Main Text
In the era of systems biology and big data, reductionism is often viewed as passé. But it can come in handy, especially when you have a theory to test. Despite decades of study, the field of biochemical signal transduction is still rife with mechanistic controversies, and one reason is that even systems of a few interacting components can exhibit complex and nonintuitive behavior. Many mechanisms are proposed, but most tests are confounded by the complexity of the underlying biological systems in which they are tested. A good, and perhaps even necessary first step to testing any proposed mechanism, is to recapitulate it in a minimal biological model that removes as many of the confounding variables as possible, such as a transfected cell. Mukhopadhyay et al. (1) followed exactly such an approach to testing their hypothetical mechanism for ultrasensitivity in T cell receptor signaling.
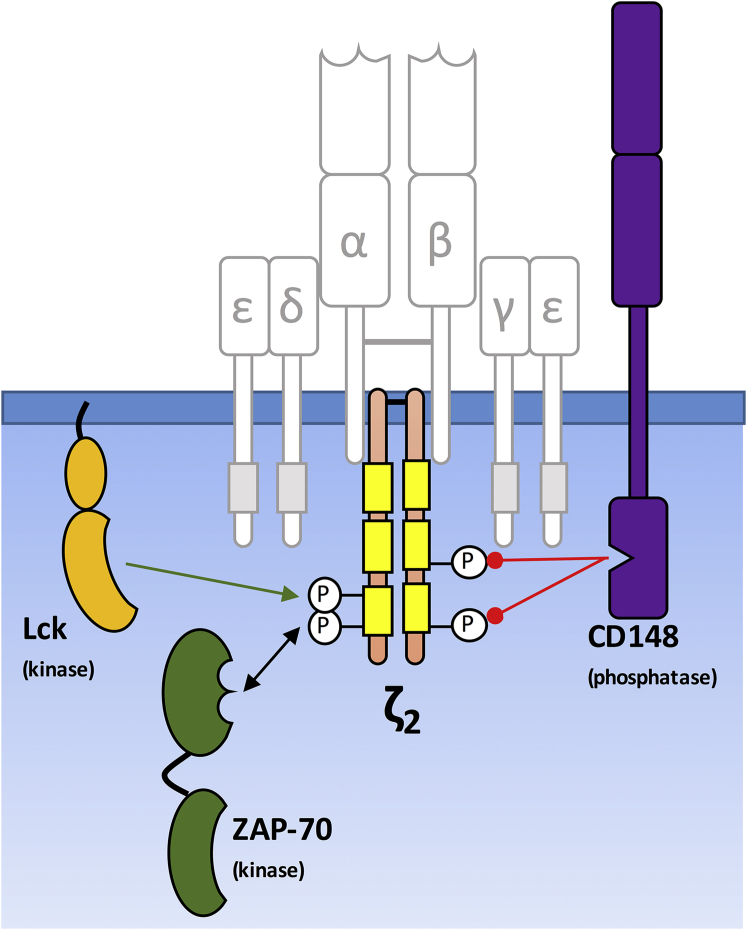
A longstanding question in immune receptor signaling is why the T cell antigen receptor (TCR; Fig. 1) is so complex, and in particular, why its cytoplasmic domains contain such a large number of tyrosine phosphorylation sites (2). In fact, multisite phosphorylation is a common feature of many receptor signaling systems, but the T cell receptor is notable for the large total number of sites—20—and that these sites occur as pairs in highly conserved regions called tyrosine-based activation motifs (ITAMs) (3). The TCR has a total of 10 ITAMs, six of which occur in the subunit comprising the ζ-chain homodimer. This high degree of apparent redundancy has motivated numerous investigations, but the functional role of the high ITAM multiplicity of the TCR remains an open question (4). Several experimental approaches have been employed to examine the role of TCR ITAMs in vivo during T cell development, but these studies have often yielded contradictory results (5, 6).
Figure 1.
A reductionist view of T cell receptor phosphorylation. Subunits shown in shading are not included in the transfectant models of Mukhodpadhyay et al. (1) The ITAMs of the TCR subunits are shown as rectangles. To see this figure in color, go online.
Taking a more mechanistic approach, Dushek et al. (7) proposed that multisite phosphorylation could enable the TCR system to generate sharp switchlike (or ultrasensitive) responses to inputs. Recent work has shown that TCR signaling can exhibit sharp response thresholds to input signals, but known mechanisms for generating such behavior involve components considerably downstream of the receptor itself (8). Dushek et al. (7) proposed a novel mechanism that incorporated diffusion-limited interactions among membrane-associated kinases and phosphatases and their multisite substrate. The TCR ζ-dimer subunit with its 12 tyrosines seemed like a natural system in which to test their model, and they proposed experiments based on a previously developed transfectant system (9).
Not long after, James and Vale (10) used a different transfectant system to investigate the mechanism of T cell activation, demonstrating that key features such as synapse formation and recruitment of the cytosolic kinase ZAP-70 could be recapitulated in a nonimmune cell with a minimal number of transfected components. Subsequently, Hui and Vale (11) went further along a reductionist path to investigate T cell signaling mechanisms, using a fully in vitro system composed of purified proteins on liposomes to characterize the dynamics of early phosphorylation events involving the TCR ζ-chain and CD45 and the kinases Lck, which is primarily responsible for ζ-chain phosphorylation, and Csk, which regulates Lck. Although both of these studies addressed mechanistic questions, they did not present a mathematical framework with which to analyze and further interpret the findings.
In the meantime, Mukhopadhyay et al. (12) further developed their computational model of multisite TCR phosphorylation building on two key hypotheses, supported by earlier experimental work: (1) that ζ-ITAMs are phosphorylated in a specific sequence and (2) that ZAP-70 exhibits increasing affinity for each ITAM in the sequence. Together, these were shown to be necessary and sufficient for ultrasensitivity to be observed, and the model predicted that ultrasensitivity would be reduced or eliminated by reduction in the number of ITAMs or in the absence of ZAP-70 expression.
Although Hui and Vale (11) did find ultrasensitive responses to variation in the levels of Lck and CD45 (11), they did not directly address the role of ζ-ITAM multiplicity, which Mukhopadhyay et al. (1) sought to address using transfectants expressing a chimera of the TCR ζ-chain with the CD2 extracellular domain. Seven forms of the CD3ζ chain were tested, including the wild-type and each of the three possible single and double ITAM knockouts. Lck was also transfected along with roughly equivalent levels of CD148, a membrane-bound protein tyrosine phosphatase, and the kinase/phosphatase activity was adjusted by titration of pervanadate, a potent phosphatase inhibitor, to generate a dose-response profile for each transfectant.
The dose response profiles obtained in these experiments indicate that the response is determined almost entirely by the number of ITAMs expressed but not their identity. In other words, the activation curves for chimeric ζ-chains with one ITAM were identical regardless of the position of the ITAM. Similar results were obtained with chimeric ζ-chains containing two ITAMs. If ITAM phosphorylation preferentially followed a specific sequence, one would expect ITAM position in the chimeras to affect the observed phosphorylation, and thus these results indicate that phosphorylation of the ζ-ITAMs occurs in a random order, contradicting previous experiments and a key assumption of the model. Hui and Vale (11) also reached the same conclusion based on the observation of a continuous shift over time in electrophoretic mobility of the phosphorylated ζ-chain, supporting the contention that ordered phosphorylation is not an intrinsic property of the ζ-chain as a substrate for Lck. It is worthwhile to mention that these reconstitution experiments do not rule out preferential phosphorylation of specific ζ-ITAMs in resting primary T cells, as has been previously observed in van Oers et al. (9). In general, it will be important in the future to validate the results obtained using reductionist approaches in T cells activated via the TCR.
Given the observed random order ITAM phosphorylation, the model predicts and experiments confirmed that the number of ITAMs does not modulate the ultrasensitivity of the response. ZAP-70 expression decreased the EC50 (i.e., increased receptor potency), but did not modulate ultrasensitivity, both of which were in agreement with previous findings (11). Thus, a central result of the article is that multisite phosphorylation is not the predominant mechanism for any observed ultrasensitivity in TCR activation. Furthermore, the transfectants expressing ZAP-70 exhibited no preferential phosphorylation of specific ζ-ITAMs, which undercuts another long-held view that ZAP-70 exhibits preferential binding to specific ζ-ITAMs and the second major assumption of the model. These results, although no doubt disappointing to the authors, emphasize the value of a reductionist approach for ruling out plausible mechanisms. The authors should also be commended for presenting their results in a straightforward way that does not obscure the discord.
On the positive side, a key finding is that ζ-ITAM multiplicity increases the receptor potency, an effect not explained by the original model or other previous models. To capture this effect the model must include an allosteric mechanism in which phosphorylation of an ITAM increases the catalytic rates for phosphorylation of other ITAMs. The authors hypothesize that this cooperative effect could arise from a reduction of entropy in the intrinsically disordered ζ-chains upon phosphorylation, producing a lower entropy change for association with the catalytic proteins. Recent work combining molecular dynamics, network modeling, and experiments supports such a mechanism (13). Interestingly, the net effect of this cooperative mechanism involving multiple sites is somewhat counterintuitive: it lowers the observed EC50 but does not affect the steepness of the switch.
We are then left with a model in which multisite phosphorylation is cooperative and explains the observed effects of ITAM mutation and ZAP-70 expression on potency, but does not give ultrasensitivity. Mukhopadhyay et al. (1) do observe ultrasensitivity in their data (which fit Hill coefficients of ∼4), but they choose to focus their analysis on relative changes in ultrasensitivity because of the artificial way in which the kinase to phosphatase ratio is adjusted with pervanadate. Hui and Vale (11) also observed ultrasensitive responses when the kinase/phosphatase was adjusted by direct alteration of either enzyme concentrations, and reported slightly higher Hill coefficients for variation of phosphatase concentration than kinase concentration. Taken together, these two studies suggest strongly that there is ultrasensitivity in ζ-chain phosphorylation, although it is not strongly dependent on the number of ITAMs, and there is then currently no model that reconciles all of the observations. Although the experiments have not confirmed the predictions of the original model, the clarity of the data provided by following a reductionist approach has enabled the elimination of several potential mechanisms and the identification of a new one, which would have been difficult to glean from a more complex system.
Acknowledgments
We thank Carlos Lopez for helpful comments on the article.
J.R.F. acknowledges support from the National Institutes of Health under award Nos. P41GM103712, R01AI107825, R01GM115805, and P01HL114453.
Editor: H. Wiley.
References
- 1.Mukhopadhyay H., De Wet B., Dushek O. Multisite phosphorylation of the T cell receptor ζ-chain modulates potency but not the switch-like response. Biophys. J. 2016;110:1896–1906. doi: 10.1016/j.bpj.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitcher L.A., van Oers N.S.C. T-cell receptor signal transmission: who gives an ITAM? Trends Immunol. 2003;24:554–560. doi: 10.1016/j.it.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Dushek O., Goyette J., van der Merwe P.A. Non-catalytic tyrosine-phosphorylated receptors. Immunol. Rev. 2012;250:258–276. doi: 10.1111/imr.12008. [DOI] [PubMed] [Google Scholar]
- 4.Guy C.S., Vignali D.A.A. Organization of proximal signal initiation at the TCR:CD3 complex. Immunol. Rev. 2009;232:7–21. doi: 10.1111/j.1600-065X.2009.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang S., Palin A.C., Love P.E. TCR ITAM multiplicity is required for the generation of follicular helper T-cells. Nat. Commun. 2015;6:6982. doi: 10.1038/ncomms7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guy C.S., Vignali K.M., Vignali D.A. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat. Immunol. 2013;14:262–270. doi: 10.1038/ni.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dushek O., van der Merwe P.A., Shahrezaei V. Ultrasensitivity in multisite phosphorylation of membrane-anchored proteins. Biophys. J. 2011;100:1189–1197. doi: 10.1016/j.bpj.2011.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zikherman J., Au-Yeung B. The role of T cell receptor signaling thresholds in guiding T cell fate decisions. Curr. Opin. Immunol. 2015;33:43–48. doi: 10.1016/j.coi.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Oers N.S., Tohlen B., Slaughter C.A. The 21- and 23-kD forms of TCR zeta are generated by specific ITAM phosphorylations. Nat. Immunol. 2000;1:322–328. doi: 10.1038/79774. [DOI] [PubMed] [Google Scholar]
- 10.James J.R., Vale R.D. Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature. 2012;487:64–69. doi: 10.1038/nature11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui E., Vale R.D. In vitro membrane reconstitution of the T-cell receptor proximal signaling network. Nat. Struct. Mol. Biol. 2014;21:133–142. doi: 10.1038/nsmb.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukhopadhyay H., Cordoba S.-P., Dushek O. Systems model of T cell receptor proximal signaling reveals emergent ultrasensitivity. PLOS Comput. Biol. 2013;9:e1003004. doi: 10.1371/journal.pcbi.1003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Travers T., Shao H., Camacho C.J. Tandem phosphorylation within an intrinsically disordered region regulates ACTN4 function. Sci. Signal. 2015;8:ra51. doi: 10.1126/scisignal.aaa1977. [DOI] [PMC free article] [PubMed] [Google Scholar]