Abstract
Dynamic water solvation is crucial to protein conformational reorganization and hence to protein structure and functionality. We report here the characterization of water dynamics on the L-asparaginase structural homology isozymes L-asparaginases I (AnsA) and II (AnsB), which are shown via fluorescence spectroscopy and dynamics in combination with molecular dynamics simulation to have distinct catalytic activity. By use of the tryptophan (Trp) analog probe 2,7-diaza-tryptophan ((2,7-aza)Trp), which exhibits unique water-catalyzed proton-transfer properties, AnsA and AnsB are shown to have drastically different local water environments surrounding the single Trp. In AnsA, (2,7-aza)Trp exhibits prominent green N(7)-H emission resulting from water-catalyzed excited-state proton transfer. In stark contrast, the N(7)-H emission is virtually absent in AnsB, which supports a water-accessible and a water-scant environment in the proximity of Trp for AnsA and AnsB, respectively. In addition, careful analysis of the emission spectra and corresponding relaxation dynamics, together with the results of molecular dynamics simulations, led us to propose two structural states associated with the rearrangement of the hydrogen-bond network in the vicinity of Trp for the two Ans. The water molecules revealed in the proximity of the Trp residue have semiquantitative correlation with the observed emission spectral variations of (2,7-aza)Trp between AnsA and AnsB. Titration of aspartate, a competitive inhibitor of Ans, revealed an increase in N(7)-H emission intensity in AnsA but no obvious spectral changes in AnsB. The changes in the emission profiles reflect the modulation of structural states by locally confined environment and trapped-water collective motions.
Introduction
In solution, proteins are not static objects but rather populate ensembles of conformations. Conformational reorganization between these states spans a range of temporal and spatial scales, and has been linked to protein structure and functionality, such as allosteric modulation and enzyme catalysis (1, 2). The elementary events of these conformational dynamics may comprise trapped-water motions as well as local constrained protein structural reorganization (3, 4, 5, 6, 7, 8). An experimental verification of molecular dynamics (MD) descriptions by time-resolved spectroscopy requires labeling of protein with a probe capable of sensing specific conformational reorganization at a well-defined time (9, 10). Typically, extrinsic dye molecules have been used as local optical probes to explore the active or functional sites, and structural relaxations were observed to range from femtoseconds to nanoseconds (11). However, the binding of such bulky dye molecules usually induces structural perturbations in proteins. To eliminate those interferences and to reveal intact environment responses, amino acid analogs have been developed that can be substituted for the specific residue for probing local protein conformational dynamics (12, 13). For instance, the intrinsic fluorescence of tryptophan (Trp) has been shown to be sensitive to its local environment, for example, to polarity of solvent or amino acids in its proximity (14). Effective utilization of Trp analogs facilitates the use of spectroscopy to explore the microenvironments within a protein and hence to investigate protein structural dynamics (15). For example, the Trp analog 7-azatryptophan ((7-aza)Trp) has been widely used as a sensor to probe the local polarity environment surrounding Trp. Upon electronic excitation, (7-aza)Trp undergoes an intramolecular charge transfer from the pyrrolic (highest occupied molecular orbital) to the pyridyl (lowest unoccupied molecular orbital) moiety. The resulting charge-transfer emission is thus strongly affected by the polarity of the environment, being red-shifted by increasing the polarity up to ∼400 nm in water (12, 16). However, although there is intensive investigation of protein structures, whether dynamic water solvation is coupled with protein conformational reorganization, which may be crucial to protein functionality, remains elusive (15).
In quest of an in situ Trp analog to probe the water molecules in protein, the recently developed 2,7-diazatryptophan ((2,7-aza)Trp; Fig. 1 a) should be a case in point. In neutral water, (2,7-aza)Trp exists predominantly in the N(1)-H form, equilibrated with a trace of N(2)-H isomer (<2%) (17, 18). Upon ultraviolet excitation, the N(1)-H form undergoes a water-catalyzed proton transfer from the N(1)-H to the N(7) site, resulting in a green N(7)-H tautomer emission (∼500 nm) (see Scheme S1 in the Supporting Material for the reaction mechanism) (17, 18). The N(2)-H isomer exhibiting a 380 nm emission band in bulk water does not exist in less polar/hydrogen-bonding solvents such as methanol (17). Also, the lack of N(2)-H isomer in (2,7-aza)Trp-substituted proteins is generally expected, because microsolvated water molecules in proteins are considered to be less polar and to provide fewer hydrogen bonds compared to bulk water (17). Any N(2)-H emission resolved in proteins may yield valuable information regarding the specific hydrogen-bonding structure formation (18). (2,7-aza)Trp thus offers powerful new dimensions for assessing the local water or hydrogen-bonding environment in the proximity of a Trp of interest in protein. Considering that using a protein with a sole Trp residue would greatly facilitate the interpretation of its associated fluorescence data, we selected the isozymes of amidohydrolase L-asparaginase (Ans), which hydrolyzes L-asparagine to L-aspartate (Asp) and ammonia (NH3) (19, 20) for comparative study. In this work, either (7-aza)Trp or (2,7-aza)Trp was substituted for the “buried” single Trp in proximity to the active site of these two types of Ans from Escherichia coli. Although the amino acid sequences substantially differ between these isozymes, the skeletons and the residues around the active site and in the vicinity of the sole Trp residue are relatively conserved (19, 20), as shown in Fig. 1. The potential role of the sole Trp66 in the structural stability of AnsB in E. coli was recently reported (21). Trp66 links to the substrate-binding residue Ser58 by just a few residues and also connects to His87, which is the neighbor of the catalytic residue Asp90 through Thr95 by a hydrogen-bond (H-bond) network (Fig. 1 c) (22). However, these two isozymes exhibit a dramatic difference in catalytic efficacy, with KM values of 1.2 mM and 12.5 μM for AnsA and AnsB, respectively (19, 20). The former (AnsA, 37.2 kDa) is a low-affinity enzyme found in the cytoplasm, whereas the latter (AnsB, 36.9 kDa) is a high-affinity enzyme localized to the periplasm (23, 24). For this reason, AnsB has been utilized in the treatment of acute lymphoblastic leukemia (25).
Figure 1.
(a) Structures of Trp and the Trp analog probes. (b and c) Comparison of the crystallography structures of E. coli (b) AnsA (PDB: 2HIM) and (c) AnsB (PDB: 3ECA) shows the local Trp environment with the relevant residues colored blue for polar and red for nonpolar amino acids, also highlighting the substrate binding-pocket residues S60 and D92 for AnsA and S58 and D90 for AnsB. The other relevant residues are represented by yellow sticks with blue and red indicating the nitrogen and oxygen positions, respectively, and the relevant water molecules appear as pink spheres. To see this figure in color, go online.
With the aim of shedding light on the distinct enzymatic activity, we next carried out in situ incorporation of (2,7-aza)Trp and (7-aza)Trp into AnsA and AnsB and studied their associated fluorescence spectroscopy and dynamics. In particular, we utilized (2,7-aza)Trp, which undergoes a water-catalyzed proton-transfer reaction, to probe the water static population and dynamics. Note that we did not intend to probe the motion of single water molecules on the femtosecond timescale, but rather to investigate the collective molecular dynamics of water relay structures in protein, which span a timescale of picoseconds to nanoseconds. The results, together with MD simulations, enable us to resolve the subtle Trp microenvironmental differences between the two Ans isozymes with structural homology. Details of the results and discussion are elaborated below.
Materials and Methods
Expression and purification of L-asparaginases
The recombinant Ans from E. coli with a 6xHis tag at the N-terminus was constructed in a pCWori-based vector for overexpression driven by the Ptac promoter. All constructs were validated by DNA sequencing before protein expression. A Trp auxotrophic E. coli strain (ATCC 23231) was utilized for protein expression. Transformed E. coli were cultured in 1 L of M9 minimal medium supplemented with 2 mM MgSO4, 0.4% glucose, 0.1 μM CaCl2, 100 μg/mL ampicillin, and 1 mM L-Trp. Growth was facilitated by shaking at 200 rpm at 37°C until the OD600 reached 0.8. The cells were then collected by centrifugation and resuspended in 1 L M9 medium supplemented as above but without L-Trp. The culture was then incubated at 30°C (30 min at 200 rpm); then, 2 mM (7-aza)Trp/(2,7-aza)Trp and lactose (40 g) were added and the culture continued to incubate at 30°C (18–20 h at 180 rpm) before harvest by centrifugation. To obtain recombinant Ans, the cell pellets were first frozen at −80°C for at least overnight and thawed, then resuspended in sodium phosphate buffer (50 mM, pH 7.5, containing 0.1 M NaCl, 10 μg/mL DNase, and 2 mM MgCl2) at a buffer solution/cell pellet ratio of 4:1 (v/w) and lysed by sonication at 4°C. After centrifugation, the supernatants were collected. The recombinant Ans were purified by Ni-NTA column (Qiagen, Hilden, Germany) and dialyzed against buffer with 50 mM NaPi and 500 mM NaCl. The purity of recombinant Ans was evaluated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and was estimated by densitometer to be 95% and higher.
Photophysical measurement
Steady-state ultraviolet/vis absorption and emission spectra were recorded by a spectrophotometer (U-3310, Hitachi, Tokyo, Japan) and a fluorimeter (FS920, Edinburgh Instruments, Livingston, United Kingdom), respectively. Detailed fluorescence-lifetime measurement has been described elsewhere (26). In brief, nanosecond lifetime studies were performed with an Edinburgh FL 900 time-correlated single-photon counting system (TCSPC) with a hydrogen lamp or a nitrogen lamp as the excitation source with a 40 kHz repetition rate. The emission decays were fitted by the sum of exponential functions with a temporal resolution of ∼300 ps by convolution of instrument response function. To achieve a faster time resolution, studies were also performed using a TCSPC coupled with excitation light from the third harmonic generation (at 306 nm) of pulse-selected femtosecond laser pulses at 920 nm (90 fs; model 3980 pulse picker, Tsunami, Spectra-Physics, Santa Clara, CA). The temporal resolution is ∼60 ps. Data were analyzed by using the nonlinear least-squares procedure in combination with an iterative convolution method.
Computational approach
All simulations were conducted using the Amber 12.0 packages (27, 28). Starting coordinates were taken from two x-ray crystallography structures of AnsA (PDB: 2HIM) (19) and AnsB (PDB: 3ECA) (20), as well as the (2,7-aza)Trp-replaced Ans structures. It is difficult to locate hydrogen atoms in x-ray crystallography, and we have added the hydrogen atoms based on the geometry specified in the residue databases of Amber 12.0. Simulations were based on a force field that extends the improved side-chain torsion potentials of the Amber ff99SB (29) protein force field and adopted a set of GAFF (30) parameters for the description of the (2,7-aza)Trp-substituted Ans ((2,7-aza)Trp-Ans). Periodic boundary conditions were imposed for solvent-solute systems contained in parallelepiped boxes. The solutes were placed in a periodic parallelepiped box containing about 10,000 TIP4P water molecules at an initial system density of ∼0.8 g/cm3. The systems were then neutralized by addition of an appropriate number of Na+ ions. The initial protein structure was subjected to primary constraint with a force constant of ∼100 kcal/mol in the explicit water-solvation box for at least 50,000 energy minimization steps, followed by cycles of fully structural relaxation with energy minimization using the steepest descent and gradient descent algorithm for other runs until the total system energy converged to within 0.01 kcal/mol. The system then underwent a 300 ps annealing NPT ensemble with equilibrated steps from 0 K to 300 K under a constant pressure of 1.0 bar. A Langevin thermostat (31) was used to maintain the system temperature by controlling the collision frequency at 1 ps−1. As the result of the optimization cycles, the system density and box lengths converged to reach equilibration; the system density was stabilized at ∼1.06 g/cm3, with temperature varying within ±3 K of the target temperature, 300 K.
After the fully optimized processes, MD simulations were carried out in the canonical (NVT) ensemble with a heat bath of 300 K at a collision frequency of 1 ps−1 using a Langevin thermostat to maintain the system temperature. The SHAKE algorithm (32) was implemented to constrain the covalent bond involving the hydrogen atom. Fourier-based Ewald summation utilized Fourier transforms to replace the summation of interaction energies in real space with an equivalent summation in Fourier space (33). The smooth particle mesh Ewald method extended this approach by using B-spline interpolation to calculate the reciprocal sum (34). In our system, the nonbonding interactions from van der Waals and long-range electrostatic interactions were carried out using the smooth particle mesh Ewald algorithm with a real-space cutoff length of 10 Å. Numerical integration was performed with a time step of 1 fs for all the MD simulations. Details of the computational approach are given in the Supporting Material.
Results and Discussion
Steady-state analyses
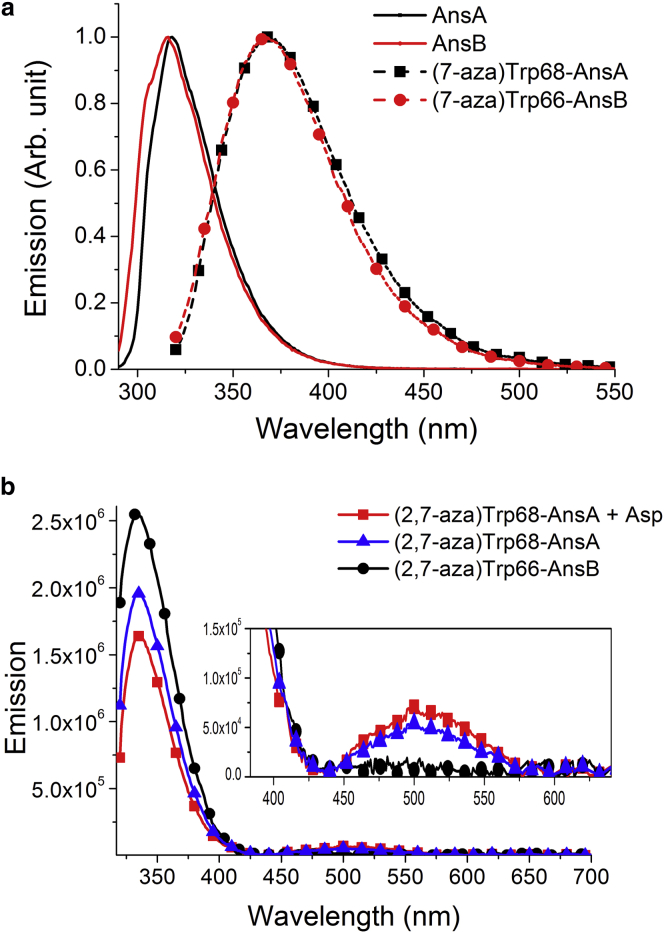
The wild-type AnsA and AnsB exhibit a Trp emission band maximized at 318 nm and 316 nm, respectively (Fig. 2). In comparison to the Trp emission of 308 nm in a low-polarity environment (35), the red shift could originate from protein elements, microsolvation by a few water molecules, bulk water, or any combination of these (36). Because of the weak excited-state charge-transfer property for Trp, i.e., less significant solvatochromism in emission, which may hide the spectroscopy-polarity relationship, adopting Trp emission alone is insufficient to unveil the Trp microenvironments.
Figure 2.
(a) Normalized (at peak wavelength) emission spectra of AnsA (black line), AnsB (red line), (7-aza)Trp68-AnsA (black squares), and (7-aza)Trp66-AnsB (red cicrcles). (b) Emission spectra of (2,7-aza)Trp68-AnsA (blue), (2,7-aza)Trp68-AnsA titrated with Asp (red), and (2,7-aza)Trp66-AnsB (black). The excitation wavelength (λex) is 310 nm. The inset in (b) shows an enlargement of the spectra in the region of 400–650 nm. To see this figure in color, go online.
To consider the factors affecting Trp emission shifts in protein, Trp was first replaced by the analog (7-aza)Trp to study the shift due to intraprotein interaction. This identity has allowed the ubiquitous use of the emission spectral shift of (7-aza)Trp in probing Trp local polarity for proteins of interest during the past several decades (13). As for (7-aza)Trp68-AnsA, the steady-state emission shown in Fig. 2 a reveals a peak wavelength at ∼370 nm. This result therefore supports a polar-intense environment surrounding Trp in AnsA. We next intended to investigate the water microsolvation around Trp. To achieve this goal, the recently developed Trp analog (2,7-aza)Trp (17) should provide a complementary examination. For (2,7-aza)Trp, the weak electron-donating strength of the pyrazolic moiety greatly suppresses the charge transfer strength, and hence its normal emission peak wavelength, is rather insensitive to the local polarity environment. Alternatively, the increase of N(1)-H acidity in (2,7-aza)Trp leads to water-catalyzed excited-state proton transfer, resulting in a green N(7)-H tautomer emission (see above). This unique photophysical property allows monitoring of any bio-water-perturbed Trp environment (17). As shown in Fig. 2 b, the emission spectrum of (2,7-aza)Trp68-AnsA reveals prominent dual emission maximized at 335 nm and 500 nm, corresponding to the N(1)-H and N(7)-H proton-transfer isomers, respectively (17). The results unequivocally confirm the existence of water molecules in the proximity of (2,7-aza)Trp in (2,7-aza)Trp68-AnsA.
In comparison, the emission spectra of wild-type AnsB, as well as those of (7-aza) and (2,7-aza)Trp66-AnsB, were then examined and are shown in Fig. 2, a and b. We notice that the emission peak wavelength of 368 nm for (7-aza)Trp66-AnsB, within the experimental error, is identical with that in (7-aza)Trp68-AnsA (370 nm), indicating that Trp66 (AnsB) and Trp68 (AnsA) are subject to a similar local polarity environment despite their different proximal protein structure (see below). The remarkable difference, however, lies in the fact that (2,7-aza)Trp66-AnsB reveals distinctly different emission spectra from that of (2,7-aza)Trp68-AnsA (see Fig. 2 b). In sharp contrast to the dual emission in (2,7-aza)Trp68-AnsA, (2,7-aza)Trp66-AnsB exhibits a single emission band maximized at ∼335 nm that clearly originates from the N(1)-H normal species. The absence of an N(7)-H emission band (∼500 nm) in AnsB represents a water-scant environment of the Trp66, such that water-catalyzed N(1)-H to N(7)-H tautomerism does not proceed in the excited state. Notably, the results seem to echo the distinction between Trp proximal water environments presented in crystallography structures. As shown in Fig. 1, the AnsA single crystal reveals a few crystallography water molecules surrounding Trp68, whereas there is virtually no water molecule in the proximity of the N(1) site of Trp66 in the AnsB crystal.
Fluorescence relaxation dynamics
We then made attempts to gain further insight into the dynamics of the emission, which should find correlation with respect to the observed steady-state spectra. Also, it may provide certain clues for the protein dynamics of local motion surrounding Trp, which is otherwise inaccessible from the static crystallography structures.
Fig. 3 a shows the fluorescence relaxation dynamics of (7-aza)Trp66-AnsB monitoring at the N(1)-H emission band of 370 nm. The resulting kinetics can be well fitted by biexponential decay with time constants of ∼2.3 ns and 10.8 ns (see Fig. 3 a and Table 1). Fig. 3 c shows the corresponding emission spectra of the short and long single-exponential decay time components of (7-aza)Trp66-AnsB by integrating the temporal decay. Clearly, the short-lived (2.3 ns) component reveals slightly blue-shifted emission (∼363 nm) with respect to that of the longer one (10.8 ns, 375 nm), indicating a slightly high polarity environment for the long-lived component. For (7-aza)Trp68-AnsA, the fluorescence relaxation (∼370 nm) is also biexponential (2.4 and 10.9 ns; see Fig. 3 b and Table 1), with a more pronounced fast component. Thus, the excited-state decay times of the (7-aza)Trp chromophore are attributed to two distinguishable emissions maximized at 367 nm and 380 nm, respectively (Fig. 3 d). In brief, upon replacing Trp with (7-aza)Trp, the (7-aza)Trp emission for both Ans can be modeled by a biexponential decay, in which the relatively higher polar environment renders a red-shifted emission peak wavelength as well as a longer lifetime (16) To further explore Trp spectroscopy/local environment correlation, the dynamics of relaxation of the corresponding emission bands in (2,7-aza)Trp68-AnsA and (2,7-aza)Trp66-AnsB were also measured (Fig. 3, e and f), with pertinent data listed in Table 1. Upon monitoring at the tautomer N(7)-H emission of 520 nm for (2,7-aza)Trp68-AnsA, the relaxation dynamics could be well fitted by a single-exponential rise (with a negative preexponential factor) and a decay component with time constants of 0.25 and 1.73 ns, respectively (see Fig. 3 f and Table 1). On the other hand, the decay dynamics at the N(1)-H emission region of, e.g., 330 nm could be fitted by two major single decay components with time constants of 0.24 and 1.34 ns. The former, within experimental error, is identical with the rise component (0.25 ns) of the 520 nm and could thus be reasonably assigned to the excited N(1)-H species of (2,7-aza)Trp68 that undergoes a water-catalyzed proton-transfer reaction, resulting in the green (500 nm) proton-transfer tautomer emission. Accordingly, we attribute the 1.34 ns decay component to the N(1)-H species, which does not undergo water-catalyzed proton-transfer reaction. This, together with the conclusion drawn about the (7-aza)Trp68-AnsA, leads us to propose that there are two (7-aza)Trp local environments in AnsA. Despite only a slight difference in polarity between these two Trp local sites, their water microsolvation is distinct, such that one should be relatively accessible in water molecules and the other essentially water scant, so that water-catalyzed proton transfer is allowed in the former but forbidden in the latter cases.
Figure 3.
(a and b) Emission decay dynamics and residues of (a) (7-aza)Trp66-AnsB and (b) (7-aza)Trp68-AnsA monitored at 380 nm. (c and d) Integrated intensity of short and long single-exponential decay-time components of (c) (7-aza)Trp66-AnsB and (d) (7-aza)Trp68-AnsA as a function of emission wavelength from 340 to 440 nm. (e and f) Emission decay dynamics and residues of (2,7-aza)Trp68-AnsA monitored at (e) 330 nm and (f) 520 nm. The instrument response and the fitting curves are marked by blue and red lines, respectively. To see this figure in color, go online.
Table 1.
Photophysical Properties of Various Azaindole and Azatryptophan Analogs in Neutral Water and L-Asparaginases
| λabs (nm) | λem (nm) | λmon (nm)a | Τ (ns)b | |
|---|---|---|---|---|
| 7-Azaindole | 288 | 386 | 0.91 | |
| (7-aza)Trp | 289 | 400 | 0.65 | |
| 2,7-Diazaindole | 295 | 335 | 320 | 0.22 |
| 370 | 400 | 10.10 | ||
| 495 | 550 | 0.21[rise], 1.30 | ||
| (2,7-aza)Trp | 300 | 340 | 320 | 0.26 |
| 380 | 380 | 0.27 (12%), 10.07 (88%) | ||
| 500 | 540 | 0.26[rise], 0.58 | ||
| AnsA | 280 | 318 | 320 | 2.71 (16%), 6.34 (86%) |
| (7-aza)Trp68-AnsA | 300 | 370 | 380 | 2.4 (65%), 10.9 (35%) |
| (2,7-aza)Trp68-AnsA | 300 | 335 | 330 | 0.24 (59%), 1.34 (41%) |
| 500 | 520 | 0.25[rise], 1.73 | ||
| (2,7-aza)Trp68-AnsA+Asp | 300 | 335 | 330 | 0.26 (73%), 1.34 (27%) |
| 500 | 520 | 0.25[rise], 1.73 | ||
| AnsB | 280 | 315 | 320 | 2.4 (15%), 4.6 (85%) |
| (7-aza)Trp66-AnsB | 300 | 368 | 380 | 2.3 (75%), 10.8 (25%) |
| (2,7-aza)Trp66-AnsB | 300 | 335 | 330 | 1.67 |
| (2,7-aza)Trp66-AnsB+Asp | 300 | 335 | 330 | 1.46 |
λmon is the wavelength at which the measurement of relaxation dynamics was monitored.
Values are ±0.03 ns in uncertainty. Values in parentheses indicate percentages and brackets denote the rise component.
In sharp contrast, only the N(1)-H 335 nm emission could be resolved in (2,7-aza)Trp66-AnsB (see above) and the N(1)-H emission dynamics was best fit by a single-exponential decay component with a lifetime of 1.46 ns. Such a long N(1)-H emission decay indicates the lack of an excited-state proton-transfer reaction, which is also supported by the absence of N(7)-H tautomer emission. Thus, although there are two different Trp polar environments, as evidenced by the photophysics of (7-aza)Trp66-AnsB, the results from the (2,7-aza)Trp66-AnsB experiment imply that both sites have almost no water molecules surrounding (2,7-aza)Trp in AnsB, or at least that the numbers of water molecules are insufficient to induce the proton-transfer reaction. This, together with the polarity-insensitive N(1)-H emission for (2,7-aza)Trp (see above), makes the decay rate of the N(1)-H emission between the two Trp66 environments indistinguishable in (2,7-aza)Trp66-AnsB, at least using our current TCSPC system (see Materials and Methods).
Up to this stage, we are able to conclude that there are two different Trp local environments and hence possibly two protein conformers in both AnsA and AnsB. In AnsA, the Trp sites in these two conformations are distinct in terms of relatively water-accessible and water-scant environments. For AnsB, both conformers lack proximal water molecules surrounding Trp. This viewpoint is also supported by the crystallography structure analysis, in which there are only a few water molecules surrounding the Trp in AnsA and virtually none in AnsB of E. coli (19, 20). However, water microenvironment does not seem to directly correlate with the polarity environment surrounding Trp. Factors other than water, such as side chains of near-by residues, may contribute more to the polarity in the proximity of Trp, as evidenced by the spectral red shift in the (7-aza)Trp experiment (see above).
For AnsA, a further attempt was then made to analyze the population ratio for the proposed two types of conformers from the spectroscopy/dynamics data. Theoretically, the electronic transition moment is governed by the electronic configuration, which should be less altered by the surrounding environment. As a result, the population ratio for two conformers can be deduced by the ratio of the fitted preexponential value (i.e., the intensity at decay time = 0) for short versus long decay components of the N(1)-H emission for (7-aza)Trp68-AnsA and (2,7-aza)Trp68-AnsA. As a result, the population ratio for short versus long decay components is calculated to be ∼65:35 and 59:41 from the results (N(1)-H emission) for (7-aza)Trp68-AnsA and (2,7-aza)Trp68-AnsA, respectively (see Table 1). These two ratios, within experimental error, are mutually consistent, supporting the validity of this approach. A similar method was applied to AnsB and a ratio of 75:25 was deduced for (7-aza)Trp66-AnsB. The results indicate that for AnsA and AnsB, two Trp local environments exist with equilibrium constants of ∼3:2 and 3:1, respectively. Furthermore, it seems likely that the internal motion of AnsA is accompanied by the motion of water molecules into and out of Trp proximity, resulting in the occurrence and prohibition, respectively, of the N(1)-H-to-N(7)-H proton transfer for (2,7-aza)Trp68-AnsA. Support of this viewpoint is elaborated in the following computational approach.
The water-coupled conformational reorganization
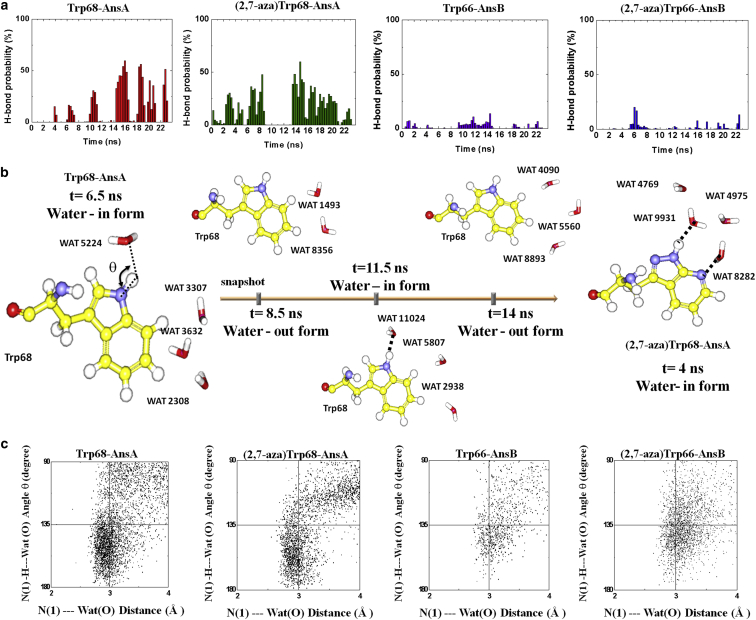
Protein-water interactions are crucial to protein conformational reorganization. To examine the conformational dynamics of the Trp site in Ans, MD simulations were used to investigate both wild-type and various Trp-analog-substituted Ans in aqueous solution. An important remark regarding our MD simulations is that these dynamics of structural modulation involve locally constrained environment of protein and the trapped-water motions within several angstroms distance from the Trp vicinity in conjunction with the active site and occur on timescales of picoseconds to nanoseconds.
Fig. 4 illustrates the radial distribution function of a Trp-water pair, which essentially describes how water density varies as a function of distance from the reference N(1)-H of the Trp site in AnsA and AnsB, as well as the trajectory time evolution. Although the MD structures started with the crystallography with or without water molecules in the vicinity of Trp, these water molecules were observed to diffuse or exchange with bulk water during the simulation time. In the 23 ns duration time of the trajectories, two major populations of water density appear in the vicinity of Trp (see Fig. 4, a and b). The results clearly reveal the water density from the Trp-N(1) site, which may correlate with the shells of water-in and water-out, respectively. On the other hand, although no water molecules are in the proximity of Trp66 in the AnsB crystallography structure (20), MD simulations show that a small number of dynamical water molecules penetrate the local region, but diffuse away from the Trp66 site quickly (see Fig. 4 b). The discrepancy between the crystallography structure and the MD simulation is thus conceivable. The crystallography structures shown in Fig. 1 are static, and no disordered water molecules could be located in these structures dynamically (37). It seems likely that the hydrophobic nature of the Trp vicinity does not favor water molecules residing inside, but that they can move in and out of the Trp vicinity. Nevertheless, the water density is significantly lower in the first hydration shell of the Trp66 of AnsB (Fig. 4 b) than in that of Trp68 in AnsA (Fig. 4 a). These results clearly suggest that penetration of water molecules in the Trp66 vicinity of AnsB is significantly lower than in the Trp68 vicinity of AnsA. This also holds true even the trajectory runs for 50 ns evolution for both wild-type and the (2,7-aza)Trp-Ans (see Fig. S7).
Figure 4.
Comparison of the radial distribution function of the Trp-water pair between wild-type AnsA(a) and AnsB (b) describes how water density varies as a function of distance from the reference N(1)-H of Trp site, by the trajectory time evolution. (c) The radial distribution function depicts the water density in the shells from the Trp-N(1) site in the reorganization of the water-in and water-out conformers. To see this figure in color, go online.
To correlate the water dynamical contribution with the structure of water-in and water-out states, we carefully analyzed the time-resolved hydrogen-bonding distributions of the Trp-water pair. As shown in Fig. 5, a and b, upon switching between the water-in and water-out states in a period of ∼4 ns, the phenomenon associated with H-bond is well recognized. The H-bond growth and relaxation time for the Trp-water pair is <∼0.5 ns in AnsA (and in AnsB). Remarkably, the probability of H-bond formation for the Trp-water pair is fivefold higher in AnsA than in AnsB. Applying the Mann−Whitney U test (38), we then evaluate the pattern of time-resolved H-bonding distributions for AnsA and AnsB. The result of statistical analysis suggests that AnsA and AnsB exhibit significantly different distribution patterns (p < 0.05). Also, as expected, the replacement of (2,7-aza)Trp does not reveal a statistically significant difference in time-resolved H-bonding distribution for AnsA (p = 0.57) or AnsB (p = 0.35).
Figure 5.
(a) Time-resolved H-bond probability of the Trp-water pair regarding the water-density variation in the Trp hydration shell for the MD trajectory span time of 23 ns. (b) Snapshots of MD-derived structures illustrate the local structures of the Trp (or (2,7-aza)Trp replacement) site with the water H-bond network switched between the water-in and water-out conformations. (c) Also shown are the contact water orientation analyses as a function of the N(1)-Wat(O) distance and the corresponding N(1)-H-Wat(O) angle distribution. To see this figure in color, go online.
The orientation of trapped water around the Trp-N(1) atom (within 4 Å) is also analyzed as a function of distance and angle, in which the H-bond formation is defined as an N(1)-Wat(O) distance of <3 Å and the N(1)-H-Wat(O) angle is between 135° and 180°. The results shown in Fig. 5 c clearly indicate that the trapped water in AnsA exhibits a denser hydrogen-bonding population than that in AnsB. This can be rationalized by the lower water content in AnsB such that water molecules are loosely bound and hence are subject to more heterogeneous orientations. This viewpoint is consistent with the recent work reported by Meral et al. (39).
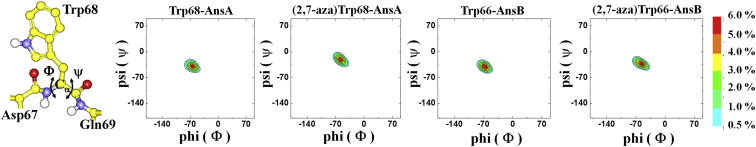
According to Meral’s work (39), the hydration dynamics of polypeptide structures with helix- and β-strand-like features could be varied upon conformational changes in the amino acid backbone. To examine this possibility, we made the Ramachandran plots in Fig. 6 from the 23 ns MD trajectories, which are long enough to observe the conformational reorganization of the Trp backbone. As a result, the difference in the dihedral angles of Φ and ψ between AnsA and AnsB is small, with an ∼5° shift in the average torsion angles (Φ = −70°, ψ = −40°for AnsA, and Φ = −75°, ψ = −45° for AnsB; see Fig. 6). It also allowed for a fair comparison between the wild-type and (2,7-aza)Trp-replaced AnsA (or AnsB). The Ramachandran plots also indicate that wild-type and (2,7-aza)Trp-replaced AnsA (or AnsB) possess a similar basin of helix feature, supporting the idea that the replacement of Trp with (2,7-aza)Trp does not disrupt its parent structure. These results suggest that the Trp backbone contribution to hydrolysis is more constricted in Ans proteins than what would perhaps be expected in polypeptide structures (39, 40). It thus highlights that factors other than the backbone structure should play a role in the difference in water microenvironment surrounding Trp in Ans, as discussed in detail below.
Figure 6.
Analysis of Ramachandran plots for the Trp (and (2,7-aza)Trp replacement) peptide dihedral-angle distributions of Φ (Φ = C-Cα-N-C) and Ψ (Ψ = N-C-Cα-N) for the MD-derived trajectories of Trp68-AnsA, (2,7-aza)Trp68-AnsA, Trp66-AnsB, and (2,7-aza)Trp66-AnsB. The dihedral-angle fluctuations and distributions were displayed by two-dimensionanl histograms with a resolution of 72 × 72 = 5184 bins. The color key at the right indicates the population density. To see this figure in color, go online.
For clarity, the relevant residues and the microenvironment surrounding the Trp residue are depicted in Fig. 7. The hydration environment of the Trp is different between AnsA and AnsB. The trapped-water motion takes place in different ways, and we were interested in how such water molecules in the Trp site could be regulated in accordance with different structural states. The work started with a structural analysis of crystallography and MD-derived structures for water channels using the software Caver (41). Interestingly, a number of different tunnels were found and we focused on the three highest-ranked channels that connect the Trp site to the protein surface as well as the Ans substrate binding site (Fig. 7 a). Water molecules were found to reside within the identified channels, and 23 ns MD simulations in a water box are sufficient to observe the movement of water molecules in the three tunnels, in support of their respective links to the substrate-binding residues.
Figure 7.
Snapshots of explicit-water MD trajectories for the microenvironment surrounding the Trp site illustrate the water channels connected to the Trp site in (a)Trp68-AnsA and (b) Trp66-AnsB. The snapshots display the highest-ranked tunnels colored in gray and the relevant water molecules within 10 Å of the Trp site, as well as the H-bond network between Trp (yellow stick with blue nitrogen) and the active-site pocket residues (cyan stick). To see this figure in color, go online.
As shown for AnsA in Fig. 7 a, the relevant water molecules connecting the binding-pocket residues Asp59, Ser60, and Asp92 are strongly associated with the trapped-water collective motions upon modulation of the structural states between the Trp68 vicinity and the active site. The trajectory at a time span of 23 ns has clearly demonstrated that in the water-in structural state, the water tunnel opened (the tunnel diameter is >1.5 Å) in connection to the Ans binding site and Trp hydration is in high water density (cf. the water-out structural state (Fig. S8 a)). In contrast to AnsA, the water-in structural state of AnsB has less water in the Trp66 vicinity and the water tunnel was revealed and switched off in a very short time of several picoseconds (see Fig. S8 b). In comparison, Trp hydration is revealed in low water density for the water-out structural state in both AnsA and AnsB (see Fig. S8 c, in which the water tunnel in connection to the active site is closed). Overall, the Trp site of AnsA is associated with substantial water motion through the relevant water tunnel such that the water-network reorganization is much stronger than in AnsB. This result further supports the time-resolved fluorescence measurement in which the N(1)-H-to-N(7)-H proton transfer takes place in (2,7-aza)Trp68-AnsA.
Fluorescence changes upon titration of the inhibitor
Finally, we sought to examine whether the modulation of structural states by constraint and trapped-water collective motions could be affected upon binding, e.g., the inhibitor and consequently influence the associated emission spectroscopy. In this approach, L-aspartate was used as an inhibitor of AnsB, with a Ki of 2.5 mM at pH 8 and 37°C (42). By monitoring the fluorescence spectroscopy and dynamics of Trp, (7-aza)Trp, and (2,7-aza)Trp, we hoped to unveil the corresponding subtle structural variation related to the active site.
As a result, upon binding of the inhibitor, AnsA and (7-aza)Trp68-AnsA showed virtually no shift of the emission peak, manifesting negligible changes of polarity amid the titration (see Fig. S3). In sharp contrast, the inhibitor-bound (2,7-aza)Trp68-AnsA revealed a significant change in the intensity ratio (see Fig. 2 b), with a decrease in the normal emission intensity (335 nm) accompanied by an increase in the N(7)-H tautomer emission (500 nm). In comparison, the inhibitor-bound (7-aza)Trp66-AnsB and (2,7-aza)Trp66-AnsB exhibited no change of spectral profile except for a decrease in emission intensity (see Fig. S3).
Moreover, upon binding of the inhibitor, dynamically, the population ratio for the short versus long decay components of the N(1)-H emission in (2,7-aza)Trp68-AnsA was increased to 3:1 (cf. 3:2 for inhibitor-free (2,7-aza)Trp68-AnsA, see Table 1). The results correlate well with the differences between the intensity ratios for N(1)-H versus N(7)-H emission before and after adding the inhibitor L-aspartate (see Fig. 2 b and the derivation of the intensity ratio using Eq. S2). Binding the inhibitor thus increases water accessibility, which modulates the constraint of the local environment and the trapped-water collective motions. In comparison, the emission relaxation dynamics of (2,7-aza)Trp for (2,7-aza)Trp66-AsnB remained unchanged, which exhibited a sole N(1)-H 335 nm emission with a long decay component of 1.46 ns, similar to that of inhibitor-free (2,7-aza)Trp66-AsnB. No signal of water-catalyzed proton transfer was observed before or after inhibitor binding of (2,7-aza)Trp66-AsnB (see Table 1). As for (7-aza)Trp66-AnsB, both short and long decay components of (7-aza)Trp were decreased upon binding to the inhibitor (see Fig. S4), suggesting the occurrence of fluorescence quenching via residues in the vicinity.
Conclusions
In the course of protein dynamics, water is presumed to play a central role in modulation of the conformation transition. This study presents a combination approach using the in situ tryptophan analogs to probe the protein dynamics. We made comparative studies of two single-Trp-containing Ans isozymes, AnsA and AnsB, with structural homology but differences in Trp microenvironments. Our observations are highlighted as follows.
-
1)
Analysis of the nearly identical emission-peak wavelength (∼370 nm) for (7-aza)Trp68-AnsA and (7-aza)Trp66-AnsB suggests a similarity of the moderately polar environment surrounding the Trp site.
-
2)
The subtle but significant difference between AnsA and AnsB lies in the hydration water environment in the Trp vicinity, which is water-accessible in AnsA, but shows very few to no water molecules in AnsB.
-
3)
The results of explicit-water MD simulations show that the Trp68-AnsA (and (2,7-aza)Trp-replaced AnsA) vicinity is hydrated by higher water density, and the water H-bond networking is modulated by the water displacement. The MD simulation also supports the water-catalyzed excited-state proton-transfer tautomerism from N(1)-H to N(7)-H in (2,7-aza)Trp68-AnsA. Conversely, the low water density around Trp66 explains the lack of N(7)-H tautomer emission in (2,7-aza)Trp66-AnsB.
-
4)
Titration of aspartate in (2,7-aza)Trp68-AnsA revealed a substantial ratiometric change of the N(1)-H-versus-N(7)-H emission intensity, implying that the hydrogen-bond network is rearranged in the vicinity of Trp upon binding the inhibitor.
In summary, using selected single-Trp-containing isozymes, AnsA and AnsB from E. coli, distinct protein dynamics, as well as conformational modulation by local constraint and trapped-water collective motions, have been unveiled. We thus demonstrate the power of combining the tryptophan analogs as the in situ sensing tool for probing polarity and water-coupled protein dynamics on a molecular structural basis for asparaginase isozymes.
Author Contributions
W.C.C. measured and analyzed the data. J.Y.S. and Y.H.C. performed the chemical syntheses. C.H.Y. and Y.K.L. performed the MD simulation. J.H.Y., L.J.L., and K.W. performed protein experiments and analysis. H.C.Y., J.F.L., J.S.W., and P.T.C. co-wrote the article. All the authors discussed the results and commented on the manuscript.
Acknowledgments
We thank the National Center for High-Performance Computing for computational resources. The designated corresponding authors Dr. Chou and Dr. Yang agree to share any relevant correspondence with Dr. Wang and Dr. Lu who supplied major contributions in the protein and genetic engineering, respectively.
This work was supported by Ministry of Science and Technology of Taiwan, a cutting-edge research grant from the National Taiwan University.
Editor: Elizabeth Rhoades.
Footnotes
Wei-Chih Chao, Jiun-Yi Shen, and Cheng-Han Yang contributed equally to this work.
Supporting Materials and Methods, Supporting Results, one scheme, and ten figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30110-2.
Contributor Information
Hsiao-Ching Yang, Email: hcyang_chem@mail.fju.edu.tw.
Pi-Tai Chou, Email: chop@ntu.edu.tw.
Supporting Citations
References (43, 44) appear in the Supporting Material.
Supporting Material
References
- 1.Wolfenden R., Snider M.J. The depth of chemical time and the power of enzymes as catalysts. Acc. Chem. Res. 2001;34:938–945. doi: 10.1021/ar000058i. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz S.D., Schramm V.L. Enzymatic transition states and dynamic motion in barrier crossing. Nat. Chem. Biol. 2009;5:551–558. doi: 10.1038/nchembio.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li T., Hassanali A.A., Singer S.J. Hydration dynamics and time scales of coupled water-protein fluctuations. J. Am. Chem. Soc. 2007;129:3376–3382. doi: 10.1021/ja0685957. [DOI] [PubMed] [Google Scholar]
- 4.Wood K., Plazanet M., Weik M. Coupling of protein and hydration-water dynamics in biological membranes. Proc. Natl. Acad. Sci. USA. 2007;104:18049–18054. doi: 10.1073/pnas.0706566104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang L.-W., Kitao A., Gō N. Ligand-induced protein responses and mechanical signal propagation described by linear response theories. Biophys. J. 2014;107:1415–1425. doi: 10.1016/j.bpj.2014.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian L., Gwizdala M., van Amerongen H. Picosecond kinetics of light harvesting and photoprotective quenching in wild-type and mutant phycobilisomes isolated from the cyanobacterium Synechocystis PCC 6803. Biophys. J. 2012;102:1692–1700. doi: 10.1016/j.bpj.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y., Qin Y., Zhong D. Ultrafast water dynamics at the interface of the polymerase-DNA binding complex. Biochemistry. 2014;53:5405–5413. doi: 10.1021/bi500810a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amaro M., Šachl R., Hof M. Time-resolved fluorescence in lipid bilayers: selected applications and advantages over steady state. Biophys. J. 2014;107:2751–2760. doi: 10.1016/j.bpj.2014.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giepmans B.N., Adams S.R., Tsien R.Y. The fluorescent toolbox for assessing protein location and function. Science. 2006;312:217–224. doi: 10.1126/science.1124618. [DOI] [PubMed] [Google Scholar]
- 10.Royer C.A. Probing protein folding and conformational transitions with fluorescence. Chem. Rev. 2006;106:1769–1784. doi: 10.1021/cr0404390. [DOI] [PubMed] [Google Scholar]
- 11.Henzler-Wildman K., Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. doi: 10.1038/nature06522. [DOI] [PubMed] [Google Scholar]
- 12.De Filippis V., De Boni S., Fontana A. Incorporation of the fluorescent amino acid 7-azatryptophan into the core domain 1-47 of hirudin as a probe of hirudin folding and thrombin recognition. Protein Sci. 2004;13:1489–1502. doi: 10.1110/ps.03542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong C.Y., Eftink M.R. Biosynthetic incorporation of tryptophan analogues into staphylococcal nuclease: effect of 5-hydroxytryptophan and 7-azatryptophan on structure and stability. Protein Sci. 1997;6:689–697. doi: 10.1002/pro.5560060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lacowicz J.R. Springer; Berlin, Germany: 2000. Protein Fluorescence. [Google Scholar]
- 15.Chaplin M. Do we underestimate the importance of water in cell biology? Nat. Rev. Mol. Cell Biol. 2006;7:861–866. doi: 10.1038/nrm2021. [DOI] [PubMed] [Google Scholar]
- 16.Negrerie M., Gai F., Petrich J.W. Photophysics of a novel optical probe: 7-azaindole. J. Phys. Chem. 1991;95:8663–8670. [Google Scholar]
- 17.Shen J.Y., Chao W.C., Chou P.T. Probing water micro-solvation in proteins by water catalysed proton-transfer tautomerism. Nat. Commun. 2013;4:2611. doi: 10.1038/ncomms3611. [DOI] [PubMed] [Google Scholar]
- 18.Chao W.C., Shen J.Y., Chou P.T. Probing water environment of Trp59 in ribonuclease T1: insight of the structure-water network relationship. J. Phys. Chem. B. 2015;119:2157–2167. doi: 10.1021/jp503914s. [DOI] [PubMed] [Google Scholar]
- 19.Yun M.K., Nourse A., Heath R.J. Crystal structure and allosteric regulation of the cytoplasmic Escherichia coli L-asparaginase I. J. Mol. Biol. 2007;369:794–811. doi: 10.1016/j.jmb.2007.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swain A.L., Jaskólski M., Wlodawer A. Crystal structure of Escherichia coli L-asparaginase, an enzyme used in cancer therapy. Proc. Natl. Acad. Sci. USA. 1993;90:1474–1478. doi: 10.1073/pnas.90.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derst C., Wehner A., Röhm K.H. States and functions of tyrosine residues in Escherichia coli asparaginase II. Eur. J. Biochem. 1994;224:533–540. doi: 10.1111/j.1432-1033.1994.00533.x. [DOI] [PubMed] [Google Scholar]
- 22.Aung H.P., Bocola M., Röhm K.H. Dynamics of a mobile loop at the active site of Escherichia coli asparaginase. Biochim. Biophys. Acta. 2000;1481:349–359. doi: 10.1016/s0167-4838(00)00179-5. [DOI] [PubMed] [Google Scholar]
- 23.Cedar H., Schwartz J.H. Localization of the two-L-asparaginases in anaerobically grown Escherichia coli. J. Biol. Chem. 1967;242:3753–3755. [PubMed] [Google Scholar]
- 24.Jennings M.P., Beacham I.R. Co-dependent positive regulation of the ansB promoter of Escherichia coli by CRP and the FNR protein: a molecular analysis. Mol. Microbiol. 1993;9:155–164. doi: 10.1111/j.1365-2958.1993.tb01677.x. [DOI] [PubMed] [Google Scholar]
- 25.Pui C.H., Campana D., Evans W.E. Childhood acute lymphoblastic leukaemia—current status and future perspectives. Lancet Oncol. 2001;2:597–607. doi: 10.1016/S1470-2045(01)00516-2. [DOI] [PubMed] [Google Scholar]
- 26.Chou P.-T., Chen Y.-C., Cheng Y.-M. Excited-state intramolecular proton transfer in 10-hydroxybenzo[h]quinoline. J. Phys. Chem. A. 2001;105:1731–1740. [Google Scholar]
- 27.Case D.A., Cheatham T.E., 3rd, Woods R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearlman D.A., Case D.A., Kollman P. AMBER, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comput. Phys. Commun. 1995;91:1–41. [Google Scholar]
- 29.Hornak V., Abel R., Simmerling C. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J., Wolf R.M., Case D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004;25:1157–1174. doi: 10.1002/jcc.20035. [DOI] [PubMed] [Google Scholar]
- 31.Loncharich R.J., Brooks B.R., Pastor R.W. Langevin dynamics of peptides: the frictional dependence of isomerization rates of N-acetylalanyl-N′-methylamide. Biopolymers. 1992;32:523–535. doi: 10.1002/bip.360320508. [DOI] [PubMed] [Google Scholar]
- 32.Miyamoto S., Kollman P.A. Settle: an analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992;13:952–962. [Google Scholar]
- 33.de Leeuw S.W., Perram J.W., Smith E.R. Simulation of electrostatic systems in periodic boundary conditions. I. Lattice Sums and Dielectric Constants. Proc. R. Soc. London Ser. A. 1980;373:27–56. [Google Scholar]
- 34.Essmann U., Perera L., Pedersen L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995;103:8577–8593. [Google Scholar]
- 35.Finazzi-Agrò A., Rotilio G., Mondovì B. Environment of copper in Pseudomonas fluorescens azurin: fluorometric approach. Biochemistry. 1970;9:2009–2014. doi: 10.1021/bi00811a023. [DOI] [PubMed] [Google Scholar]
- 36.Vivian J.T., Callis P.R. Mechanisms of tryptophan fluorescence shifts in proteins. Biophys. J. 2001;80:2093–2109. doi: 10.1016/S0006-3495(01)76183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wlodawer A., Minor W., Jaskolski M. Protein crystallography for non-crystallographers, or how to get the best (but not more) from published macromolecular structures. FEBS J. 2008;275:1–21. doi: 10.1111/j.1742-4658.2007.06178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann H.B., Whitney D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947;18:50–60. [Google Scholar]
- 39.Meral D., Toal S., Urbanc B. Water-centered interpretation of intrinsic pPII propensities of amino acid residues: in vitro-driven molecular dynamics study. J. Phys. Chem. B. 2015;119:13237–13251. doi: 10.1021/acs.jpcb.5b06281. [DOI] [PubMed] [Google Scholar]
- 40.Toal S., Meral D., Schweitzer-Stenner R. pH-Independence of trialanine and the effects of termini blocking in short peptides: a combined vibrational, NMR, UVCD, and molecular dynamics study. J. Phys. Chem. B. 2013;117:3689–3706. doi: 10.1021/jp310466b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chovancova E., Pavelka A., Damborsky J. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLOS Comput. Biol. 2012;8:e1002708. doi: 10.1371/journal.pcbi.1002708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Citri N., Zyk N. Stereospecificity of the catalytic reaction of L-asparaginase. Biochemistry. 1972;11:2103–2109. doi: 10.1021/bi00761a017. [DOI] [PubMed] [Google Scholar]
- 43.Kuethe J.T., Zhong Y.-L., Davies I.W. Development of practical syntheses of potent non-nucleoside reverse transcriptase inhibitors. Tetrahedron. 2009;65:5013–5023. [Google Scholar]
- 44.Levine B.G., Stone J.E., Kohlmeyer A. Fast analysis of molecular dynamics trajectories with graphics processing units—radial distribution function histogramming. J. Comput. Phys. 2011;230:3556–3569. doi: 10.1016/j.jcp.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.