Main Text
Just as you move to the kitchen in the morning when you smell coffee brewing, single-cell animals move to their meal once they sense nutrients. In both cases, you and the amoeboids use surface sensory molecules, such as G protein-coupled receptors, to detect critical nutrients (i.e., coffee), which trigger a series of protein associations and reactions on the inner surface of the plasma membrane that ultimately allow for actin rearrangement and forward movement.
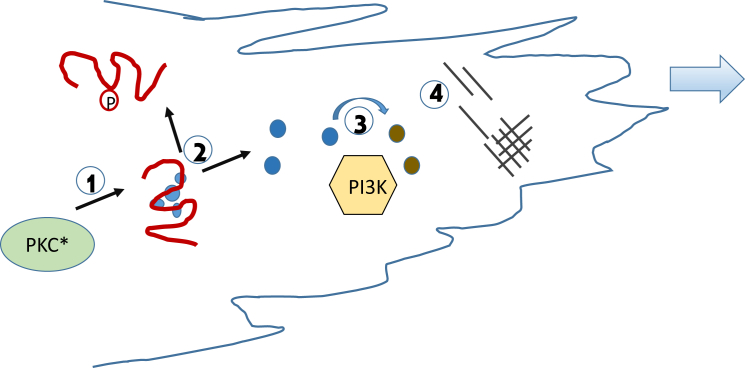
In this article, Ziemba et al. (1) quantitatively describe an early portion of the reaction circuit that propagates chemotaxis of amoeboids using single-molecule fluorescence. Specifically, they focus on four key steps of the reaction pathway (see Fig. 1) that are initiated when the cell commits to forward movement by reconstituting the purified components and quantifying the association and dissociation kinetics as the reaction proceeds. Their analysis begins after external stimuli have mobilized the signaling pathway that activates PKC (protein kinase C). PKC has many targets and one of these is MARCKS (myristoylated alanine-rich C kinase substrate) (2). The unstructured effector domain of MARCKS is very basic (+13) and can sequester ∼3 molecules of the acidic signaling lipid PIP2 (phosphatidylinositol 4,5 bisphosphate) by nonspecific electrostatic interactions (3, 4). It is therefore not surprising that phosphorylation of the PIP2 binding region of MARCKS by PKC (step 1, Fig. 1), releases it from the plasma membrane while concurrently releasing sequestered PIP2 in the plasma membrane (step 2, Fig. 1). The released PIP2 is a target for the key regulatory enzyme, PI3K (phosphatidylinositol 3 kinase), which phosphorylates PIP2 to PIP3 (step 3, Fig. 1). PIP3 then recruits a number of cellular proteins, causing actin rearrangement at the leading edge of the chemotaxing cell (step 4, Fig. 1). Previously this reaction scheme was purely hypothetical because it was based on measurements of the individual steps, on cell biology studies of cell movement under different physiological conditions and treatments, and on inference (5, 6). This study provides clear and quantitative support for the series of concerted events. The authors placed fluorescence tags on purified full-length PKCα and PI3K, the effector domain of MARCKS, and PIP2 and PIP3-sensors. They then followed the diffusion trajectories of the species, the activities of the individual enzymes in real time, and the generation of lipid substrates and products, all under buffer conditions that closely mimic the physiological. By direct observation, they find that activated PKCα drives PI3K activity but only in the presence of membrane-bound MARCKS and PIP2, and that membrane dissociation of MARCKS by PKCα activity short-circuits the reaction.
Figure 1.
Surface view of a plasma membrane of a chemotaxing cell showing the portion of the feed-forward events studied. (1) Activated PKCα (PKC∗, green ellipse) phosphorylates the effector domain of MARCKS (red squiggly line) on at least one site resulting in (2) dissociation of MARCKS from the membrane surface and the dissociation of the sequestered PIP2 (blue circles) into other regions of the plasma membrane. (3) PIP2 acts as an activator and substrate of PI3K (yellow hexagon) to produce PIP3 (brown circles), causing a further series of events that results in actin rearrangement (4) and forward movement. To see this figure in color, go online.
Considering the combined rates of the reaction pathway, their results support the putative biological pathway (Fig. 1), and pave the way for future studies in cellular systems. In a more general sense, these studies demonstrate that a working concerted biological pathway can be tested using single-molecule studies to give quantitative support for a proposed biological pathway. Additionally, these studies readily show how the system can easily be short-circuited, like when you finally reach that coffee pot.
Editor: Claudia Steinem.
References
- 1.Ziemba B.P., Burke J.E., Falke J.J. Regulation of phosphoinositide-3-kinase by PKC and MARCKS: single molecule analysis of a reconstituted signaling pathway. Biophys. J. 2016;110:1811–1825. doi: 10.1016/j.bpj.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohmori S., Sakai N., Saito N. Importance of protein kinase C targeting for the phosphorylation of its substrate, myristoylated alanine-rich C-kinase substrate. J. Biol. Chem. 2000;275:26449–26457. doi: 10.1074/jbc.M003588200. [DOI] [PubMed] [Google Scholar]
- 3.Gambhir A., Hangyás-Mihályné G., McLaughlin S. Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins. Biophys. J. 2004;86:2188–2207. doi: 10.1016/S0006-3495(04)74278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rauch M.E., Ferguson C.G., Cafiso D.S. Myristoylated alanine-rich C kinase substrate (MARCKS) sequesters spin-labeled phosphatidylinositol 4,5-bisphosphate in lipid bilayers. J. Biol. Chem. 2002;277:14068–14076. doi: 10.1074/jbc.M109572200. [DOI] [PubMed] [Google Scholar]
- 5.Artemenko Y., Lampert T.J., Devreotes P.N. Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes. Cell. Mol. Life Sci. 2014;71:3711–3747. doi: 10.1007/s00018-014-1638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens L., Milne L., Hawkins P. Moving towards a better understanding of chemotaxis. Curr. Biol. 2008;18:R485–R494. doi: 10.1016/j.cub.2008.04.048. [DOI] [PubMed] [Google Scholar]