced cytoprotection against oxidative stress, whereas inhibition of AKT or knockdown of NRF2 only produced a minor effect. Blocking PI3K activity or knocking down COX2 expression did not alter the protective effect of DETA-NO on cell survival. The crucial roles of STAT3 and NFκB in NO-mediated signaling pathways were further confirmed by stable expression of gene-specific shRNAs in hCSCs. Thus, preconditioning hCSCs with DETA-NO promotes cell survival and resistance to oxidative stress by activating multiple cell survival signaling pathways. These results will potentially provide a simple and effective strategy to enhance survival of hCSCs after transplantation and increase their efficacy in repairing infarcted myocardium.
Keywords: apoptosis, cell therapy, heart failure, myocardial infarction, nitric oxide, preconditioning, cardiac stem cell, cell survival
Introduction
Ischemic heart disease is a common cardiac disorder with great prevalence and mortality worldwide. Current surgical treatment for acute myocardial infarction is relatively effective but invariably induces further cardiomyocyte death by ischemia-reperfusion injury (1). Stem cells, with the unique capacity of tissue regeneration, have been widely investigated for cardiac repair in the past, although some types of cells showed only negative, transient, or modest effects (2, 3). The discovery of c-Kit+/Lin− endogenous cardiac stem cells (CSCs)2 was striking because of their great potential of differentiating into multiple cell types presented in the heart (4). Subsequent studies demonstrated that c-Kit+ CSCs were proliferative in culture, and delivery of these cells into an infarcted heart improved heart function in various animal models (5–8). A recent early clinical trial has announced the beneficial effect of CSC transplantation in patients with better heart function, which holds great promise as a treatment after myocardial infarction (9). However, there is still space to improve the efficacy: one of the main challenges associated with cell transplantation is the fact that more than 90% of transplanted cells cannot survive within the ischemic heart (8, 10, 11). Therefore, approaches to enhance CSC survival would lead to great therapeutic implications for patients with ischemic heart disease.
Nitric oxide (NO) is a gaseous signaling molecule that plays important roles in many physiological and pathological processes. It is endogenously biosynthesized from l-arginine, oxygen, and NADPH by nitric-oxide synthase (NOS) enzymes. There are three NOS isoforms: endothelial NOS, neuronal NOS, and inducible NOS. Endothelial NOS and neuronal NOS are constitutively expressed to produce a physiological level of NO, whereas inducible NOS is a stress-inducible protein in response to variable stimuli. NO has been implicated in myriad biological functions and contributes to vascular homeostasis in the cardiovascular system by inhibiting smooth muscle contraction (12). NO has also been found to function in preventing myocardial ischemia-reperfusion injury (13, 14). Nevertheless, humans with atherosclerosis, diabetes, or hypertension often exhibit impaired NO pathways, suggesting an important preventive role of NO in cardiovascular disease (15–17). At the molecular level, the regulatory functions of NO have been noticed to be associated with several survival signaling molecules, including PI3K/AKT, ERK, NFκB, and STAT3 (18–21), as well as antiapoptotic genes such as BCL-2, BCL-xL, and MCL-1 (22, 23). Although the protective effect of NO in the cardiovascular system has been extensively studied, little is known about its active functional roles in CSCs.
Diethylenetriamine nitric oxide adduct (DETA-NO) is a chemical-based NO releaser with a half-life of 20 h. Here we demonstrate that preconditioning human cardiac stem cells (hCSCs) with DETA-NO promotes cell survival and resistance to oxidative stress via activating multiple cell survival signaling molecules. As noted, oxidative stress can be induced by ischemia-reperfusion during cardiac surgery, which is one of the main causes of death of native cardiomyocytes as well as transplanted cells. By pretreating hCSCs with this NO donor, we expect to provide a simple and effective strategy to enhance hCSC survival after cell transplantation and to improve efficacy in cardiac repair for patients with ischemic heart disease.
Materials and Methods
Reagents
Collagenase II was from Worthington Biochemical. Ham's F-12 medium was from Invitrogen. Fetal bovine serum (FBS) was obtained from Hyclone. Antibodies to p-ERK, ERK, HO-1, COX2, NRF2, NFκB, and STAT3 were from Santa Cruz Biotechnology; antibodies to BCL-xL, MCL-1, BCL-2, p-p65, p-STAT3, and p-AKT were from Cell Signaling Technology; and p-NRF2 was from Abcam. Quantitative PCR primers for target genes were obtained from Real Time Primers, LLC. Unless indicated otherwise, chemicals used in experiments were purchased from Sigma.
Harvesting of Human c-Kit+ CSCs
hCSCs expressing c-Kit cell surface marker were isolated and purified using atrial appendages from patients during open heart surgery at Albany Medical Center. Our protocol has been approved by the Institutional Committee on Research Involving Human Subjects (Institutional Review Board). All participants in the study provided written informed consent. The procedures were exactly followed as described previously (24, 25). Briefly, right atrial tissues (100–400 mg) were minced and enzymatically digested with collagenase II (30 units/ml) at 37 °C in a shaking water bath. During the incubation, chopped tissues were mechanically disturbed by gently pipetting several times. After 1 h of incubation, undigested clumps were separated by gravity on ice for 10 min, and the supernatant was carefully transferred into a 15-ml tube. Dissociated cells from digestion were collected by centrifugation at 1200 rpm for 5 min. The cell pellet was suspended and cultured in Ham's F-12 medium supplemented with 10% FBS, 10 ng/ml human basic FGF, 0.005 unit/ml human erythropoietin, 0.2 mm l-glutathione, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained in a humidified environment at 37 °C and 5% CO2. The next day, the CSC growth medium was refreshed, and adherent cells were cultured with a medium change every other day. Upon reaching around 80% confluence, cells were sorted using the c-Kit MACS kit according to the manufacturer's instructions (Miltenyi Biotec), expanded, and characterized by FACS analysis to obtain lineage-negative hCSCs. Cell characterization was performed as described previously (24–26). Human CSCs from the donor AMC1 were used at passages 8–15 to perform all the in vitro experiments in this study. The lactate dehydrogenase (LDH) release assay was performed in three extra preparations of hCSCs (AMC3, AMC6, and AMC9).
Priming hCSCs with DETA-NO
hCSCs were trypsinized and subcultured at a density of 2000–3000 cells/cm2 in normal 10% FBS, F-12 medium without growth factors prior to experiments. The next day, cells were treated with or without DETA-NO at the indicated dosage for the indicated time period. To determine the optimal conditions, a dose at the range of 5–500 μm DETA-NO was applied to prime cells for 12 h and challenged with H2O2 in F-12 serum-free medium thereafter. The cytoprotective effect of DETA-NO preconditioning against oxidative stress was detected by LDH release assay. The following time-dependent experiments were performed by treating cells with 250 μm DETA-NO for several time points within 24 h. Based on optimized dose and time for DETA-NO preconditioning, the efficacy of cytoprotection was also examined by withdrawal of DETA-NO for up to 24 h.
Cell Viability Assay
An LDH release assay, a simple approach to measure cellular membrane integrity, was applied to determine the oxidative stress-induced cell death in hCSCs. The procedures were exactly followed according to the manufacturer's instructions from the Cytotoxicity LDH Detection kit (Takara). The day before DETA-NO preconditioning, hCSCs were seeded at a density of 1 × 104/well in a 96-well plate. After treatment, cells were exposed to 2 mm H2O2 for 3 h, an optimized condition following pretesting shown in the Fig. 1A. The plate was then centrifuged at 250 × g for 10 min. 100 μl of the supernatant was collected and mixed with an equal volume of pre-prepared solution (catalyst/dye buffer = 1:45) for 30 min at room temperature in a 96-well plate. The absorbance of samples at 490 nm was measured using a Bio-Rad iMarkTM microplate reader. The percentage of LDH release for each sample was determined by comparing with the absorbance value from cells pretreated with 0.5% Triton X-100.
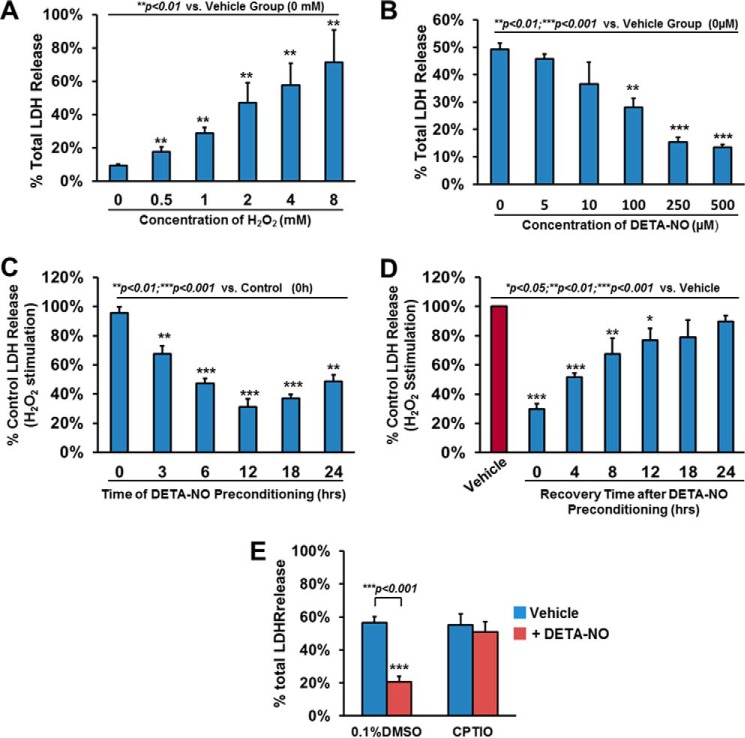
FIGURE 1.
Preconditioning with nitric oxide donors, DETA-NO, enhances hCSC survival. A, hCSCs were challenged with the indicated dose of H2O2 for 3 h and evaluated by LDH assay. B, hCSCs were preconditioned with various concentrations of DETA-NO for 12 h, challenged with H2O2, and evaluated by LDH assay. C, hCSCs were preconditioned with 250 μm DETA-NO for the indicated time, challenged with H2O2, and evaluated by LDH assay. D, after hCSCs were preconditioned with 250 μm DETA-NO for 12 h, medium was refreshed for a period of time as indicated. Cells were then challenged with H2O2 followed by LDH assay. E, hCSCs were pretreated with 250 μm DETA-NO in the presence or absence of 10 μm 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (CPTIO) for 12 h. Cells were then challenged with H2O2 and evaluated by LDH assay. Data are presented as mean ratio of H2O2-induced LDH release to total LDH in cells with S.D. (means ± S.D.). Error bars represent S.D. * indicates p < 0.05 versus vehicle; ** indicates p < 0.01 versus vehicle; *** indicates p < 0.001 versus vehicle; n = 4 independent experiments. DMSO, dimethyl sulfoxide.
Apoptosis Assay
Cell apoptosis was investigated by dual staining of hCSCs with FITC-conjugated Annexin V and propidium iodide (PI) using an Annexin V/Dead Cell Apoptosis kit (Invitrogen). Briefly, hCSCs were plated at a density of 2 × 105/well in a 6-well plate prior to preconditioning. After 12 h of 250 μm DETA-NO pretreatment, hCSCs were challenged with or without 1 mm H2O2 in serum-free F-12 medium for 90 min. Cells were then detached by TrypLE solution (Invitrogen), washed twice with PBS, and suspended in 100 μl of 1× annexin binding buffer supplemented with 1 μg/ml PI and 5 μl of Alexa Fluor 488-Annexin V solution. After 15-min incubation at room temperature, 400 μl of 1× annexin binding buffer was added with gentle mixing, and samples were immediately analyzed by LSRII flow cytometry (BD Biosciences).
BrdU Cell Proliferation Assay
hCSCs incorporated with BrdU were analyzed by flow cytometry to determine the effect of DETA-NO on cell proliferation ability. Briefly, hCSCs were preconditioned with either vehicle or DETA-NO for 12 h and then refreshed with growth medium supplemented with 10 μm BrdU. 48 h later, cells were detached by trypsinization, washed with PBS twice, and fixed with 70% ice-cold ethanol for 1 h on ice. Cells were then permeabilized with 0.5% Triton X-100 for 20 min and treated with 2 m HCl for another 30 min. After sufficient 4× PBS washes, cells were stained with BrdU antibody in 1% BSA, PBS for 2 h followed by the application of a secondary mouse FITC-conjugated antibody for 1 h. On the final PBS wash, 1 μg/ml PI was applied to 100 μl of suspended cells for 5 min. Cells were subsequently diluted to an appropriate volume in PBS for flow cytometry analysis.
MTT-based Cell Growth Determination Assay
The effect of DETA-NO on cellular growth was evaluated by measuring mitochondrial activity in living cells. The procedures were carried out according to a protocol provided in the MTT assay kit (Sigma). Generally, hCSCs were seeded in a 96-well plate at a density of 0.5 × 104/100 μl of culture medium. The next day, cells were preconditioned with vehicle or DETA-NO for 12 h, and medium was then replaced with 100 μl of fresh growth medium. 48 h later, 10 μl of MTT reconstituted in balanced salt solution (provided by kit) was added to cells in each well (10% of the culture medium volume), and the plate was returned to the incubator for another 3 h of culture. After the incubation period, cultures were removed, and the resulting formazan crystals were dissolved in 100 μl of MTT solubilization solution followed by spectrophotometrically measuring absorbance at a wavelength of 570 nm.
Inhibitory Exclusion Assay
To screen and evaluate the important roles of activated survival signaling molecules by DETA-NO preconditioning, pharmaceutical inhibition of ERK (10 μm U0126), STAT3 (1 μm STATTIC), NFκB (5 μm Bay117085), PI3K (10 μm LY492002), and AKT (0.2 μm AKT IV inhibitor) were applied to hCSCs with or without DETA-NO preconditioning. The effects of blocking these signaling molecules were examined by LDH release assay under H2O2-induced oxidative stress. The roles of NRF2, COX2, and HO-1 were also examined using previously established hCSCs with stable expression of shRNA of these genes.
ROS Measurement
Intracellular ROS levels were detected using the Cellular ROS Detection Assay kit (Abcam). Procedures were performed according to the manufacturer's instructions. hCSCs were detached by TrypLE solution and stimulated with H2O2 (2 mm) for 30 min. The cell-permeable ROS Deep Dye was then added to suspended cells with appropriate dilution (1:1000) as indicated in the protocol, and cells were further maintained at 37 °C for another 1 h. After two PBS washes, the intensity of red fluorescence was determined at excitation/emission of 650/675 nm.
Lentiviral Infection of hCSCs
Specific knockdown of STAT3 and NFκB in hCSCs was carried out using shRNA lentiviral particles according to the manufacturer's instructions (Santa Cruz Biotechnology). Both scrambled shRNA and GFP lentiviral particles were also used to infect hCSCs as negative and positive controls. hCSCs were seeded at a density of 1 × 105/well in a 6-well plate 24 h prior to infection. At 50–60% confluence, cells were refreshed with 2 ml of the culture medium followed by the direct addition of 5 μg/ml Polybrene and 20 μl of lentiviral particles with gentle orbital shaking. The next day, medium was replaced with normal growth medium for recovery. 48 h after infection, 5 μg/ml puromycin was applied to the culture medium in both infected and non-infected hCSCs until all non-infected cells died eventually (2–3 days). To obtain stable transfected cells, hCSCs were further cultured in puromycin-containing growth medium for up to 10 days. By that time, almost 100% of cells with GFP fluorescence were observed in the positive control group. The efficiency of gene knockdown was subsequently confirmed by Western blotting.
RNA Isolation and Quantitative Analysis
Real time PCR was performed to determine the change of antiapoptotic or NFκB pathway-related genes in response to DETA-NO preconditioning in hCSCs. Primers used in this study were obtained from RealTimePrimers, LLC. The total RNA of each sample was extracted and purified using the AurumTM Total RNA minikit (Bio-Rad). The quality and quantity of RNA were detected by a NanoDrop 2000C spectrophotometer (Thermo Scientific). The reverse transcription of 1 μg of RNA to cDNA was established using the Bio-Rad iScriptTM cDNA Synthesis kit. Samples for real time PCR were prepared according to the manufacturer's instructions in the iQ SYBR Green Supermix kit (Bio-Rad), and real time PCR was run with a Bio-Rad iQ5 optical module. Cycling conditions were as follows: 95 °C for 2 min as initial denaturation, 40 cycles of denaturation at 95 °C for 15 s, and annealing/extension at 60 °C for 40 s. Melt curve analysis was set between 65 and 95 °C with 0.5 °C increments at 5 s per step. In these experiments, GAPDH was used as internal control gene for quantitative RT-PCR expression analysis.
Immunoblotting
Western blotting analysis was carried out according to a protocol as described previously (27). After two ice-cold PBS washes, cells were lysed in ice-cold modified immunoprecipitation assay buffer (150 mm NaCl, 5 mm EDTA, 1% Nonidet P-40, 20 mm Tris-HCl, pH 7.5) with addition of protease and phosphatase inhibitor mixtures. 20 μg of protein from each sample was separated on 10% polyacrylamide gels and transferred to PVDF membranes. After blocking with 5% milk in TBST (50 mm Tris·HCl, pH 7.4, 150 mm NaCl, 0.1% Tween 20) buffer for 1 h, primary antibodies against specific genes of interest were applied followed by HRP-conjugated secondary antibodies. The chemiluminescence signals were detected using ECL-Plus reagent (GE Healthcare). The α-tubulin detected in the same sample was used as an equal loading control. Band density analysis was performed by ImageJ software. All data were normalized to the band density for tubulin.
Statistics
Data are presented as means ± S.D. of results taken from at least three independent experiments. Statistical significance was assessed by analysis of variance using Bonferroni/Dunn test or unpaired Student's t test. A p value less than 0.05 was considered statistically significant.
Results
NO Preconditioning Enhanced hCSC Survival against Oxidative Stress
NO has been shown to have an antiapoptotic role in many types of cells (28–31). However, little is known about whether it also plays a preventive role against oxidative stress in cardiac stem cells. Hydrogen peroxide is one of the physiological oxidants in cells, and its excessive production caused by ischemia-reperfusion is capable of inducing further cardiac damage during surgery. To evaluate the effect of H2O2-induced cellular damage in hCSCs, the levels of LDH release were examined at a dose of 0–8 mm H2O2. As shown in Fig. 1A, there was a dose-dependent increase of LDH release, and an induction of 50% of total LDH release was observed when hCSCs were challenged with 2 mm H2O2 for 3 h, the condition used for all the following experiments unless specifically indicated.
To address our hypothesis that NO is cytoprotective for hCSCs, we examined the effect of DETA-NO preconditioning on promoting hCSC survival under H2O2-induced oxidative stress. By preconditioning hCSCs with DETA-NO (5–500 μm) for 12 h, the H2O2-induced LDH release was significantly alleviated at the dose of 100 μm and was further reduced by 70% at the dose of 250 μm and stabilized afterward compared with the control group (Fig. 1B). To assess the optimal preconditioning time, we pretreated hCSCs with 250 μm DETA-NO for several time points up to 24 h. As shown in Fig. 1C, pretreating hCSCs with DETA-NO showed a time-dependent decrease of LDH release under H2O2-induced oxidative stress. The rapid drop of LDH release was detected at 3 h of preconditioning followed by a further significant reduction (∼50%) of LDH release at 6 h. The most reduction (∼70%) of LDH release was observed at 12 h of preconditioning, and this cytoprotection remained effective between 12 and 24 h. The LDH release assay was further performed in three extra preparations of hCSCs isolated from different donors/patients whose basic demographic information is listed in supplemental Table 1. As shown in supplemental Fig. 2, the LDH release was significantly decreased about 1-fold for these hCSCs preconditioned with 250 μm DETA-NO for 12 h compared with the vehicle control, confirming the cytoprotective effect of preconditioning hCSCs with NO donor in multiple lines.
To evaluate the time length of the cytoprotective effect after DETA-NO preconditioning, pretreated hCSCs (250 μm DETA-NO for 12 h) were refreshed with normal cell culture medium for the indicated time points up to 24 h. Compared with the vehicle group with H2O2 stimulation, we observed a gradual decrease in cytoprotection starting from 70% reduction of LDH release at 0 h to a 25% reduction at 12 h (Fig. 1D). There was no significant difference of LDH release between the vehicle group and the DETA-NO preconditioned hCSCs with more than 12-h recovery. In a parallel study using another NO donor, S-nitroso-N-acetyl-dl-penicillamine, we found that preconditioning hCSCs with S-nitroso-N-acetyl-dl-penicillamine exhibited the similar dose- and time-dependent cytoprotective effect against H2O2-induced cell death (supplemental Fig. 1). To verify the specific role of NO in hCSCs, we also introduced 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (NO scavenger) into cells and found that the protective effect of NO was completely diminished (Fig. 1E). These data indicate that preconditioning hCSCs with 250 μm DETA-NO for 12 h is the most effective in prevention of cell death under oxidative stress. Therefore, these optimized conditions for hCSC pretreatment were applied in the following experiments (unless otherwise specified).
To further evaluate the functional role of DETA-NO preconditioning in promoting hCSC survival, we applied an effective approach to examine cell apoptosis by staining cells with Annexin V-FITC and PI. Flow cytometry analysis showed live cells with double negative staining in the Q3 area and early apoptotic cells with single annexin V staining in the Q4 area, whereas cells with dually positive Annexin V/PI staining in the Q2 area indicated necrosis or later apoptosis (Fig. 2A). In the normal condition (without H2O2 stimulation), there was no significant change of live cells (92.9 ± 0.25 versus 93.7 ± 0.99%), apoptotic cells (3.9 ± 0.78 versus 3.6 ± 0.53%), and necrotic cells (2.8 ± 0.88 versus 2.3 ± 0.88%) between the DETA-NO-preconditioned group (hCSCs-PC) and the control group (hCSCs). With 1 mm H2O2 stimulation for 90 min, 25 ± 2.19% total cell death (defined as the difference between live cells and total cells) was observed in the control group, whereas the preconditioned group exhibited a significant reduction of total cell death (17.1 ± 1.65%). To specifically look at the type of cell death under oxidative stress, we observed that H2O2-induced cell apoptosis in the preconditioned group was significantly decreased by 50% compared with the control group. However, there was no significant difference for necrotic cells between these groups (Fig. 2B). Taken together with data from LDH release assays, these results suggest that preconditioning hCSCs with DETA-NO greatly improves cell survival.
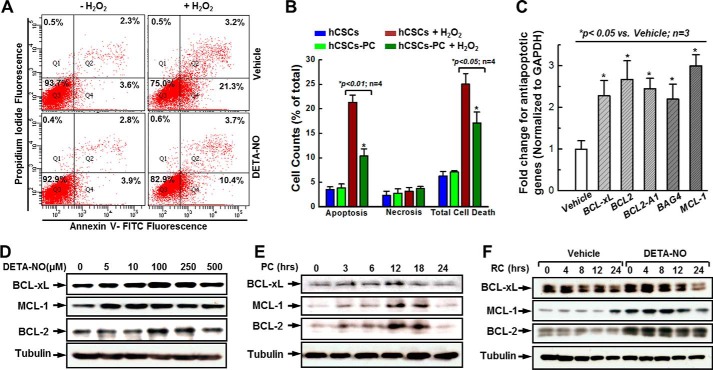
FIGURE 2.
DETA-NO preconditioning promotes hCSC anti-apoptotic effect. A, representative FACS analysis with annexin V/PI staining showing H2O2-induced apoptosis in both preconditioned (PC) (250 μm DETA-NO for 12 h) and vehicle-treated groups. B, quantitative data analysis for A. C, examination of antiapoptotic gene expression at the mRNA level in response to DETA-NO preconditioning. D, representative images of Western blot showing the dose-dependent up-regulation of antiapoptotic gene expression in response to DETA-NO preconditioning. E, representative images of Western blot showing the time-related up-regulation of antiapoptotic gene expression with 250 μm DETA-NO preconditioning. F, representative images of Western blot showing antiapoptotic gene expression levels after DETA-NO withdrawal over 24 h. The quantitative data for Western blots shown in D–F are provided in supplemental Fig. 4. Values are means ± S.D. Error bars represent S.D. * indicates p < 0.05 versus control; n = 3 independent experiments. RC, recovery.
Based on the functional study, it seemed that DETA-NO preconditioning efficiently attenuated H2O2-induced cell death, mainly via antiapoptotic mechanism. To confirm this, antiapoptotic gene expression was investigated by quantitative PCR using a PCR primer library screening. As shown in Fig. 2C, a 2–3-fold increase was detected in the gene expression of BCL-xL, BCL-2, BCL2-A1, BAG4, and MCL-1 in response to DETA-NO preconditioning. Protein levels of the most up-regulated genes, BCL-xL, BCL-2, and MCL-1, were subsequently examined by Western blotting. Consistent with LDH release assay results, DETA-NO preconditioning seemed to influence the expression of these three proteins in a highly dose- and time-dependent manner (Fig. 2, D and E, and supplemental Fig. 3, A and B). We observed that these antiapoptotic genes reached their peak expression levels when hCSCs were pretreated with 250 μm DETA-NO for 12 h, which is the same condition that exhibited the best survival ability in the aforementioned functional assays. Moreover, gradual decreases of BCL-xL and MCL-1 expression, along with extended recovery time, were also observed when preconditioning medium was withdrawn (Fig. 2F and supplemental Fig. 3C). These data indicate that the promotion of hCSC survival by DETA-NO preconditioning may be influenced by the activation of antiapoptotic genes.
DETA-NO Preconditioning Has No Effect on Cellular Abilities of hCSC Proliferation and Differentiation
The purpose of this in vitro work is to explore a strategy to improve hCSC survival and its efficacy for cardiac repair after cell transplantation. To exclude the concern that this pretreatment could lead to side effects on cell regenerative abilities, cardiac lineage gene expressions were first examined following DETA-NO preconditioning. As shown in Fig. 3A, there were no significant differences in the expression of cardiac genes, including cardiac troponin T (cTNT), GATA4, MEF2C, NKX2.5, MYH6, and RYR2, between preconditioned and non-conditioned hCSCs. To evaluate the effect of DETA-NO on cell proliferation ability, both a BrdU cell proliferation assay and MTT assay were performed. After DETA-NO preconditioning, hCSCs were cultured in the normal growth medium supplemented with 10 μm BrdU for 3 days. As presented in Fig. 3B, there was no significant difference of BrdU+/PI+ cells between non-conditioned and preconditioned groups (85.68 ± 1.88 versus 81.78 ± 2.09%). This result is consistent with what was found in the MTT assay (Fig. 3C).
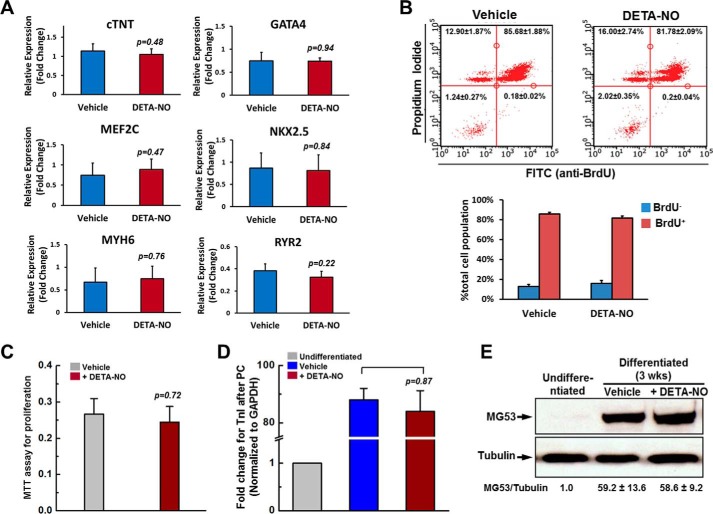
FIGURE 3.
DETA-NO preconditioning did not alter the capacities of hCSC cardiac gene expression, cell proliferation and proliferation. A, quantitative PCR of cardiac gene expressions in hCSCs pretreated with vehicle or DETA-NO. cTNT, cardiac troponin T. B, BrdU incorporation assay examined by flow cytometry. hCSCs were preconditioned with or without DETA-NO for 12 h, and cells were then cultured in growth medium supplemented with 10 μm BrdU for 3 days. C, MTT assay. hCSCs were preconditioned with vehicle or DETA-NO for 12 h, and cells were then cultured in growth medium 3 days. D and E, hCSCs were preconditioned (PC) with vehicle or DETA-NO, and cells were then cultured in differentiation medium for 3 weeks (wks). The expression level of Troponin I was examined by quantitative PCR (D). Expression of MG53 protein was examined by Western blotting (E). Data are shown as mean ± S.D. from three independent experiments. Error bars represent S.D.
To examine whether DETA-NO preconditioning alters cellular capacity for differentiation in vitro, hCSCs were preconditioned with either vehicle or DETA-NO, and then cells were refreshed in the differentiation medium for continuous culture for 3 weeks (4). In comparison with undifferentiated hCSCs, there was a dramatic increase in gene expression of Troponin I after differentiation in both DETA-NO-treated and vehicle-treated hCSCs with no difference between these two groups (Fig. 3D). A similar result was also observed for expression of MG53 protein, which is known to be highly expressed in mature muscle cells but not in cells at stemness stage (Fig. 3E). Taken together, these results offer assurance that DETA-NO preconditioning has no adverse effect on the cell regenerative abilities in terms of proliferation and differentiation in hCSCs.
DETA-NO Preconditioning Activated Multiple Cell Survival Signaling Pathways
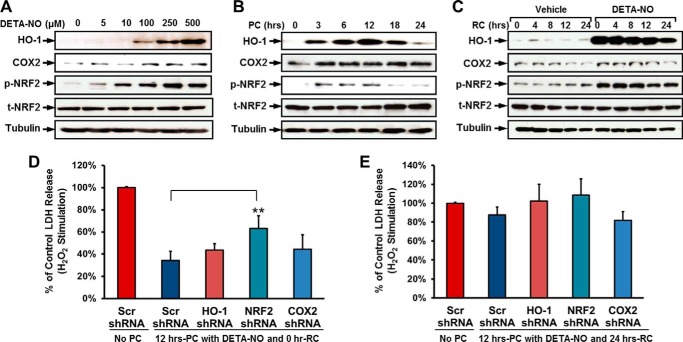
Although up-regulation of antiapoptotic genes induced by DETA-NO pretreatment was observed, it remained unknown which specific cell survival signaling pathway was involved in the NO-induced antiapoptotic effect. In our previous study, we demonstrated that preconditioning hCSCs with cobalt protoporphyrin (CoPP), an HO-1 inducer, enhanced cell survival via activation of NRF2 and COX2 signaling pathways (27). However, it was unclear whether these two signaling molecules were also activated by DETA-NO preconditioning and played similar roles in mediating hCSC survival. In response to DETA-NO preconditioning, expression of COX2 and HO-1 and p-NRF2 activation were found to be concomitantly elevated in both dose- and time-dependent manners, reaching their peak levels when hCSCs were treated with 250 μm DETA-NO for 12 h (Fig. 4, A and B, and supplemental Fig. 4, A and B). In the recovery experiments, DETA-NO-induced up-regulation of COX2 and HO-1 expression, and p-NRF2 activation also appeared to gradually decrease with the extension of time after DETA-NO withdrawal (Fig. 4C and supplemental Fig. 4C). The following functional study was carried out using previously established hCSCs with stable expression of shRNAs to knock down NRF2, COX2, and HO-1 (27). Interestingly, knockdown of COX2 and HO-1 did not show a significant effect on NO-induced cytoprotection. Inhibition of NRF2 partially diminished the NO-induced cytoprotective effect with significance, but the effort seemed to be marginal (Fig. 4D). When hCSCs were recovered after DETA-NO preconditioning for 24 h, the NO-induced antiapoptotic effect was not detected, and knockdown of NRF2, COX2, and HO-1 also did not exhibit a significant difference in H2O2-induced LDH release compared with the control group (Fig. 4E). These results indicate that NRF2, COX2, and HO-1 are up-regulated by DETA-NO preconditioning but may not play a major role in mediating NO-induced ant-apoptotic effects in hCSCs.
FIGURE 4.
The up-regulation of HO-1, COX2, and NRF2 is indirectly associated with NO-induced cytoprotective effect in hCSCs. A, representative images of Western blot showing the dose-dependent increase in expression levels of HO-1 and COX2 and phosphorylation levels of NRF2 in response to DETA-NO preconditioning. B, representative images of Western blot showing the time-dependent up-regulation of HO-1, COX2, and NRF2 with 250 μm DETA-NO preconditioning (PC). C, representative images of Western blot showing expression levels of HO-1 and COX2 and phosphorylation levels of NRF2 after DETA-NO withdrawal over 24 h. The quantitative data for Western blots shown in A–C are provided in supplemental Fig. 4. D, hCSCs expressing shRNAs of HO-1, COX2, and NRF2 were preconditioned with 250 μm DETA-NO for 12 h and then stimulated with H2O2. The cytotoxicity induced by oxidative stress was evaluated by LDH release assay. E, after preconditioning (250 μm DETA-NO for 12 h), hCSCs expressing shRNAs of HO-1, COX2, and NRF2 were recovered (RC) for 24 h by refreshing medium. The cytotoxicity induced by oxidative stress was evaluated by LDH release assay. Data (both D and E) are presented as the mean percentage by comparison with scrambled (Scr) shRNA control with S.D. (means ± S.D.). Error bars represent S.D. ** indicates p < 0.01 versus scrambled shRNA group; n = 3 independent experiments. RC, recovery.
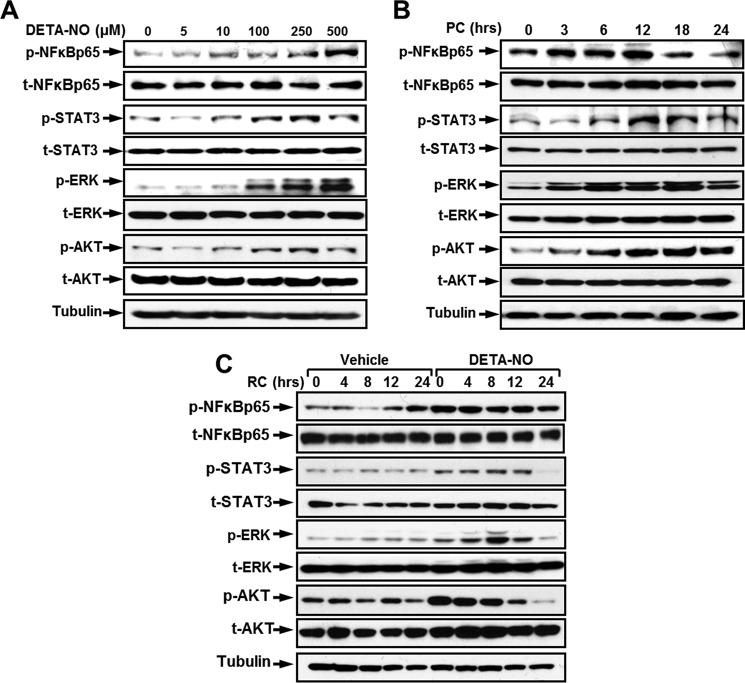
Despite up-regulation of COX2 and HO-1 expression as well as activation of NRF2 by the NO donor, it seems that promoting hCSC survival by carbon monoxide (CO) donor or NO donor preconditioning is mediated via activating divergent survival signaling pathways. To reveal the key signaling pathway involved in the NO-induced antiapoptotic effect, several well established survival signaling molecules were screened by Western blotting (Fig. 5 and supplemental Fig. 5). A dose-dependent activation of NFκB-p65, STAT3, ERK, and AKT was detected when hCSCs were preconditioned with increasing concentrations of DETA-NO (Fig. 5A and supplemental Fig. 5A). Elevated activities of phosphorylation of these molecules also appeared in a time-dependent manner in response to DETA-NO preconditioning (Fig. 5B and supplemental Fig. 5B). In addition, the activity of each individual survival molecule was examined at different recovery time points after withdrawal of DETA-NO. As shown in Fig. 5C and supplemental Fig. 5C, ERK activity was significantly increased at 0 h in comparison with the non-treated group. Its phosphorylation level was further elevated at 4 h, peaked at 8 h, and returned to the basal level at 24 h after preconditioning medium was refreshed. In contrast, the phosphorylation of AKT rapidly decreased immediately after preconditioning. NO-induced activation of p65 and STAT3 appeared to be rather stable within 12 h of recovery, but their phosphorylation levels were sharply decreased at 24 h after recovery. Western blotting analysis shows that preconditioning hCSCs with the NO donor is capable of activating the phosphorylation of NFκB-p65, STAT3, ERK, and AKT. The NO donor-induced beneficial effects thus may be caused by up-regulation of these and other survival signaling molecules.
FIGURE 5.
DETA-NO preconditioning elevates phosphorylation of ERK, AKT, STAT3, and NFκB-p65 in hCSCs. A, representative images of Western blot showing the activation of ERK, AKT, STAT3, and NFκB-p65 phosphorylation in a dose-dependent manner in response to DETA-NO preconditioning. B, representative images of Western blot showing the time-dependent up-regulation of ERK, AKT, STAT3, and NFκB-p65 phosphorylation with 250 μm DETA-NO preconditioning (PC). C, representative images of Western blot showing phosphorylation levels of ERK, AKT, STAT3, and NFκB-p65 after DETA-NO withdrawal over 24 h. The quantitative data for Western blots shown in A–C are provided in supplemental Fig. 5. Experiments were performed at least three times (n = 3 independent experiments). t, total; RC, recovery.
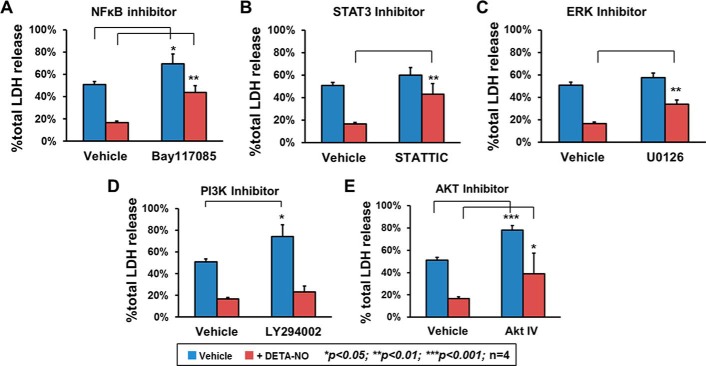
To evaluate the functional roles of survival signaling proteins in NO-induced cytoprotection, an inhibition exclusion assay was performed by introducing pharmaceutical inhibitors to block activity of each of the aforementioned survival signaling molecules, and then effects were examined by LDH release assay. The H2O2-induced cytotoxicity (∼50% of total LDH release) was dramatically decreased by 70% when hCSCs were pretreated with DETA-NO. The preventive effect of DETA-NO preconditioning was greatly diminished by an NFκB blocker (Fig. 6A). When Bay117085, an NFκB inhibitor, was applied to hCSCs, an increased induction of H2O2-induced cytotoxicity (∼70% of total LDH release) was detected. Blocking STAT3 and ERK activation by STATTIC and U0126 also significantly impaired the NO-induced cytoprotective effect, although their effect on non-preconditioned hCSCs did not appear to be significantly different (Fig. 6, B and C). We also examined the PI3K-AKT signaling pathway, which is well known for its important role in supporting cell survival. As shown in Fig. 6, D and E, compared with the positive vehicle group, inhibiting the activities of both PI3K and AKT by LY294002 and AKT IV caused greater induction of cellular cytotoxicity, resulting in about 70 and 75% total LDH release, respectively. However, in hCSCs with DETA-NO preconditioning, the inhibitor for AKT, but not that for PI3K, showed an adverse effect on NO-induced cytoprotection, indicating that PI3K is not involved in NO-induced AKT activation. According to results obtained from the inhibitory exclusion assays, blocking NFκB and STAT3 activation diminished the NO-induced cytoprotective effect with the most efficiency, suggesting that these two molecules have important roles in the regulation of cell antiapoptosis by DETA-NO preconditioning.
FIGURE 6.
Activation of ERK, AKT, STAT3, and NFκB-p65 are involved in NO-mediated hCSC survival with essential functional roles. hCSCs were treated with various pharmaceutical inhibitors to specifically block cell survival signaling activity. Cells treated with vehicle or DETA-NO preconditioning were challenged with H2O2 and then examined by LDH release assay. A, Bay117085 (5 μm) was applied to inhibit NFκB activity. B, STATTIC (1 μm) was applied to inhibit STAT3 activity. C, U0126 (10 μm) was applied to inhibit ERK activity. D, LY294002 (10 μm) was applied to inhibit PI3K activity. E, AKT IV (0.2 μm) was applied to inhibit AKT activity. Data are presented as means ± S.D. from four independent experiments. Error bars represent S.D. *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus the indicated control group.
Both STAT3 and NFκB Played Essential Roles in Promotion of DETA-NO-mediated hCSC Survival
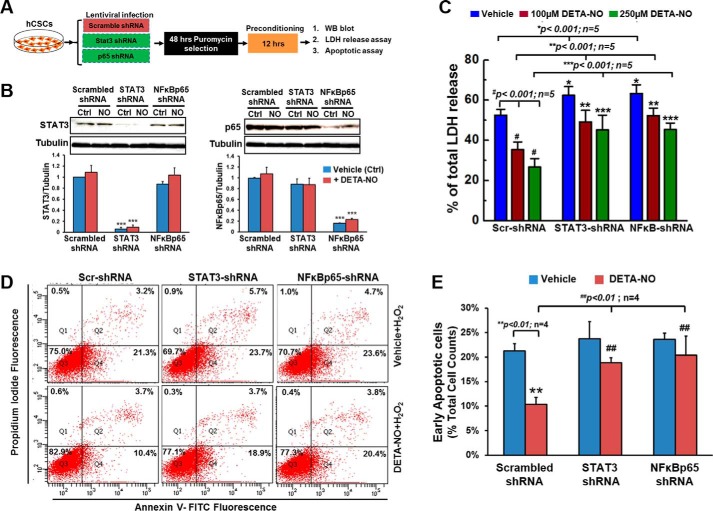
Activation of phosphorylation of STAT3 and NFκB by NO donor seemed to be important in promotion of hCSC survival against oxidative stress. To further confirm their functional roles and investigate the potential mechanisms involved in NO-induced cytoprotective effect, hCSCs with specific gene knockdown by shRNA were applied in the following study. As described in Fig. 7A, hCSCs were infected with lentivirus expressing scrambled shRNA, STAT3 shRNA, or NFκB-p65 shRNA. The efficiency of knocking down STAT3 and NFκB-p65 was evaluated and confirmed by Western blotting, which showed about 90 and 80% decrease of gene expression, respectively (Fig. 7B). Compared with hCSCs infected with scrambled shRNA, cells expressing STAT3 shRNA and NFκB-p65 shRNA showed a slightly higher induction of H2O2-induced cell death as assessed by LDH release assay. The H2O2-induced cytotoxicity was significantly lowered when exposed to 100 μm DETA-NO preconditioning and was further decreased with the 250 μm dose. However, the NO-induced cytoprotective effect was significantly attenuated in hCSCs with stable expression of knockdown shRNAs for STAT3 and NFκB-p65 (Fig. 7C). This result was similar to the findings from the inhibition exclusion assays using pharmaceutical inhibitors of STAT3 and NFκB-p65.
FIGURE 7.
Knockdown of STAT3 and NFκB-p65 expression diminishes the NO-mediated cytoprotective effect in hCSCs. A, schematic protocol to describe procedures for lentiviral infection and the subsequent experiments. B, representative images and quantitative analysis of Western blots (WB) confirming the efficiency of knockdown of STAT3 and NFκB-p65 expression by shRNA. C, hCSCs expressing scrambled (Scr), STAT3, or NFκB-p65 shRNA were preconditioned with vehicle or DETA-NO and then challenged with H2O2. Oxidative stress-induced cytotoxicity was evaluated by LDH release assay. Data are presented as mean ratio of H2O2-induced LDH release to total LDH release in cells with S.D. D, representative FACS analysis with annexin V/PI staining showing H2O2-induced apoptosis in both DETA-NO-treated (250 μm for 12 h) and vehicle-treated hCSCs expressing scrambled, STAT3, or NFκB-p65 shRNA. E, quantitative data for early apoptosis induced by H2O2 shown in D. Values are means ± S.D. Error bars represent S.D. *, p < 0.05; **, p < 0.01, and ***, p < 0.001 versus the indicated control (Ctrl) group.
By gene silencing, the functional roles of STAT3 and NFκB in NO-induced antiapoptosis pathways were further investigated using an Annexin V/PI apoptosis assay. Under normal condition without oxidative stress, hCSCs expressing scrambled shRNA, STAT3 shRNA, or NFκB-p65 shRNA showed more than 90% live cells with no significant difference regardless of exposure to DETA-NO pretreatment (data not shown). However, oxidative stress induced by H2O2 caused 21.3 ± 1.45% of cells in the scrambled control group to be apoptotic and 23.7 ± 3.53 and 23.6 ± 1.30% apoptotic cells in the STAT3 knockdown and NFκB knockdown groups, respectively. In DETA-NO-preconditioned hCSCs, there was a 50% of reduction of apoptotic cells (10.9 ± 1.42%) in the scrambled control group. The antiapoptotic effect was significantly impaired in cells with knockdown of STAT3 and NFκB, both of which showed 19.6 ± 1.03 and 20.8 ± 3.84% apoptotic cells, respectively (Fig. 7, D and E). Combined with data from LDH release assays, this evidently confirmed that STAT3 and NFκB are two essential signaling molecules involved in NO-mediated hCSC survival.
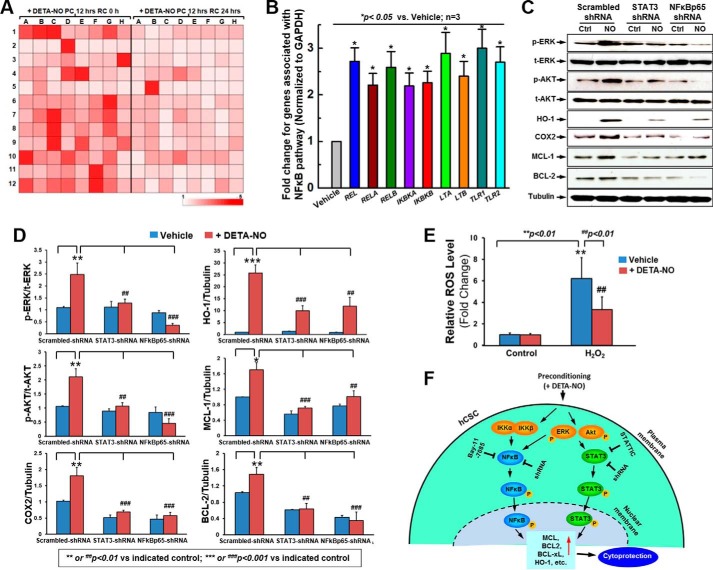
The functional results show that inhibition or knockdown of the NFκB survival signal molecule most effectively impeded NO-mediated cell survival. Knowing this, we then utilized quantitative PCR array to examine NFκB-related gene expression in response to DETA-NO preconditioning. As shown in Fig. 8A, there was a global increase in NFκB-related gene expression in hCSCs after 12-h DETA-NO preconditioning compared with the vehicle group. These up-regulated genes were dramatically decreased after 24 h of recovery, which was consistent with our aforementioned functional experiments. A screening assay showed that mRNA levels of multiple cell survival genes were elevated when hCSCs were preconditioned. Quantitative PCR analysis confirmed that DETA-NO preconditioning in hCSCs led to a significant 2–3-fold increase of expression in the genes REL/RELA/RELB, IKBKA/IKBKB, LTA/LTB, and TLR1/TLR2 (Fig. 8B).
FIGURE 8.
NFκB library screening in response to DETA-NO preconditioning and proposed molecular mechanism. A, heat map of NFκB library screening by real time PCR array in response to DETA-NO preconditioning (PC) at 0 and 24 h of recovery (RC). B, quantitative data to confirm up-regulated genes (mRNA level) screened from NFκB library array after preconditioning hCSCs with DETA-NO. C, representative images of Western blot showing the NO-induced expression of HO-1, COX2, MCL-1, and BCL-2 as well as phosphorylation of ERK and AKT in hCSCs expressing scrambled, STAT3, or NFκB-p65 shRNA. D, quantitative data for Western blots shown in C. E, ROS measurement. F, descriptive diagram of proposed molecular mechanism for DETA-NO-induced hCSC survival. Values are means ± S.D. Error bars represent S.D. ** or ##, p < 0.01 versus the indicated control (Ctrl); *** or ###, p < 0.001 versus the indicated control. t, total.
Although activation of STAT-3 and NFκB was required in the NO-induced antiapoptotic effect, the potential regulatory pathway in this process remained unknown. To elucidate this mechanism, hCSCs with stable knockdown of STAT3 and NFκB-p65 were preconditioned with or without DETA-NO for 12 h, and relevant protein expression and activation were determined by Western blotting analysis. As presented in Fig. 8, C and D, disruption of STAT3 expression completely abolished the NO-induced activation of ERK and AKT but did not alter their total protein expression levels compared with the scrambled shRNA control group. Nevertheless, knockdown of STAT3 significantly decreased expression (both basal and NO-induced) of HO-1 and COX2 as well as of the antiapoptotic genes BCL-2 and MCL-1. Inhibition of NFκB-p65 similarly showed a significant attenuation in both basal and NO-induced expression of HO-1, COX2, and MCL-1. However, it was of great interest to find that knockdown of NFκB-p65 further impaired the activities of ERK and AKT as well as BCL-2 rather than simply inhibiting their phosphorylation and protein expression in DETA-NO-preconditioned hCSCs (Fig. 8, C and D). This indicates that NO may play an apoptotic role in the absence of NFκB. Nevertheless, it was also noticed that preconditioning hCSCs with DETA-NO significantly decreased H2O2-induced excessive ROS production (Fig. 8E), indicating the antioxidant effect after preconditioning with DETA-NO.
Based on the results collected in this study, we propose that DETA-NO preconditioning is capable of promoting hCSC survival against oxidative stress. The mechanism involved seems to initially phosphorylate ERK and AKT, subsequently activate of STAT3 and NFκB, and eventually up-regulate antioxidant proteins HO-1 and COX2 as well as antiapoptotic genes BCL-2, BCL-xL, and MCL-1 (Fig. 8F). Disruption of STAT3 and NFκB impairs downstream antioxidant and antiapoptotic gene expression but also inhibits upstream ERK and AKT activity via a negative feedback loop, demonstrating that STAT3 and NFκB both have essential roles in regulation of NO-mediated cell survival.
Discussion
Divergent strategies to enhance the survival of donor cells have been reported, including hypoxia or anoxia conditioning (32), overexpression of growth factor (33), induction of anti-inflammation, and heat shock treatment (34) among others. In our previous study, we demonstrated that hCSCs exposed to CoPP exhibited a better performance against apoptosis (27). Here, we use an alternative approach to prime hCSCs with an NO donor, and our results support our initial hypothesis that preconditioning hCSCs with DETA-NO is capable of activating multiple survival signaling molecules, including ERK, AKT, STAT3, and NFκB, as well as mediating antiapoptotic gene expression (BCL-2, BCL-xL, and MCL-1) and eventually inhibiting apoptosis under oxidative stress.
NO and its functional roles in the cardiovascular system have been well studied in recent years. Since it was revealed as a signaling regulator for vasodilation in early studies, numerous other biological functions of NO have been characterized, including inhibition of smooth muscle cell proliferation and migration (35, 36), suppression of platelet aggregation and leukocyte adhesion to endothelium (37, 38), and prevention of endothelial cell apoptosis (39). Substantial generation of NO induced by ischemic preconditioning, cardiac overexpression of inducible NOS, or extracellular superoxide dismutase has been reported to reduce infarct size after ischemia-reperfusion injury in animal model (40–42), suggesting a proactive role in facilitating myocyte resistance to cell death. However, the role of NO in stem cells is less understood. There are studies on embryonic stem cells showing the beneficial effect of NO in promoting cell survival and facilitating stem cell differentiation into myocardial cells (43, 44). No report to date has looked at the precise role of NO in CSCs. Here, we demonstrate that exposure of hCSCs to NO donor effectively improves cell ability to resist oxidative stress-induced cell death by both LDH release assay and cell apoptosis assay with Annexin/PI staining. Meanwhile, the up-regulated expression of BCL-2, BCL-xL, and MCL-1 confirmed that this cytoprotection was due to an NO-induced antiapoptotic effect. The role of NO in promotion of hCSC survival therefore is quite convincing. Although NO has also been reported to induce apoptosis in some studies, it is worth noting that this adverse effect may be due to the different dose of NO or exposure time used in the specific experiments (45, 46). Based on cumulative studies to date, the discrepancy is now rather resolved and seems to indicate that NO has important roles in modulation of cell proliferation and antiapoptotic response at a low level and that a high level of NO likely induces the opposite effects (47). Thus, it is of the utmost importance to optimize dose and time for DETA-NO preconditioning.
As noted, our strategy to improve hCSC survival is via DETA-NO preconditioning. It is necessary to evaluate whether preconditioning hCSCs with DETA-NO alters cellular capacities such as proliferation and differentiation. To answer the question, the expression of cardiac genes was examined in hCSCs. Our data indicated that short term exposure of hCSCs to NO donor has no effect on these gene expressions. Both BrdU incorporation assay and MTT assay also demonstrated that there was no difference in cell proliferation ability between non-preconditioned and preconditioned groups. The cell differentiation capacity was confirmed by examining mature cardiac gene and protein expression, which showed that there was no side effect of DETA-NO preconditioning. Collective results suggest that short term preconditioning of hCSCs improved cell survival without alteration of cell proliferation and differentiation capacities.
Knowing that NO is capable of promoting hCSC survival via antiapoptotic effects, it then was essential to understand the molecular mechanism behind this process. Our previous study revealed that exposure of hCSCs to CoPP enhanced cell resistance to oxidative stress-induced apoptosis via activation of NRF2 as well as overexpression of COX2 and HO-1 (27). Upon review of the literature, it was found that these CoPP-up-regulated molecules may also be involved in the NO signaling pathway. Several studies have demonstrated that NO activates NRF2 through S-nitrosylation of KEAP-1, leading to expression of detoxifying enzyme and protective genes, including HO-1 (48–51). NO also serves as a signal messenger to induce COX2 expression in different cell types (52–54) as well as ERK/p38-dependent expression of HO-1 (55, 56). Our data showing that DETA-NO preconditioning induced overexpression of COX2 and HO-1 as well as phosphorylation of NRF2 in hCSCs were consistent with these studies. Interestingly, disruption of COX2 and HO-1 expression did not exhibit any attenuation of the cytoprotective effect, and knockdown of NRF2 only slightly impaired the NO-induced antiapoptotic role. There are additional in vivo studies confirming that COX2 and HO-1 play important roles in NO-mediated cardioprotection (57, 58). Although these molecules are up-regulated by NO in hCSCs, our results suggest that NRF2, COX2, and HO-1 may not play dominant roles in NO-mediated antiapoptosis.
To determine the main survival signaling molecules involved in NO-induced antiapoptosis, ERK, AKT, STAT3, and NFκB-p65 were selected as targets because these molecules have been implicated involving in the NO signaling pathway in other studies (18–21). The Western blotting results showed that DETA-NO preconditioning up-regulated phosphorylation of ERK, AKT, STAT3, and NFκB-p65, and their levels were increased in a dose- and time- dependent manner, consistent with similar patterns observed in regulated antiapoptotic gene expression and functional assays. Blocking activity of these molecules by pharmaceutical inhibitors elucidated their importance in supporting cell survival via the NO signaling pathway. Particularly, inhibition of STAT3 and NFκB exhibited significantly impaired NO-mediated antiapoptosis, demonstrating their dominant roles in this cytoprotective mechanism.
STAT3 and NFκB are transcriptional factors involved in regulating the expression of a variety of genes in response to cell stimuli. STAT3 belongs to the STAT family of proteins with at least seven members identified so far, and NFκB is recognized as a stress-regulated transcription factor belonging to the REL family. Numerous studies on these two transcription factors have unmasked their divergent roles at various conditions, and emerging evidence suggests that both STAT3 and NFκB are able to mediate cell survival in various types of cells (59–62). However, their roles in hCSCs are poorly understood. By specific knockdown of STAT3 and NFκB-p65, we confirmed that suppressing expression of both genes abrogated the DETA-NO-induced antiapoptotic effect at the functional level. Previous studies looking at the molecular level have shown that STAT3 and NFκB are two vital factors for inhibiting cellular apoptosis via mediation of antiapoptotic gene expression (63–65). Moreover, these two proteins seem to act as upstream regulators to induce COX2 and HO-1 expression, eventually enhancing cell survival (66–69). Consistent with these findings, our results revealed that knockdown of STAT3 and NFκB-p65 substantially decreased NO-modulated COX2 and HO-1 expression as well as expression of BCL-2 and MCL-1 in hCSCs. Suppression of STAT3 also completely inhibited NO-induced phosphorylation of ERK and AKT, both of which are upstream of STAT3, suggesting that a negative feedback regulatory mechanism was involved. Suppression, rather than inhibition, of NFκB-p65 further impaired the basal phosphorylation levels of ERK and AKT. The cause of this phenomenon is unclear.
It is likely that NO could induce cellular damage via an unknown mechanism in the absence of NFκB. An NFκB library screening assay showed a global increase in mRNA levels of NFκB family members (REL, RELA, RELB, IKBKA, and IKBKB), TNF family members (LTA and LTB), and Toll-like receptor members (TLR1 and TLR2). These up-regulated genes have been implicated to play roles in cell survival as well, further demonstrating the beneficial effect of DETA-NO preconditioning in conferring hCSCs with apoptosis resistance abilities. It is worth noting that DETA-NO preconditioning also exhibited the beneficial effect of lowering H2O2-induced ROS levels. Although its regulatory mechanism remains unidentified, the cytoprotective effect of NO via up-regulation of antiapoptotic pathways as well as the reduction of oxidative stress in hCSCs is clearly illustrated.
To understand the potential molecular mechanism, the current study was mainly focused on the above specific survival signal pathways because our aim is to examine whether preconditioning hCSCs with NO donor will enhance the cell survival ability. However, we cannot exclude many other pathways that were potentially associated with the NO donor-mediated cytoprotective effect in hCSCs. It would be very interesting to further explore the other pathways in a separate study in the future. Meanwhile, the cytoprotective effect of preconditioning hCSCs with DETA-NO has been confirmed in multiple cell lines. It was noted that different lines of hCSCs exhibited different levels of cytoprotective effect following DETA-NO preconditioning, which may be due to the distinct medical or genetic background for different patients, including age, sex, diabetes, etc. It would be interesting to further study how these different factors affect the effectiveness of stem cell therapy with or without preconditioning with NO donor.
Since the discovery of c-Kit+/Lin− CSCs with commitment to cardiac cells, these cells have become a potentially promising cell population for cardiac repair. In the past decade, a series of CSC transplantation assays in vivo with different models of myocardial damage have been tested using various mammals (5–8) have shown positive results, demonstrating the beneficial effect of cell transplantation for cardiac function improvement. An early stage human clinical trial has also shown encouraging results in the use of autologous CSCs in patients with heart failure (9). With emerging evidence, c-Kit+ CSCs are thought to be necessary and sufficient for cardiac regeneration and repair (70). However, those results have shown small albeit significant functional improvements in the heart. One of the main challenges the loss of cells during transplantation and subsequent poor cell survival rate. Here, we demonstrate a simple and efficient strategy to enhance donor cell survival by preconditioning with an NO releaser. This temporary exposure of CSCs to NO can effectively improve cell viability with no adverse effect on its proliferation and differentiation abilities.
Recently, there has been a controversial debate about CSC commitment to cardiomyocytes. A study by Molkentin and co-workers (71) has shown that c-Kit+ cells minimally contribute to cardiomyogenesis but amply generate cardiac endothelial cells. A genetic c-Kit lineage tracing study from another laboratory has also shown that these c-Kit+ cells exhibit endothelial markers (72). These results suggest that the proportion of cardiomyocytes derived from c-Kit+ cells are limited. Although these interesting findings urge us to redefine the physiological role of c-Kit+ CSCs in the heart, it is still evident without a doubt that there is a beneficial effect of using c-Kit+ donor cells to improve cardiac function. Therefore, enhancing survival and maintenance of cells in the heart after transplantation will definitely improve the efficacy for cardiac repair.
Our recently published data (26) also showed that limited, newly formed cardiomyocytes were derived from transplanted hCSCs. Although preconditioning hCSCs with the HO-1 inducer CoPP led to more in vivo cell survival/retention after cell transplantation, the majority of surviving hCSCs were negative for staining of mature cardiac markers (26). Thus, the therapeutic effect of cardiac stem cell therapy may mainly be due to the mechanism of paracrine effect because a global increase of cytokine expression was observed in preconditioned cells (27). The focus of our current research is to enhance the effectiveness of cardiac stem cell therapy by improving cell survival with the NO donor. Our rationale is that greater cell survival will result in greater cytokine release, which may lead to the promotion of endogenous cardiac stem cell survival or regeneration. However, the other possibilities, such as the release of microRNA and exosomes, cannot be excluded as potential mechanisms to explain the therapeutic effect of cardiac stem cell therapy. Elucidating this very important issue will require a considerable effort, likely spanning many years, which is beyond the scope of the present study.
This is the first conclusive study to address the pivotal role of NO in suppression of oxidative stress-induced cell apoptosis for hCSCs. We are also the first to suggest that the regulatory mechanism involved activates STAT3/NFκB signaling pathways and regulates downstream antiapoptotic gene expression. Our functional assay demonstrated clearly that in vitro preconditioning of hCSCs with the NO donor DETA-NO substantially enhances cell survival, suggesting that this may be utilized as a simple and effective strategy to improve the efficacy of CSC-based therapies for heart disease. In the future, it will be interesting to examine the in vivo survival of hCSCs enhanced by DETA-NO preconditioning and conduct a thorough analysis of the molecular mechanisms elucidated here in immunodeficient mouse hearts following acute myocardial infarction.
Author Contributions
C. C. and L. T. contributed to conception and design, data analysis and interpretation, and manuscript writing and revision. L. T. conducted the majority of experiments. E. B. obtained consent from patients and collected human cardiac tissues.
Supplementary Material
Acknowledgments
We appreciate all of our colleagues from the Division of Cardiothoracic Surgery at the Albany Medical Center, including Lynn Martin and Kelly VanDyke, for assistance in obtaining consent from patients for the cardiac tissue collection. We also thank Roshni Khatiwala for help editing the manuscript.
This work was supported by National Institutes of Health Grant R01HL114951 (to C. C.) and American Heart Association Research Grant 12BGIA9090005 (to C. C.). The authors indicate no potential conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.

This article contains supplemental Figs. 1–5 and Table 1.
- CSC
- cardiac stem cell
- AKT
- v-akt murine thymoma viral oncogene homolog 1
- BCL-2
- B-cell chronic lymphocytic leukemia/lymphoma 2
- BCL-xL
- BCL-2-like 1
- MCL-1
- myeloid cell leukemia 1
- COX2
- cyclooxygenase-2
- DETA-NO
- diethylenetriamine nitric oxide adduct
- HO-1
- heme oxygenase 1
- hCSC
- human cardiac stem cell
- LDH
- lactate dehydrogenase
- NRF2
- nuclear factor (erythroid-derived 2)-like 2
- NFκB
- nuclear factor κ light chain enhancer of activated B cells
- PI
- propidium iodide
- REL
- v-rel avian reticuloendotheliosis viral oncogene homolog
- RELA
- v-rel avian reticuloendotheliosis viral oncogene homolog A
- RELB
- v-rel avian reticuloendotheliosis viral oncogene homolog B
- IKBKA
- conserved helix-loop-helix ubiquitous kinase
- IKBKB
- inhibitor of κ light polypeptide gene enhancer in B cells, kinase β
- LTA
- lymphotoxin α
- LTB
- lymphotoxin B
- TLR1
- Toll-like receptor 1
- TLR2
- Toll-like receptor 2
- p-
- phospho-
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ROS
- reactive oxygen species
- CoPP
- cobalt protoporphyrin.
References
- 1. Hausenloy D. J., and Yellon D. M. (2013) Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J. Clin. Investig. 123, 92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansson E. M., Lindsay M. E., and Chien K. R. (2009) Regeneration next: toward heart stem cell therapeutics. Cell Stem Cell 5, 364–377 [DOI] [PubMed] [Google Scholar]
- 3. Murry C. E., Soonpaa M. H., Reinecke H., Nakajima H., Nakajima H. O., Rubart M., Pasumarthi K. B., Virag J. I., Bartelmez S. H., Poppa V., Bradford G., Dowell J. D., Williams D. A., and Field L. J. (2004) Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature 428, 664–668 [DOI] [PubMed] [Google Scholar]
- 4. Beltrami A. P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S., Kasahara H., Rota M., Musso E., Urbanek K., Leri A., Kajstura J., Nadal-Ginard B., and Anversa P. (2003) Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114, 763–776 [DOI] [PubMed] [Google Scholar]
- 5. Dawn B., Stein A. B., Urbanek K., Rota M., Whang B., Rastaldo R., Torella D., Tang X. L., Rezazadeh A., Kajstura J., Leri A., Hunt G., Varma J., Prabhu S. D., Anversa P., and Bolli R. (2005) Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc. Natl. Acad. Sci. U.S.A. 102, 3766–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tang X. L., Rokosh G., Sanganalmath S. K., Yuan F., Sato H., Mu J., Dai S., Li C., Chen N., Peng Y., Dawn B., Hunt G., Leri A., Kajstura J., Tiwari S., Shirk G., Anversa P., and Bolli R. (2010) Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 121, 293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolli R., Tang X. L., Sanganalmath S. K., Rimoldi O., Mosna F., Abdel-Latif A., Jneid H., Rota M., Leri A., and Kajstura J. (2013) Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 128, 122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Q., Guo Y., Ou Q., Chen N., Wu W. J., Yuan F., O'Brien E., Wang T., Luo L., Hunt G. N., Zhu X., and Bolli R. (2011) Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res. Cardiol. 106, 849–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolli R., Chugh A. R., D'Amario D., Loughran J. H., Stoddard M. F., Ikram S., Beache G. M., Wagner S. G., Leri A., Hosoda T., Sanada F., Elmore J. B., Goichberg P., Cappetta D., Solankhi N. K., Fahsah I., Rokosh D. G., Slaughter M. S., Kajstura J., and Anversa P. (2011) Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378, 1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Laflamme M. A., and Murry C. E. (2005) Regenerating the heart. Nat. Biotechnol. 23, 845–856 [DOI] [PubMed] [Google Scholar]
- 11. Tang Y. L., Tang Y., Zhang Y. C., Qian K., Shen L., and Phillips M. I. (2005) Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J. Am. Coll. Cardiol. 46, 1339–1350 [DOI] [PubMed] [Google Scholar]
- 12. Grange R. W., Isotani E., Lau K. S., Kamm K. E., Huang P. L., and Stull J. T. (2001) Nitric oxide contributes to vascular smooth muscle relaxation in contracting fast-twitch muscles. Physiol. Genomics 5, 35–44 [DOI] [PubMed] [Google Scholar]
- 13. Phillips L., Toledo A. H., Lopez-Neblina F., Anaya-Prado R., and Toledo-Pereyra L. H. (2009) Nitric oxide mechanism of protection in ischemia and reperfusion injury. J. Invest. Surg. 22, 46–55 [DOI] [PubMed] [Google Scholar]
- 14. Kanno S., Lee P. C., Zhang Y., Ho C., Griffith B. P., Shears L. L. 2nd, and Billiar T. R. (2000) Attenuation of myocardial ischemia/reperfusion injury by superinduction of inducible nitric oxide synthase. Circulation 101, 2742–2748 [DOI] [PubMed] [Google Scholar]
- 15. Napoli C., de Nigris F., Williams-Ignarro S., Pignalosa O., Sica V., and Ignarro L. J. (2006) Nitric oxide and atherosclerosis: an update. Nitric Oxide 15, 265–279 [DOI] [PubMed] [Google Scholar]
- 16. Lin K. Y., Ito A., Asagami T., Tsao P. S., Adimoolam S., Kimoto M., Tsuji H., Reaven G. M., and Cooke J. P. (2002) Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 106, 987–992 [DOI] [PubMed] [Google Scholar]
- 17. Hermann M., Flammer A., and Lüscher T. F. (2006) Nitric oxide in hypertension. J. Clin. Hypertens. 8, 17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shiojima I., and Walsh K. (2002) Role of Akt signaling in vascular homeostasis and angiogenesis. Circ. Res. 90, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 19. Nandagopal K., Dawson T. M., and Dawson V. L. (2001) Critical role for nitric oxide signaling in cardiac and neuronal ischemic preconditioning and tolerance. J. Pharmacol. Exp. Ther. 297, 474–478 [PubMed] [Google Scholar]
- 20. Dawn B., and Bolli R. (2002) Role of nitric oxide in myocardial preconditioning. Ann. N.Y. Acad. Sci. 962, 18–41 [DOI] [PubMed] [Google Scholar]
- 21. Bolli R., Dawn B., and Xuan Y. T. (2003) Role of the JAK-STAT pathway in protection against myocardial ischemia/reperfusion injury. Trends Cardiovasc. Med. 13, 72–79 [DOI] [PubMed] [Google Scholar]
- 22. Delikouras A., Hayes M., Malde P., Lechler R. I., and Dorling A. (2001) Nitric oxide-mediated expression of Bcl-2 and Bcl-xl and protection from tumor necrosis factor-α-mediated apoptosis in porcine endothelial cells after exposure to low concentrations of xenoreactive natural antibody. Transplantation 71, 599–605 [DOI] [PubMed] [Google Scholar]
- 23. Wang G., Liem D. A., Vondriska T. M., Honda H. M., Korge P., Pantaleon D. M., Qiao X., Wang Y., Weiss J. N., and Ping P. (2005) Nitric oxide donors protect murine myocardium against infarction via modulation of mitochondrial permeability transition. Am. J. Physiol. Heart Circ. Physiol. 288, H1290–H1295 [DOI] [PubMed] [Google Scholar]
- 24. Bearzi C., Rota M., Hosoda T., Tillmanns J., Nascimbene A., De Angelis A., Yasuzawa-Amano S., Trofimova I., Siggins R. W., Lecapitaine N., Cascapera S., Beltrami A. P., D'Alessandro D. A., Zias E., Quaini F., Urbanek K., Michler R. E., Bolli R., Kajstura J., Leri A., and Anversa P. (2007) Human cardiac stem cells. Proc. Natl. Acad. Sci. U.S.A. 104, 14068–14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He J. Q., Vu D. M., Hunt G., Chugh A., Bhatnagar A., and Bolli R. (2011) Human cardiac stem cells isolated from atrial appendages stably express c-kit. PLoS One 6, e27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cai C., Guo Y., Teng L., Nong Y., Tan M., Book M. J., Zhu X., Wang X. L., Du J., Wu W. J., Xie W., Hong K. U., Li Q., and Bolli R. (2015) Preconditioning human cardiac stem cells with an HO-1 inducer exerts beneficial effects after cell transplantation in the infarcted murine heart. Stem Cells 33, 3596–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cai C., Teng L., Vu D., He J. Q., Guo Y., Li Q., Tang X. L., Rokosh G., Bhatnagar A., and Bolli R. (2012) The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J. Biol. Chem. 287, 33720–33732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim Y. M., Chung H. T., Kim S. S., Han J. A., Yoo Y. M., Kim K. M., Lee G. H., Yun H. Y., Green A., Li J., Simmons R. L., and Billiar T. R. (1999) Nitric oxide protects PC12 cells from serum deprivation-induced apoptosis by cGMP-dependent inhibition of caspase signaling. J. Neurosci. 19, 6740–6747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li J., and Billiar T. R. (1999) The anti-apoptotic actions of nitric oxide in hepatocytes. Cell Death Differ. 6, 952–955 [DOI] [PubMed] [Google Scholar]
- 30. Kim Y. M., Talanian R. V., and Billiar T. R. (1997) Nitric oxide inhibits apoptosis by preventing increases in caspase-3-like activity via two distinct mechanisms. J. Biol. Chem. 272, 31138–31148 [DOI] [PubMed] [Google Scholar]
- 31. Kang-Decker N., Cao S., Chatterjee S., Yao J., Egan L. J., Semela D., Mukhopadhyay D., and Shah V. (2007) Nitric oxide promotes endothelial cell survival signaling through S-nitrosylation and activation of dynamin-2. J. Cell Sci. 120, 492–501 [DOI] [PubMed] [Google Scholar]
- 32. Shirai T., Rao V., Weisel R. D., Ikonomidis J. S., Li R. K., Tumiati L. C., Merante F., and Mickle D. A. (1998) Preconditioning human cardiomyocytes and endothelial cells. J. Thorac. Cardiovasc. Surg. 115, 210–219 [DOI] [PubMed] [Google Scholar]
- 33. Shintani S., Kusano K., Ii M., Iwakura A., Heyd L., Curry C., Wecker A., Gavin M., Ma H., Kearney M., Silver M., Thorne T., Murohara T., and Losordo D. W. (2006) Synergistic effect of combined intramyocardial CD34+ cells and VEGF2 gene therapy after MI. Nat. Clin. Pract. Cardiovasc. Med. 3, Suppl. 1, S123–S128 [DOI] [PubMed] [Google Scholar]
- 34. Haider H., and Ashraf M. (2008) Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J. Mol. Cell. Cardiol. 45, 554–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cornwell T. L., Arnold E., Boerth N. J., and Lincoln T. M. (1994) Inhibition of smooth muscle cell growth by nitric oxide and activation of cAMP-dependent protein kinase by cGMP. Am. J. Physiol. Cell Physiol. 267, C1405–C1413 [DOI] [PubMed] [Google Scholar]
- 36. Sarkar R., Meinberg E. G., Stanley J. C., Gordon D., and Webb R. C. (1996) Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ. Res. 78, 225–230 [DOI] [PubMed] [Google Scholar]
- 37. McCall T. B., Boughton-Smith N. K., Palmer R. M., Whittle B. J., and Moncada S. (1989) Synthesis of nitric oxide from L-arginine by neutrophils. Release and interaction with superoxide anion. Biochem. J. 261, 293–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kubes P., Suzuki M., and Granger D. N. (1991) Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 88, 4651–4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DeMeester S. L., Qiu Y., Buchman T. G., Hotchkiss R. S., Dunnigan K., Karl I. E., and Cobb J. P. (1998) Nitric oxide inhibits stress-induced endothelial cell apoptosis. Crit. Care Med. 26, 1500–1509 [DOI] [PubMed] [Google Scholar]
- 40. Wang Y., Guo Y., Zhang S. X., Wu W. J., Wang J., Bao W., and Bolli R. (2002) Ischemic preconditioning upregulates inducible nitric oxide synthase in cardiac myocyte. J. Mol. Cell. Cardiol. 34, 5–15 [DOI] [PubMed] [Google Scholar]
- 41. Obal D., Dai S., Keith R., Dimova N., Kingery J., Zheng Y. T., Zweier J., Velayutham M., Prabhu S. D., Li Q., Conklin D., Yang D., Bhatnagar A., Bolli R., and Rokosh G. (2012) Cardiomyocyte-restricted overexpression of extracellular superoxide dismutase increases nitric oxide bioavailability and reduces infarct size after ischemia/reperfusion. Basic Res. Cardiol. 107, 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Li Q., Guo Y., Wu W. J., Ou Q., Zhu X., Tan W., Yuan F., Chen N., Dawn B., Luo L., O'Brien E., and Bolli R. (2011) Gene transfer as a strategy to achieve permanent cardioprotection I: rAAV-mediated gene therapy with inducible nitric oxide synthase limits infarct size 1 year later without adverse functional consequences. Basic Res. Cardiol. 106, 1355–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mujoo K., Sharin V. G., Bryan N. S., Krumenacker J. S., Sloan C., Parveen S., Nikonoff L. E., Kots A. Y., and Murad F. (2008) Role of nitric oxide signaling components in differentiation of embryonic stem cells into myocardial cells. Proc. Natl. Acad. Sci. U.S.A. 105, 18924–18929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kanno S., Kim P. K., Sallam K., Lei J., Billiar T. R., and Shears L. L. 2nd. (2004) Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc. Natl. Acad. Sci. U.S.A. 101, 12277–12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shen Y. H., Wang X. L., and Wilcken D. E. (1998) Nitric oxide induces and inhibits apoptosis through different pathways. FEBS Lett. 433, 125–131 [DOI] [PubMed] [Google Scholar]
- 46. Taylor E. L., Megson I. L., Haslett C., and Rossi A. G. (2003) Nitric oxide: a key regulator of myeloid inflammatory cell apoptosis. Cell Death Differ. 10, 418–430 [DOI] [PubMed] [Google Scholar]
- 47. Napoli C., Paolisso G., Casamassimi A., Al-Omran M., Barbieri M., Sommese L., Infante T., and Ignarro L. J. (2013) Effects of nitric oxide on cell proliferation: novel insights. J. Am. Coll. Cardiol. 62, 89–95 [DOI] [PubMed] [Google Scholar]
- 48. Dhakshinamoorthy S., and Porter A. G. (2004) Nitric oxide-induced transcriptional up-regulation of protective genes by Nrf2 via the antioxidant response element counteracts apoptosis of neuroblastoma cells. J. Biol. Chem. 279, 20096–20107 [DOI] [PubMed] [Google Scholar]
- 49. Liu X. M., Peyton K. J., Ensenat D., Wang H., Hannink M., Alam J., and Durante W. (2007) Nitric oxide stimulates heme oxygenase-1 gene transcription via the Nrf2/ARE complex to promote vascular smooth muscle cell survival. Cardiovasc. Res. 75, 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li C. Q., Kim M. Y., Godoy L. C., Thiantanawat A., Trudel L. J., and Wogan G. N. (2009) Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc. Natl. Acad. Sci. U.S.A. 106, 14547–14551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Um H. C., Jang J. H., Kim D. H., Lee C., and Surh Y. J. (2011) Nitric oxide activates Nrf2 through S-nitrosylation of Keap1 in PC12 cells. Nitric Oxide 25, 161–168 [DOI] [PubMed] [Google Scholar]
- 52. Patel R., Attur M. G., Dave M., Abramson S. B., and Amin A. R. (1999) Regulation of cytosolic COX-2 and prostaglandin E2 production by nitric oxide in activated murine macrophages. J. Immunol. 162, 4191–4197 [PubMed] [Google Scholar]
- 53. Park S. W., Lee S. G., Song S. H., Heo D. S., Park B. J., Lee D. W., Kim K. H., and Sung M. W. (2003) The effect of nitric oxide on cyclooxygenase-2 (COX-2) overexpression in head and neck cancer cell lines. Int. J. Cancer 107, 729–738 [DOI] [PubMed] [Google Scholar]
- 54. Yang T., Zhang A., Pasumarthy A., Zhang L., Warnock Z., and Schnermann J. B. (2006) Nitric oxide stimulates COX-2 expression in cultured collecting duct cells through MAP kinases and superoxide but not cGMP. Am. J. Physiol. Renal Physiol. 291, F891–F895 [DOI] [PubMed] [Google Scholar]
- 55. Durante W., Kroll M. H., Christodoulides N., Peyton K. J., and Schafer A. I. (1997) Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ. Res. 80, 557–564 [DOI] [PubMed] [Google Scholar]
- 56. Chen K., and Maines M. D. (2000) Nitric oxide induces heme oxygenase-1 via mitogen-activated protein kinases ERK and p38. Cell. Mol. Biol. 46, 609–617 [PubMed] [Google Scholar]
- 57. Li Q., Guo Y., Tan W., Ou Q., Wu W. J., Sturza D., Dawn B., Hunt G., Cui C., and Bolli R. (2007) Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-κB dependent pathway. Circulation 116, 1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li Q., Guo Y., Ou Q., Cui C., Wu W. J., Tan W., Zhu X., Lanceta L. B., Sanganalmath S. K., Dawn B., Shinmura K., Rokosh G. D., Wang S., and Bolli R. (2009) Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-κB-dependent pathway. Circulation 120, 1222–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baetz D., Regula K. M., Ens K., Shaw J., Kothari S., Yurkova N., and Kirshenbaum L. A. (2005) Nuclear factor-κB-mediated cell survival involves transcriptional silencing of the mitochondrial death gene BNIP3 in ventricular myocytes. Circulation 112, 3777–3785 [DOI] [PubMed] [Google Scholar]
- 60. Gancz D., Lusthaus M., and Fishelson Z. (2012) A role for the NF-κB pathway in cell protection from complement-dependent cytotoxicity. J. Immunol. 189, 860–866 [DOI] [PubMed] [Google Scholar]
- 61. Lin L., Fuchs J., Li C., Olson V., Bekaii-Saab T., and Lin J. (2011) STAT3 signaling pathway is necessary for cell survival and tumorsphere forming capacity in ALDH+/CD133+ stem cell-like human colon cancer cells. Biochem. Biophys. Res. Commun. 416, 246–251 [DOI] [PubMed] [Google Scholar]
- 62. Liu J., Wang H., Wang Y., Yin Y., Du Z., Liu Z., Yang J., Hu S., Wang C., and Chen Y. (2014) The stem cell adjuvant with Exendin-4 repairs the heart after myocardial infarction via STAT3 activation. J. Cell. Mol. Med. 18, 1381–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bhattacharya S., Ray R. M., and Johnson L. R. (2005) STAT3-mediated transcription of Bcl-2, Mcl-1 and c-IAP2 prevents apoptosis in polyamine-depleted cells. Biochem. J. 392, 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Catz S. D., and Johnson J. L. (2001) Transcriptional regulation of bcl-2 by nuclear factor κB and its significance in prostate cancer. Oncogene 20, 7342–7351 [DOI] [PubMed] [Google Scholar]
- 65. Liu H., Yang J., Yuan Y., Xia Z., Chen M., Xie L., Ma X., Wang J., Ouyang S., Wu Q., Yu F., Zhou X., Yang Y., Cao Y., Hu J., and Yin B. (2014) Regulation of Mcl-1 by constitutive activation of NF-κB contributes to cell viability in human esophageal squamous cell carcinoma cells. BMC Cancer 14, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lo H. W., Cao X., Zhu H., and Ali-Osman F. (2010) Cyclooxygenase-2 is a novel transcriptional target of the nuclear EGFR-STAT3 and EGFRvIII-STAT3 signaling axes. Mol. Cancer Res. 8, 232–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang X., Shan P., Jiang G., Zhang S. S., Otterbein L. E., Fu X. Y., and Lee P. J. (2006) Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J. 20, 2156–2158 [DOI] [PubMed] [Google Scholar]
- 68. Lee K. M., Kang B. S., Lee H. L., Son S. J., Hwang S. H., Kim D. S., Park J. S., and Cho H. J. (2004) Spinal NF-κB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity. Eur. J. Neurosci. 19, 3375–3381 [DOI] [PubMed] [Google Scholar]
- 69. Wijayanti N., Huber S., Samoylenko A., Kietzmann T., and Immenschuh S. (2004) Role of NF-κB and p38 MAP kinase signaling pathways in the lipopolysaccharide-dependent activation of heme oxygenase-1 gene expression. Antioxid. Redox Signal. 6, 802–810 [DOI] [PubMed] [Google Scholar]
- 70. Ellison G. M., Vicinanza C., Smith A. J., Aquila I., Leone A., Waring C. D., Henning B. J., Stirparo G. G., Papait R., Scarfò M., Agosti V., Viglietto G., Condorelli G., Indolfi C., Ottolenghi S., Torella D., and Nadal-Ginard B. (2013) Adult c-kitpos cardiac stem cells are necessary and sufficient for functional cardiac regeneration and repair. Cell 154, 827–842 [DOI] [PubMed] [Google Scholar]
- 71. van Berlo J. H., Kanisicak O., Maillet M., Vagnozzi R. J., Karch J., Lin S. C., Middleton R. C., Marbán E., and Molkentin J. D. (2014) c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature 509, 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sultana N., Zhang L., Yan J., Chen J., Cai W., Razzaque S., Jeong D., Sheng W., Bu L., Xu M., Huang G. Y., Hajjar R. J., Zhou B., Moon A., and Cai C. L. (2015) Resident c-kit+ cells in the heart are not cardiac stem cells. Nat. Commun. 6, 8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.