Summary
The quest to extend healthspan via pharmacological means is becoming increasingly urgent, both from a health and economic perspective. Here we show that lithium, a drug approved for human use, promotes longevity and healthspan. We demonstrate that lithium extends lifespan in female and male Drosophila, when administered throughout adulthood or only later in life. The life-extending mechanism involves the inhibition of glycogen synthase kinase-3 (GSK-3) and activation of the transcription factor nuclear factor erythroid 2-related factor (NRF-2). Combining genetic loss of the NRF-2 repressor Kelch-like ECH-associated protein 1 (Keap1) with lithium treatment revealed that high levels of NRF-2 activation conferred stress resistance, while low levels additionally promoted longevity. The discovery of GSK-3 as a therapeutic target for aging will likely lead to more effective treatments that can modulate mammalian aging and further improve health in later life.
Keywords: GSK-3, NRF-2, aging, xenobiotic stress, Keap1, dietary restriction, triglycerides
Graphical Abstract
Highlights
-
•
Lithium extends Drosophila lifespan independent of sex and genetic background
-
•
Lithium reduces triglycerides and confers stress-resistance
-
•
Genetic or pharmacological inhibition of GSK-3 activates NRF-2
-
•
NRF-2 activation is required for the longevity effects of lithium
The mood stabilizer lithium has been shown to extend lifespan in organisms ranging from yeast to flies. Castillo-Quan et al. show that lithium promotes longevity through GSK-3 inhibition and subsequent NRF-2 activation, suggesting that GSK3 is a possible drug target that might affect aging.
Introduction
Lithium is the most commonly prescribed drug for the treatment of bipolar disorder. It also improves disease phenotypes in animal models of many clinical conditions including Alzheimer disease, depression, and stroke (Chiu and Chuang, 2010). The effects of lithium on aging have been documented in yeast and Caenorhabditis elegans, with lithium extending lifespan (McColl et al., 2008, Zarse et al., 2011, Tam et al., 2014, Sofola-Adesakin et al., 2014). The effects of lithium on Drosophila aging have previously been inconclusive, with demonstration of both positive and negative effects on survival (Matsagas et al., 2009, Zhu et al., 2015). Moreover, lithium concentration in the drinking water of a large Japanese population has been associated with reduced all-cause mortality (Zarse et al., 2011), suggesting that lithium may be a bona fide anti-aging drug. However, the mechanisms by which lithium acts in humans remain poorly understood.
In vitro studies have reported that lithium can protect against several forms of oxidative and xenobiotic stressors (Lai et al., 2006, Schäfer et al., 2004), but in vivo evidence for such protective effects of lithium is lacking. Longevity has been extensively correlated with resistance to stress (Minois, 2000, Rattan, 2008, Calabrese et al., 2011, Epel and Lithgow, 2014). Transcriptomic analysis of interventions known to extend lifespan have identified particular genes likely to be involved in stress resistance (McElwee et al., 2007, Steinbaugh et al., 2012). Upregulation of the transcription factor cap’n’collar C (CncC, an NRF-2 homolog) has been shown not only to confer resistance to toxic compounds, but also to promote longevity in C. elegans and flies (Tullet et al., 2008, Sykiotis and Bohmann, 2008, Ewald et al., 2015). In flies and mammals, NRF-2/CncC is negatively inhibited through cytosolic sequestration and proteasomal degradation by the canonical Keap1 (Hayes and Dinkova-Kostova, 2014, Pitoniak and Bohmann, 2015). However, a second emerging upstream regulator of NRF-2/CncC is GSK-3, a well-documented target of lithium (Jope, 2003, Hayes and Dinkova-Kostova, 2014, Cuadrado, 2015, Hayes et al., 2015, Blackwell et al., 2015). GSK-3 regulates NRF-2 by phosphorylation and nuclear exclusion, an effect that is evolutionarily conserved from invertebrates to mammals (Salazar et al., 2006, An et al., 2005). Interestingly, GSK-3 inhibition has been shown to phenocopy the effects of lithium for protection against xenobiotic stress in vitro (Lai et al., 2006, Schäfer et al., 2004).
Activation of NRF-2/CncC produces hormetic effects on lifespan, such that at low level NRF-2/CncC activity extends lifespan while higher levels of activation limit it (Mattson, 2008, Maher and Yamamoto, 2010). Interestingly a hormetic signature was recently reported for the survival of a mammalian cell line treated with lithium (Suganthi et al., 2012), suggesting that lithium and GSK-3 inhibition could influence animal lifespan and stress resistance through activation of NRF-2.
Here we show that lithium supplementation in the diet can modulate longevity, stress resistance, and metabolism in Drosophila through the inhibition of GSK-3. Correspondingly, genetic downregulation of GSK-3 and lithium treatment are epistatic, suggesting a common molecular pathway. We also show that lithium and the genetic inhibition of GSK-3 promote xenobiotic stress resistance and lifespan extension through the activation of a transcriptional response mediated by CncC/NRF-2. Furthermore, lithium protects against a high-sucrose diet and acts through mechanisms that only partially overlap with those mediating lifespan extension by dietary restriction (DR). These findings demonstrate an alternative genetic and pharmacological target for the promotion of longevity and stress resistance, and emphasize the potential of pharmacological inhibitors of GSK-3 as viable anti-aging treatments.
Results
Lithium Extends Healthy Lifespan in Drosophila
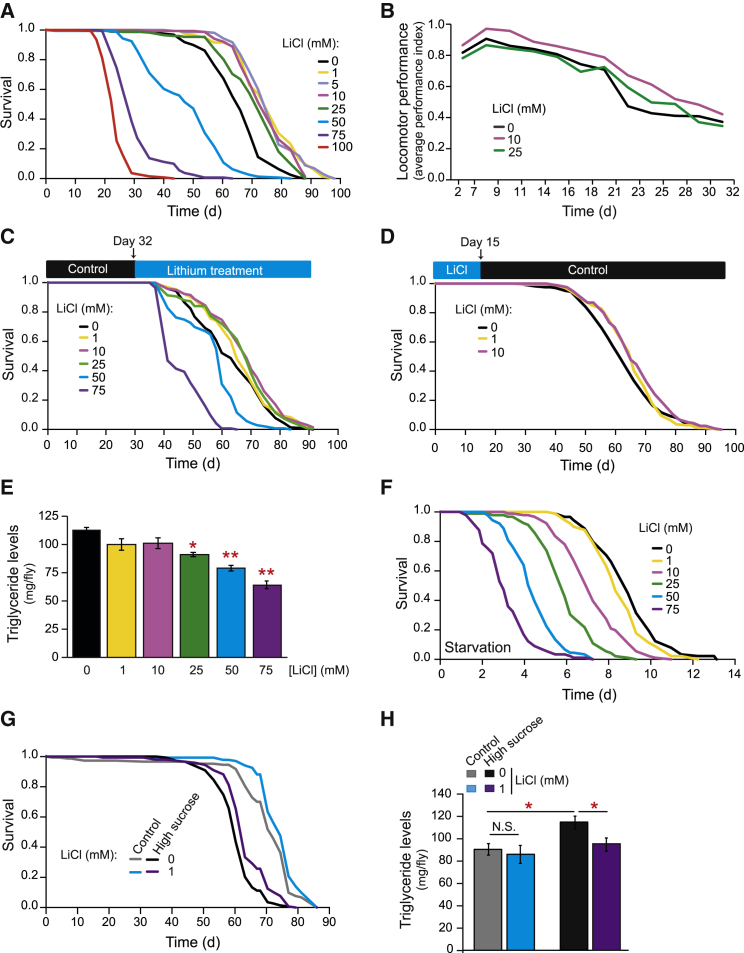
To assess the role of lithium in Drosophila aging, we treated adult female flies with lithium chloride (LiCl) by supplementation in their food. Lithium treatment in the range of 1 to 25 mM resulted in lifespan extension, whereas higher doses (50–100 mM) shortened lifespan (Figure 1A). These effects of lithium treatment on lifespan extension were also observed in an independent genetic background (Figure S1A) and in males (Figure S1B). Thus, lithium treatment extended Drosophila lifespan independently of genetic background and sex.
Figure 1.
Lithium Regulated Longevity and Metabolism in Drosophila
(A) Lithium extended lifespan of wDahDrosophila females (n = 160 flies per condition) at concentrations between 1 and 25 mM (+16% and +18% median and maximum lifespan extension; p < 0.001), but resulted in a dose-dependent reduction in lifespan at concentrations between 50 and 100 mM (p < 0.001).
(B) Lithium treated female w1118 flies showed a significant improvement and protection against age-related locomotor decline (p < 0.01, two-way ANOVA for 10 mM).
(C) Lithium extended lifespan of aged, 32-day-old female wDah flies at concentrations from 1 to 25 mM (30 days later than in Figure 1A): 1 mM extended median lifespan by 5% (4 days) and maximum lifespan by 13% (8 days; p < 0.05); 10 and 25 mM lithium increased median lifespan by 9% (6 days); 10 mM increased maximum lifespan by 4.5% (3.5 days); wherease 25 mM lengthened it by 8% or 6 days (p < 0.01); and 50 and 75 mM significantly shortened lifespan (p < 0.01). n = 150 flies per condition.
(D) Brief treatment with lithium for 15 days early in adulthood extended lifespan of female wDah flies (p < 0.05 for 1 mM and p < 0.01 for 10 mM; n = 150 flies per condition).
(E) Lithium induced a dose-dependent reduction in triglyceride levels. Bars represent means of six replicas of five flies per condition ± SEM. ∗p < 0.01, ∗∗p < 0.001.
(F) Female wDah flies pre-treated with lithium for 15 days were subsequently sensitive to starvation in a dose-dependent manner (n = 90 flies per condition).
(G) Lithium treatment significantly extended the lifespan of w1118 female flies exposed to a four times higher sucrose concentration (2g/L; p < 0.001; n = 120 flies per condition).
(H) The increase of triglycerides observed on a high-sucrose diet was completely blocked after 15 days of treatment with 1 mM lithium. Bars represent means of six replicas of five w1118 female flies per condition ± SEM. ∗p < 0.01.
To ensure that the increased lifespan observed with lithium supplementation was dependent on the addition of lithium itself, we treated flies with equivalent molar concentrations of sodium chloride (NaCl) and found no lifespan extension (Figures S1C and S1D). Thus, the pro-longevity effect of LiCl is specific to lithium and not its chloride counterion.
Interestingly, we observed that, unlike with many other genetic and pharmacological interventions (e.g., DR, insulin/IGF downregulation, rapamycin, or trametinib treatment), lithium did not reduce fecundity at life-extending doses or compromise feeding behavior (Figures S1E and S1F). Moreover, it delayed locomotor decline at two concentrations that extend lifespan (Figure 1B). Thus, lithium promotes healthspan in adult Drosophila with limited side effects.
Lithium Extends Lifespan in Mid-life or with Short-Term Treatment in Young Flies
To limit the side effects of long-term use, a drug that improves lifespan and healthspan will ideally do so with late-onset administration (Castillo-Quan et al., 2015, Longo et al., 2015). We therefore assessed the effect of commencing lithium treatment at older ages. Flies were switched onto food containing a range of lithium concentrations (1–75 mM) at 32 days of age (Figure 1C). Lower doses (1–25 mM) of lithium extended lifespan, whereas higher doses (50 and 75 mM) significantly reduced lifespan, similar to the dose-dependent effects we observed in younger flies.
We also tested whether transient lithium treatment early in life could increase lifespan. We therefore exposed young flies to 1 or 10 mM lithium for 15 days and then switched them to control food for the remainder of their lifespans. Early treatment with these doses of lithium extended lifespan (Figure 1D). Lithium treatment early in life, and for a transient period, can therefore increase survival later in life.
Lithium Alters Lipid Metabolism and Promotes Survival under a High-Sugar Diet
Genetic and environmental interventions that extend lifespan often induce abnormalities in carbohydrate and lipid metabolism (Barzilai et al., 2012, Wang et al., 2014, Lamming et al., 2013). We therefore examined the effects of lithium on whole body trehalose, glycogen, and triglyceride levels. Following 15 days of lithium treatment, and over a wide range of lithium concentrations, we were unable to detect a significant change in the levels of either trehalose or glycogen (Figures S1G and S1H). However, we observed a dose-dependent reduction in whole body triglycerides, the main lipid storage in flies (Ballard et al., 2008, Skorupa et al., 2008) (Figures 1E and S1I). In keeping with the lowered triglyceride levels (Ballard et al., 2008, Ulgherait et al., 2014), lithium treatment reduced survival under starvation conditions in a dose-dependent manner (Figures 1F and S1J). Moreover, lithium also extended lifespan under dietary conditions that promote triglyceride accumulation (Skorupa et al., 2008). Flies fed a high-sucrose diet were short lived and lithium was able to partially rescue this defect (Figure 1G) while completely blocking the increase in triglycerides observed with a sucrose-rich diet (Figure 1H). Therefore, lithium can extend lifespan under obesogenic dietary conditions.
Lithium and DR Extend Lifespan via Partially Overlapping Mechanisms
We next investigated whether lithium treatment was acting as a DR mimetic. DR is a well-established anti-aging intervention that extends healthy lifespan in diverse species (de Cabo et al., 2014, Fontana and Partridge, 2015), and some pharmacological and genetic interventions that extend lifespan have features of DR mimetics (Ingram and Roth, 2015, de Cabo et al., 2014). To determine whether lithium and DR extend lifespan by similar mechanisms, we assessed whether lithium could extend lifespan beyond the maximum achievable by DR. To maximize lifespan under DR, we varied the yeast concentration in the food while maintaining a constant concentration of sucrose (Bass et al., 2007), resulting in a typical tent-shaped response, with peak lifespan at food containing a 1.0 yeast concentration (Figures 2A and S2A–S2D). If lithium treatment and DR share overlapping pathways, then lithium would not be able to further extend lifespan already maximized by DR (Gems et al., 2002, Castillo-Quan et al., 2015). All lithium doses tested significantly extended median lifespan in both the yeast condition that maximized lifespan (1.0 yeast; Figures 2A and S2C) and under full feeding (2.0 yeast; Figures 2A and S2D), with greatest extension of median lifespan with 10 mM lithium under full feeding. However, under reduced yeast concentrations that shorten lifespan (0.2 and 0.5 yeast), 10 mM lithium either significantly reduced lifespan (Figure S2B) or did not confer a significant lifespan benefit (Figure S2A). Cox proportional hazards analysis showed a significant interaction between lithium and yeast concentrations for lifespan (interaction term p < 0.0001). The extension of lifespan from lithium increased with the level of yeast in the fly diet, suggesting partially overlapping mechanisms to those of DR.
Figure 2.
Lithium Extended Lifespan beyond Dietary Restriction by Inhibiting Sgg/GSK-3
(A) Median lifespans at different lithium concentrations (0, 1, 2.5, 5, or 10 mM) are plotted for four different yeast concentrations (0.2×, 0.5×, 1.0×, and 2.0× yeast): 1–5 mM lithium extended lifespan under all dietary conditions tested. Although 10 mM lithium prolonged life at 1.0× and 2.0×, it showed no effect at 0.2× and significantly shortened lifespan at 0.5× yeast. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, from 0 lithium; n = 160 flies per condition. Complete survival curves are shown in Figures S2A–S2D.
(B) Lithium treatment for 15 days significantly increased the inhibitory phosphorylation of Sgg/GSK-3 in a dose-dependent manner. Bars represent means of triplicates of ten flies per biological repeat ± SEM, ∗p < 0.05, ∗∗p < 0.01.
(C) Ubiquitous overexpression of wild-type sgg significantly shortened lifespan (p < 0.001) and this was partially rescued by lithium treatment at two concentrations (10 and 25 mM; p < 0.001). See Figure S2E for the interaction of sgg(S9A) and lithium treatment on lifespan.
(D) Ubiquitous RNAi-mediated downregulation of sgg extended lifespan (p < 0.001) and no further extension occurred when the flies were treated with 1 or 5 mM lithium (p > 0.05), whereas 10 mM lithium treatment restored the lifespan to control levels (p > 0.05), and 25 mM was significantly toxic (p > 0.05). See Figure S2F for lifespan extension obtained with an independent RNAi line.
Lithium Extends Lifespan through Inhibition of GSK-3
A well-known target of lithium is GSK-3 (Phiel and Klein, 2001, Jope, 2003, Eldar-Finkelman and Martinez, 2011). We therefore evaluated the phosphorylation status of the fly ortholog of GSK-3, Shaggy (Sgg), in response to lithium treatment. Lithium addition to the fly medium resulted in a dose-dependent increase in the inhibitory phosphorylation (Serine 9 or S9) of Sgg (Figure 2B). To evaluate the role of Sgg in lithium-mediated lifespan extension, we directly manipulated its activity in adult flies. Ubiquitous overexpression of wild-type or constitutively active Sgg (SggS9A) significantly reduced lifespan by ∼30% and 50%, respectively (Figures 2C and S2E). This reduction in lifespan was almost completely reversed by lithium treatment. Furthermore, RNAi-mediated reduction in sgg expression using two independent dsRNA-expressing transgenes significantly increased lifespan (Figures 2D and S2F). Importantly, lithium was unable to further increase the lifespan of these sgg RNAi knockdown mutants flies (Figure 2D). Taken together, these findings suggest that Sgg/GSK-3 inhibition and lithium treatment increase lifespan by acting on the same downstream targets.
Lithium Activates the Cap’n’Collar C/NRF-2 Transcription Factor
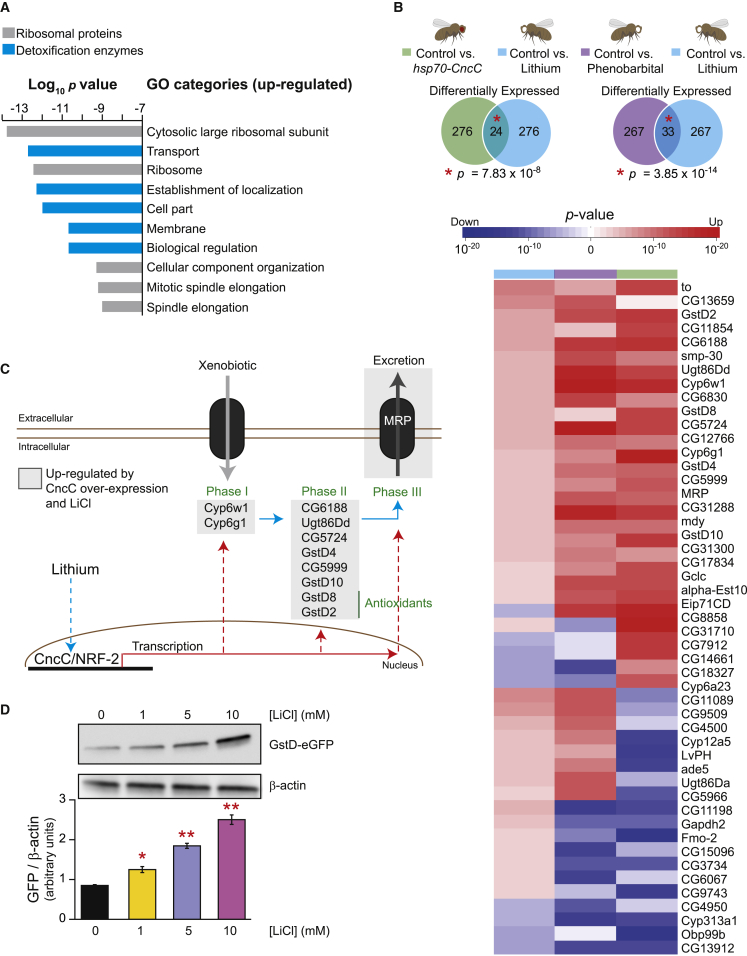
To identify downstream mediators of lifespan extension by lithium and of GSK-3 inhibition, we analyzed the genome-wide transcript profiles of lithium-treated flies using microarrays. Genes encoding ribosomal proteins were among the most upregulated (Figure 3A) and downregulated (Figure S3A) gene ontology (GO) categories in lithium-treated flies. This transcriptional response could underlie the translational repression following lithium treatment that has been previously observed in fission yeast and Drosophila heads (Sofola-Adesakin et al., 2014). In addition, five GO terms for genes encoding enzymes in the detoxification pathway were also in the ten most upregulated categories (Figure 3A).
Figure 3.
Lithium Activated a Transcriptional Response Similar to that of CncC/NRF-2
(A) Ten most significantly upregulated GO categories induced by lithium treatment of w1118 female flies. See Figure S3A for downregulated GO categories.
(B) Lithium treatment of w1118 females flies induced a transcriptional response that significantly overlapped with that induced by cncC overexpression (p = 7.83 × 10−8) or phenobarbital treatment (p = 3.85 × 10−14) (Misra et al., 2011). Heatmap showing the 57 genes most significantly changed by lithium or phenobarbital treatment and overexpression of cncC.
(C) Genes upregulated by lithium treatment mapped to the three phases of the xenobiotic detoxification pathway in flies.
(D) Lithium treatment of wDah female flies upregulated Gst-D protein levels. Bars represent means of triplicates of ten flies per condition ± SEM. ∗p < 0.05, ∗∗p < 0.01.
The responses to xenobiotics and oxidative stress in Drosophila are regulated by the transcription factors dFOXO, CncC, and DHR96 (Salih and Brunet, 2008, Sykiotis and Bohmann, 2010, Tullet, 2015, Hoffmann and Partridge, 2015, Blackwell et al., 2015). We therefore assessed whether the transcriptional responses to activation of these transcription factors overlapped with that of lithium treatment. The transcriptomic response to lithium did not overlap with that of dFOXO-dependent or -independent transcriptional regulation downstream of IIS (Figures S3B and S3C) (Alic et al., 2011). Furthermore, although we detected a significant overlap in the transcriptional signatures of lithium and DHR96 (King-Jones et al., 2006), they did not share the same directionality (Figure S4). However, we found a significant overlap (Figure 3B) between the genes that were upregulated by lithium and cncC overexpression (Misra et al., 2011), but not between genes downregulated by both treatments (Figure S5A), suggesting that lithium might activate a CncC transcriptional response downstream of GSK-3. The barbiturate phenobarbital activates CncC and induces a similar transcriptional response to that of cncC overexpression (Misra et al., 2011). We therefore analyzed the overlap between the transcriptional profiles induced by lithium and phenobarbital treatment, and again found a significant overlap (Figure 3B) between upregulated, but not downregulated, genes (Figure S5B). The genes upregulated in common between lithium treatment, phenobarbital treatment and cncC overexpression (Figures 3B and S5C) encoded enzymes that participate in all three phases of xenobiotic metabolism (Figure 3C). To further confirm the activation of CncC by lithium, we used a previously generated CncC reporter that responds to both chemical and genetic inducers of CncC (Sykiotis and Bohmann, 2008). Flies carrying the GstD-eGFP CncC reporter showed a dose-dependent increase in GFP expression with increasing concentrations of lithium (Figure 3D). Taken together, our results suggest that lithium activates CncC to upregulate the expression of genes in the detoxification pathway.
Lithium Induces Lifespan-Extension, Hormesis, and Protection against Xenobiotics via CncC-Dependent Mechanisms
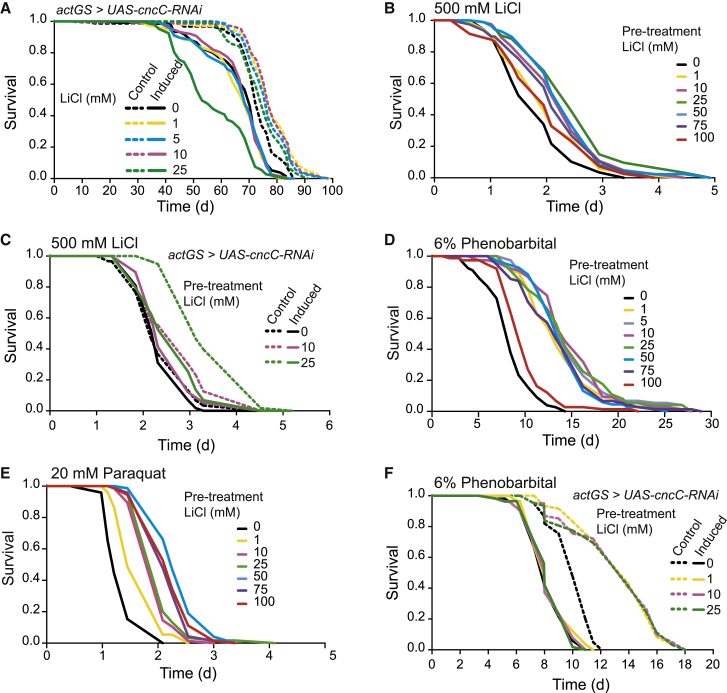
We next assessed whether CncC activity is required for the pro-longevity effects of lithium. Ubiquitous, RNAi-mediated knockdown of cncC expression blocked the lifespan extension of 1 to 10 mM lithium, but was detrimental to survival in flies treated with 25 mM lithium, the highest dose that extends lifespan under basal conditions, albeit to a lesser extent (Figure 4A). Thus, lithium treatment requires CncC activity to confer its longevity benefits.
Figure 4.
Lithium-Induced Xenobiotic Resistance and Longevity Were Mediated by CncC
(A) Ubiquitous knockdown of cncC blocked lifespan extension by lithium.
(B) Pre-treatment with increasing concentrations of lithium protected against a subsequent toxic dose of lithium (500 mM; p < 0.01 for doses from 10 to 100 mM; p < 0.05 for 1 mM).
(C) Ubiquitous downregulation of cncC blocked the protective effect of 10 mM lithium pre-treatment against a subsequent toxic dose and partially blocked the protective effect of 25 mM lithium pre-treatment.
(D) 1 to 100 mM lithium pre-treatment protected against a 6% phenobarbital (p < 0.001 for all doses).
(E) Lithium pre-treatment (for 15 days) protected against the herbicide paraquat in a dose-dependent manner (p < 0.001 for all doses, with maximal protection at 50 mM).
(F) RNAi-mediated downregulation of cncC completely blocked the protective effect of lithium against phenobarbital.
Because CncC/NRF-2 can induce hormesis (Mattson, 2008, Maher and Yamamoto, 2010), we assessed whether lithium can also do this. To test for a hormetic effect of lithium at low doses, we pre-treated flies with a range of concentrations of lithium and then challenged them with a toxic dose of 500 mM. Most pre-treatment doses of lithium induced subsequent resistance to the toxic dose (Figure 4B). To assess whether the hormetic response of lithium was mediated by CncC, we knocked down expression of cncC using RNAi, and treated the flies with 1–25 mM lithium. Reduction in cncC expression completely blocked the hormetic response induced by 10 mM lithium pre-treatment, and significantly reduced the effect of 25 mM lithium (Figure 4C).
We next assessed the ability of lithium pre-treatment to protect against other xenobiotics. Flies pre-treated with increasing concentrations of lithium ranging from 1 to 100 mM were significantly resistant to a toxic concentration of phenobarbital, with lithium doses between 1 and 75 mM almost doubling survival (Figure 4D). Lower doses of lithium also protected against a toxic dose of the anti-malarial drug, chloroquine (Figure S5D; 1–10 mM), and the pesticide paraquat (Figure 4E). Thus, low to intermediate concentrations of lithium protect against xenobiotic toxicity. To determine the role of CncC activity in lithium-mediated protection against phenobarbital, we used RNAi to knock down expression of cncC, which sensitized the flies to phenobarbital and completely abrogated the protection against phenobarbital afforded by lithium supplementation (Figure 4F). Thus, CncC is at least partly responsible for the hormetic effect induced by low-level treatment with lithium.
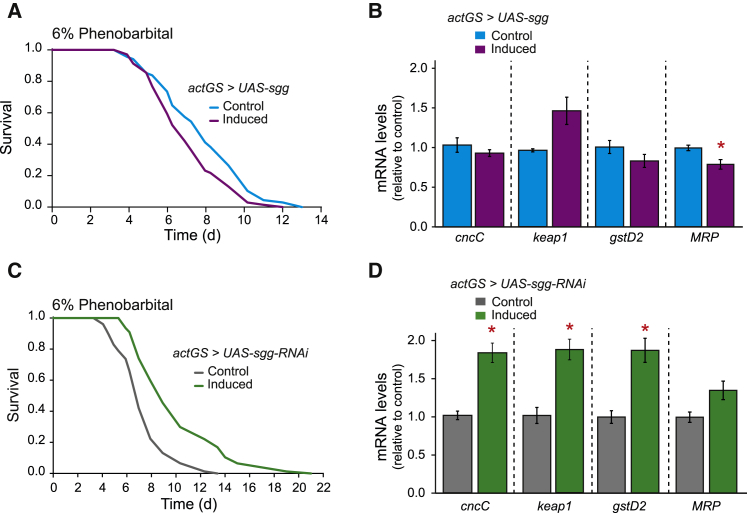
To confirm that Sgg, upstream of CncC, is also necessary for the resistance to xenobiotic stress (Blackwell et al., 2015, Cuadrado, 2015, Hayes et al., 2015), we assessed the effect of ubiquitous overexpression of wild-type sgg or the constitutively active Sgg(S9A) on xenobiotic resistance. Both significantly sensitized flies to phenobarbital (Figures 5A and S5E). We confirmed that sgg or sgg(S9A) overexpression regulated CncC by showing significantly lower levels of MRP and keap1 (Figures 5B and S5F), both CncC target genes. Correspondingly, RNAi-mediated knockdown of sgg resulted in resistance to phenobarbital (Figure 5C), and paraquat (Figure S5G). An increase of mRNA levels of cncC, keap1, and gstD2 confirmed that CncC was active in sgg knockdown flies (Figure 5D). Thus, increased Sgg activity sensitizes against xenobiotic stressors, whereas its inhibition protects against them.
Figure 5.
Reduced Activity of GSK-3 Increased Resistance to Xenobiotics
(A) Ubiquitous overexpression of wild-type sgg significantly (p < 0.05) reduced survival under xenobiotic stress with phenobarbital. n = 75 flies per condition.
(B) Overexpression of wild-type sgg significantly reduced multidrug-resistance like protein 1 (MRP) mRNA levels (p < 0.05, paired t test), whereas non-significant trends were detected for glutathione S transferase D2 (gstD2) and cncC mRNA levels (p > 0.05). A non-significant increase of keap1 mRNA levels was observed.
(C) RNAi-mediated knockdown of sgg protected against phenobarbital stress (p < 0.001). n = 75 flies per condition.
(D) Knockdown of sgg increased mRNA levels of cncC, keap1 and gstD2 (p < 0.05), while a non-significant increase was observed for MRP mRNA levels.
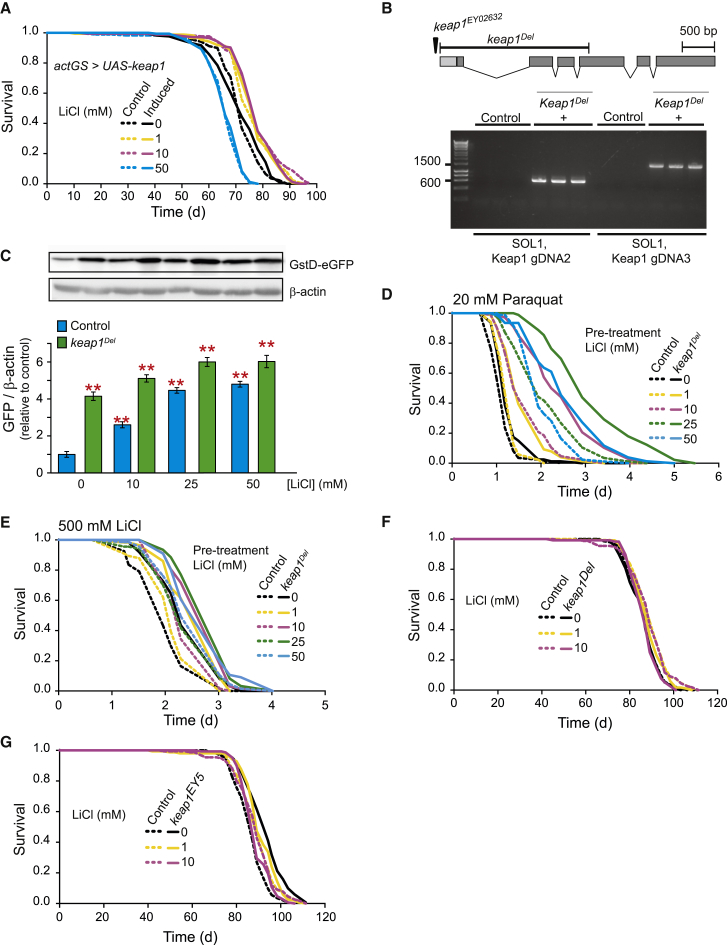
Lifespan and Stress Resistance Depend on the Degree of Activation of CncC by Keap1 and Lithium Treatment
In addition to activating CncC by repressing Sgg/GSK-3, lithium could potentially increase CncC activity by inhibiting its canonical repressor Keap1 (Cuadrado, 2015, Pitoniak and Bohmann, 2015). Hence, we analyzed the interaction between lithium treatment and Keap1. Overexpression of Keap1, which inhibits CncC activity in vivo (Sykiotis and Bohmann, 2008), was unable to prevent the lifespan-extending properties of lithium (Figure 6A), suggesting that the longevity effect of lithium treatment is independent of Keap1. Next, we analyzed the interaction of loss of Keap1 and lithium treatment. We generated a deletion of the keap1 coding sequence by P-element-mediated male recombination using a previously described P-element insertion line (Sykiotis and Bohmann, 2008) (Figure 6B). The keap1 deletion (keap1Del) was homozygous lethal, but activated CncC 4-fold in the heterozygous state, as measured by the CncC reporter (Figure 6C). Lithium treatment of the keap1Del flies further activated CncC (Figure 6C). We next tested whether this effect on CncC activation protected against paraquat and lithium toxicity. keap1Del flies were significantly resistant to both paraquat and lithium (Figures 6D and 6E), and pre-treatment with lithium further protected them. We confirmed these findings using a previously described heterozygous loss-of-function mutation in the keap1 gene (keap1EY5) (Sykiotis and Bohmann, 2008) (Figures S6A and S6B). Thus, the combination of loss of keap1 and lithium treatment further protected against paraquat and lithium-induced toxicity, suggesting that stronger CncC activation results in greater protection against these xenobiotics.
Figure 6.
Higher Activation Levels of CncC Promote Xenobiotic Resistance but Not Lifespan
(A) Overexpression of keap1 did not prevent the lifespan-modulatory effects of lithium treatment. n = 150 flies per condition.
(B) Schematic of the keap1 gene showing the portion deleted in the keap1Del mutant (top) and agarose gel showing start and end of P-element disrupting keap1 coding sequence in the keap1Del mutant (bottom).
(C) Combination of heterozygous deletion of keap1 and lithium treatment showed a greater activation of CncC than on their own. Bars represent means of four replicas of five flies per repeat ± SEM. ∗∗p < 0.01.
(D) Deletion of keap1 in flies treated with lithium showed greater protection against paraquat than either treatment on its own, with maximal effects observed at 25 mM (p < 0.001).
(E) The keap1 deletion protected against toxic concentrations of lithium (500 mM), and this protection was augmented with lithium pre-treatment (p < 0.01).
(F) Deletion of keap1 did not extend lifespan: 1 mM lithium (p < 0.05), but not 10 mM (p > 0.05), treatment of keap1 flies resulted in a small but significant extension. n = 150 flies per condition.
(G) keap1EY5 mutant flies showed significant lifespan extension (p < 0.001), that was dose-dependently abolished (p > 0.05) by lithium, likely as a result of over-activation of CncC. n = 150 flies per condition.
We subsequently evaluated the interaction between loss of keap1 and lithium treatment for longevity. Survival analysis showed that the lifespan of keap1Del mutant flies was indistinguishable from controls, but that addition of 1 mM lithium marginally, yet significantly, extended lifespan (Figure 6F). Increasing the dose of lithium to 10 mM restored longevity to control levels. The keap1EY5 mutant flies showed a significant lifespan extension (Figure 6G). However, supplementation of either 1 or 10 mM lithium to the keap1EY5 mutant shortened lifespan in a dose-dependent manner. These results suggest that the level of activation of CncC that maximizes extension of lifespan is considerably lower than that which maximizes protection against toxic doses of lithium and paraquat.
Lithium Does Not Induce or Require Autophagy to Promote Longevity
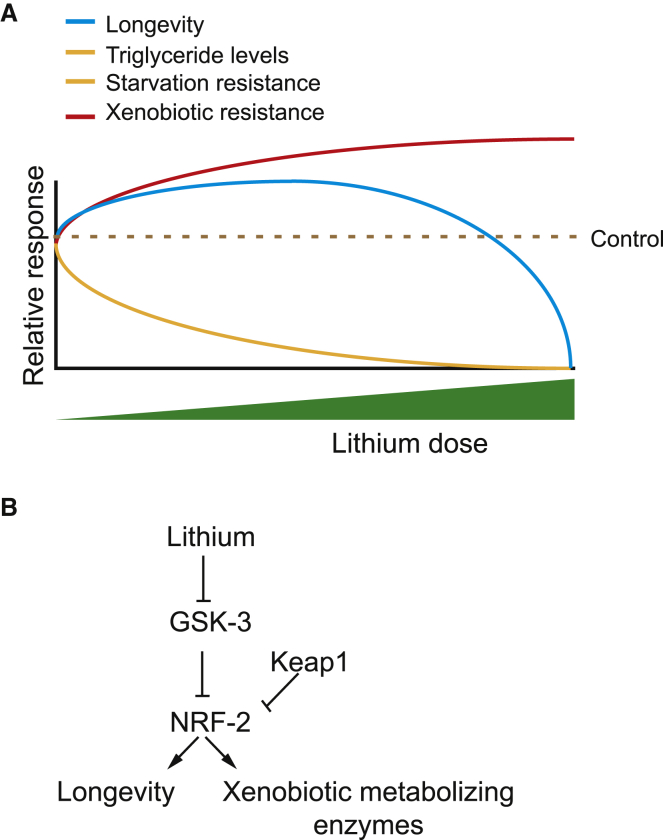
Activation of autophagy has been proposed as a mechanism for the beneficial effects of lithium (Sarkar et al., 2005). We therefore analyzed the induction of autophagy by LC3-I/LC3-II (Atg8 in Drosophila) levels without detecting statistically significant changes. Indeed, there was a tendency for lower LC3-I that did not reach statistical significance (Figure S7A). Moreover, lithium treatment was able to extend the lifespan of flies with autophagy defects due to heterozygous loss of atg1 (Figure S7B) (Lee et al., 2007). Thus, taken together our results do not immediately support a role for autophagy in the pro-longevity effects of lithium treatment, and strengthen our conclusion that they are mediated through the inhibition of GSK-3 and the subsequent activation of CncC/NRF-2 (Figure 7). However, it remains possible that induction of autophagy occurs in atg1-deficient flies, or that lithium induces autophagy in a tissue-specific manner.
Figure 7.
Lithium Regulates Longevity, Metabolism, and Stress Resistance by Inhibiting GSK-3 and Activating NRF-2
(A) Summary of findings with lithium for longevity, stress resistance, starvation, and triglyceride levels.
(B) Proposed model showing the mechanism by which lithium, Sgg/GSK-3, and CncC/NRF-2 act in the same pathway to modulate longevity and xenobiotic resistance.
Discussion
Lithium Acts as a Pro-longevity Drug
Drug repurposing is the most promising approach for developing pharmacological agents to improve healthy aging. So far, two medically approved drugs, metformin and rapamycin, have been reported to promote longevity and provide health benefits across species from invertebrates to mammals (de Cabo et al., 2014, Madeo et al., 2014, Riera and Dillin, 2015). We and others have shown that lithium can extend lifespan in fission yeast, C. elegans, and Drosophila (McColl et al., 2008, Matsagas et al., 2009, Sofola-Adesakin et al., 2014). We also showed that this effect was common between two different laboratory strains and, unlike other interventions that seem to be more effective in females (Austad and Bartke, 2015), lithium similarly extended lifespan in both sexes.
Lifespan-extending drugs can often act like DR mimetics (Madeo et al., 2014, Ingram and Roth, 2015); hence, it was important to determine whether lithium was acting in a similar manner. While low doses of lithium were able to extend lifespan at all dietary levels tested, median lifespan extension was greatest under full feeding conditions. Our data thus suggest that lithium and DR act via partially overlapping mechanisms and confirms the observation made in C. elegans that lithium extends lifespan of eat-2 mutants (McColl et al., 2008), a genetic model of DR in worms. Lithium also extended the lifespan of flies fed a diet enriched with sucrose, possibly by modulating lipid metabolism (Sykiotis et al., 2011, Pang et al., 2014, Karim et al., 2015, Steinbaugh et al., 2015). However, the role of CncC in modulating the triglyceride phenotype of lithium remains to be explored. Overall, our observations strongly suggest that lithium is a pro-longevity drug capable of extending lifespan at low doses independent of sex and genetic background, and under a variety of dietary conditions.
Lithium Toxicity, Hormesis, and Stress Resistance
In humans, the therapeutic window for lithium treatment of bipolar disorder lies between 0.5 and 1 mM in serum, whereas concentrations of 1.5 mM and above severely increase the risk of tissue damage (Malhi and Tanious, 2011). Previous work in Drosophila suggests that the dose range at which we observed lifespan extension (0.5–25 mM) translates to Drosophila tissue concentrations below 0.5 mM (Dokucu et al., 2005). As previously reported for C. elegans and Drosophila (McColl et al., 2008, Zhu et al., 2015), concentrations above 50 mM were highly toxic.
Drug interventions to promote healthy lifespan are less likely to have side effects if started late in life (Castillo-Quan et al., 2015). Only a handful of drugs approved by the US Food and Drug Administration, namely rapamycin, metformin, and the Ras inhibitor trametinib, induce lifespan extension when commenced at later ages in model organisms (Harrison et al., 2009, Cabreiro et al., 2013, Martin-Montalvo et al., 2013, Slack et al., 2015). We found that lithium extends lifespan when first administered in mid-late life. In humans, long-term treatment with lithium for psychiatric disorders is associated with progressive and permanent renal damage (Malhi and Tanious, 2011). We showed that short treatment periods in Drosophila, 15 days during early adulthood, are sufficient to prolong life. Taken together, our data suggest that when testing lithium as a pro-longevity drug in mammals, lower doses than those used in psychiatric disorders are likely to be sufficient, and other strategies such as alternate-day dosing or transient treatment periods (either early or late in life), may be sufficient to reduce undesirable side effects and maximize the potential health benefits.
Interestingly, doses of lithium that shortened lifespan were protective against certain forms of xenobiotic stress. In vitro studies in mammalian cells have shown that lithium, and other GSK-3 inhibitors, protect against cell death caused by rotenone-induced oxidative stress (Lai et al., 2006), glutamate excitotoxicity, and H2O2 (Schäfer et al., 2004). This is likely mediated through a hormetic response (Suganthi et al., 2012), in this case orchestrated by NRF-2 activation. We observed that while simultaneous activation of CncC by loss of Keap1 and lithium treatment is additive and confers greater stress resistance to xenobiotics, the threshold for lifespan extension is perhaps considerably lower. A similar situation has been observed in C. elegans in which strong activation of the endoplasmic reticulum unfolded protein response conferred stress resistance benefits, while shortening lifespan (Taylor and Dillin, 2013). Our findings thus suggest that while NRF-2 activation either by loss of Keap1 or inhibition of GSK-3 is beneficial for longevity and stress resistance, at low levels of activation, stronger induction is detrimental for lifespan. This suggests that the hormetic benefits of lithium are more likely to occur at low levels under basal non-stress conditions (Calabrese, 2013). Hence, when testing for GSK-3 inhibitors or NRF-2 activators in modulating animal (and especially mammalian) aging, the degree of NRF-2 activation within the hormetic curve will determine positive or negative longevity outcomes. Future work studying the convergence of the salutary and damaging effects of lithium will aid in understanding to what extent the molecular mechanisms are shared (Calabrese and Mattson, 2011, Calabrese et al., 2013, Epel and Lithgow, 2014). Additionally, our microarray analysis was performed in heads and thoraces; therefore, it remains to be explored to what extent systemic or localized activation of NRF-2 modulates longevity, stress resistance, and lipid metabolism at the tissue level (Douglas et al., 2015).
GSK-3 and NRF-2 as Drug Targets for Aging
Complete absence of GSK-3 in C. elegans, Drosophila, and mice shortens lifespan or prevents development (Hoeflich et al., 2000, McColl et al., 2008, Bourouis, 2002), while moderate inhibition has been associated with most of its positive effects (Avrahami et al., 2013). GSK-3 is upregulated in many disease states, including neurodegeneration, diabetes, inflammatory conditions, and some cancers (Takahashi-Yanaga, 2013). We have shown that adult-specific genetic manipulation of the fly ortholog of GSK-3, Sgg, affects longevity. Downregulation of Sgg prolonged lifespan and lithium was unable to further extend the lifespan, suggesting that lithium and inhibition of Sgg act through a common molecular pathway to extend lifespan.
In C. elegans and mammalian cells, GSK-3 directly interacts with NRF-2 to repress its activity, independently of Keap1 (An et al., 2005, Salazar et al., 2006, Rojo et al., 2008, Rada et al., 2012). Therefore, we hypothesized that lithium might act via Sgg/GSK-3, to de-repress CncC, the fly ortholog of NRF-2 and activate the oxidative and xenobiotic stress transcriptional signature (An et al., 2005, Hayes et al., 2015), which in turn would induce a CncC/NRF-2-dependent protective response (Jones et al., 2015, Blackwell et al., 2015). GO enrichment analysis identified a transcriptional signature that indeed suggested that lithium acts via CncC/NRF-2. CncC activity was indispensable for the lifespan extension conferred by lithium. In keeping with our results, work in rodents and mammalian cell lines has shown that lithium treatment and GSK-3 inhibition activate NRF-2 (Lee et al., 2014, Rizak et al., 2014). Because activation of CncC/NRF-2 modulates longevity in C. elegans and Drosophila (Tullet et al., 2008, Sykiotis and Bohmann, 2008, Ewald et al., 2015), our results provide evidence that GSK-3 is a viable therapeutic target to promote longevity via activation of NRF-2.
To date, the only GSK-3 inhibitor approved for human use is lithium (Williams and Harwood, 2000, Meijer et al., 2004, Martinez et al., 2011). However, researchers and pharmaceutical companies have developed more selective GSK-3 inhibitors, some of which have already entered the early stages of clinical trials for obesity, Alzheimer disease, and progressive supranuclear palsy (Eldar-Finkelman and Martinez, 2011). Our results call for a reassessment of the potential use of GSK-3 inhibitors and NRF-2 activators as potential anti-aging compounds.
Experimental Procedures
Fly Stocks and Husbandry
The w1118 stock was obtained from Bloomington Drosophila Stock Center. The control white Dahomey (wDah) stock has been maintained in large population cages with overlapping generations since 1970. The wDah stock was initially derived by incorporation of the w1118 mutation into the outbred Dahomey background by backcrossing (Bass et al., 2007). Further details concerning fly mutants can be found in the Supplemental Experimental Procedures.
Lithium Treatment
LiCl (Sigma) or NaCl (Sigma) were dissolved in ddH2O at a concentration of 5 M before supplementing to the medium. Equivalent volumes of vehicle were supplemented to the medium to compensate for dilution.
Dietary Restriction Protocol
The DR protocol was performed as described previously (Bass et al., 2007).
Statistical Analyses
Statistical analyses were performed using Excel, GraphPad Prism, or JMP software version 9 (SAS Institute). Survival experiments were analyzed using log rank test. Other data were tested by one-way analyses of variance (ANOVA) and planned comparisons of means were made using Tukey-Kramer HSD test. Cox proportional hazards analysis was performed to compare interactions for survival.
Author Contributions
J.I.C.-Q. and I.B. conceived the experiments. J.I.C.-Q., I.B., L.L., K.J.K., L.S.T., T.N., and F.K. performed the experiments. D.K.I. analyzed the microarray data. C.S. and I.B. contributed reagents. J.I.C.-Q., I.B., J.T., J.H., and L.P. supervised experiments/project. J.I.C.-Q. and L.P. wrote the manuscript. All authors approved the final submission.
Acknowledgments
We thank Profs. D. Gems, M. Murphy, and D. Rubinsztein, and Drs. H. Cochemé, F. Cabreiro, S. Emran, Y. de la Guardia, M. Piper, N. Woodling, A. Gilliat, and O. Sofola-Adesakin for insightful advice and comments; Dr. N. Alic for advice and assistance with statistical testing; and Dr. D. Wieser for initial analysis of microarray data. We thank the Max Planck-Genome-centre Cologne (http://mpgc.mpipz.mpg.de/home/) for microarray analysis. We acknowledge funding from UCL Scholarships (to J.I.C.-Q.), the Max Planck Society (to J.I.C.-Q., L.S.T., C.S., T.N., and L.P.), European Research Council (to I.B. and L.P.), Research Into Ageing (to I.B. and L.P.), Wellcome Trust (to D.K.I., K.J.K., J.T., J.H., and L.P.), Parkinson’s UK (to L.L. and L.P.), and Alzheimer’s Research UK (to F.K. and L.P.).
Published: April 7, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2016.03.041.
Accession Numbers
The accession number reported for the microarray data in this paper is ArrayExpress: E-MTAB-3809.
Supplemental Information
References
- Alic N., Andrews T.D., Giannakou M.E., Papatheodorou I., Slack C., Hoddinott M.P., Cochemé H.M., Schuster E.F., Thornton J.M., Partridge L. Genome-wide dFOXO targets and topology of the transcriptomic response to stress and insulin signalling. Mol. Syst. Biol. 2011;7:502. doi: 10.1038/msb.2011.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J.H., Vranas K., Lucke M., Inoue H., Hisamoto N., Matsumoto K., Blackwell T.K. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc. Natl. Acad. Sci. USA. 2005;102:16275–16280. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad S.N., Bartke A. Sex Differences in Longevity and in Responses to Anti-Aging Interventions: A Mini-Review. Gerontology. 2015;62:40–46. doi: 10.1159/000381472. [DOI] [PubMed] [Google Scholar]
- Avrahami L., Licht-Murava A., Eisenstein M., Eldar-Finkelman H. GSK-3 inhibition: achieving moderate efficacy with high selectivity. Biochim. Biophys. Acta. 2013;1834:1410–1414. doi: 10.1016/j.bbapap.2013.01.016. [DOI] [PubMed] [Google Scholar]
- Ballard J.W.O., Melvin R.G., Simpson S.J. Starvation resistance is positively correlated with body lipid proportion in five wild caught Drosophila simulans populations. J. Insect Physiol. 2008;54:1371–1376. doi: 10.1016/j.jinsphys.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass T.M., Grandison R.C., Wong R., Martinez P., Partridge L., Piper M.D.W. Optimization of dietary restriction protocols in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T.K., Steinbaugh M.J., Hourihan J.M., Ewald C.Y., Isik M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015;88(Pt B):290–301. doi: 10.1016/j.freeradbiomed.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouis M. Targeted increase in shaggy activity levels blocks wingless signaling. Genesis. 2002;34:99–102. doi: 10.1002/gene.10114. [DOI] [PubMed] [Google Scholar]
- Cabreiro F., Au C., Leung K.-Y., Vergara-Irigaray N., Cochemé H.M., Noori T., Weinkove D., Schuster E., Greene N.D.E., Gems D. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153:228–239. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E.J. Hormesis: Toxicological foundations and role in aging research. Exp. Gerontol. 2013;48:99–102. doi: 10.1016/j.exger.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Mattson M.P. Hormesis provides a generalized quantitative estimate of biological plasticity. J. Cell Commun. Signal. 2011;5:25–38. doi: 10.1007/s12079-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V., Cornelius C., Cuzzocrea S., Iavicoli I., Rizzarelli E., Calabrese E.J. Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity. Mol. Aspects Med. 2011;32:279–304. doi: 10.1016/j.mam.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Iavicoli I., Calabrese V. Hormesis: its impact on medicine and health. Hum. Exp. Toxicol. 2013;32:120–152. doi: 10.1177/0960327112455069. [DOI] [PubMed] [Google Scholar]
- Castillo-Quan J.I., Kinghorn K.J., Bjedov I. Genetics and pharmacology of longevity: the road to therapeutics for healthy aging. Adv. Genet. 2015;90:1–101. doi: 10.1016/bs.adgen.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Chiu C.-T., Chuang D.-M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol. Ther. 2010;128:281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP. Free Radic. Biol. Med. 2015;88(Pt B):147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- de Cabo R., Carmona-Gutierrez D., Bernier M., Hall M.N., Madeo F. The search for antiaging interventions: from elixirs to fasting regimens. Cell. 2014;157:1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokucu M.E., Yu L., Taghert P.H. Lithium- and valproate-induced alterations in circadian locomotor behavior in Drosophila. Neuropsychopharmacology. 2005;30:2216–2224. doi: 10.1038/sj.npp.1300764. [DOI] [PubMed] [Google Scholar]
- Douglas P.M., Baird N.A., Simic M.S., Uhlein S., McCormick M.A., Wolff S.C., Kennedy B.K., Dillin A. Heterotypic signals from neural HSF-1 separate thermotolerance from longevity. Cell Rep. 2015;12:1196–1204. doi: 10.1016/j.celrep.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Finkelman H., Martinez A. GSK-3 inhibitors: preclinical and clinical focus on CNS. Front. Mol. Neurosci. 2011;4:32. doi: 10.3389/fnmol.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E.S., Lithgow G.J. Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(Suppl 1):S10–S16. doi: 10.1093/gerona/glu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald C.Y., Landis J.N., Porter Abate J., Murphy C.T., Blackwell T.K. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature. 2015;519:97–101. doi: 10.1038/nature14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Partridge L. Promoting health and longevity through diet: from model organisms to humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gems D., Pletcher S., Partridge L. Interpreting interactions between treatments that slow aging. Aging Cell. 2002;1:1–9. doi: 10.1046/j.1474-9728.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Hayes J.D., Chowdhry S., Dinkova-Kostova A.T., Sutherland C. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochem. Soc. Trans. 2015;43:611–620. doi: 10.1042/BST20150011. [DOI] [PubMed] [Google Scholar]
- Hoeflich K.P., Luo J., Rubie E.A., Tsao M.S., Jin O., Woodgett J.R. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Hoffmann J.M., Partridge L. Nuclear hormone receptors: roles of xenobiotic detoxification and sterol homeostasis in healthy aging. Crit. Rev. Biochem. Mol. Biol. 2015;50:380–392. doi: 10.3109/10409238.2015.1067186. [DOI] [PubMed] [Google Scholar]
- Ingram D.K., Roth G.S. Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res. Rev. 2015;20:46–62. doi: 10.1016/j.arr.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Desai C., Darby T.M., Luo L., Wolfarth A.A., Scharer C.D., Ardita C.S., Reedy A.R., Keebaugh E.S., Neish A.S. Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep. 2015;12:1217–1225. doi: 10.1016/j.celrep.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope R.S. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 2003;24:441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- Karim M.R., Taniguchi H., Kobayashi A. Constitutive activation of Drosophila CncC transcription factor reduces lipid formation in the fat body. Biochem. Biophys. Res. Commun. 2015;463:693–698. doi: 10.1016/j.bbrc.2015.05.126. [DOI] [PubMed] [Google Scholar]
- King-Jones K., Horner M.A., Lam G., Thummel C.S. The DHR96 nuclear receptor regulates xenobiotic responses in Drosophila. Cell Metab. 2006;4:37–48. doi: 10.1016/j.cmet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Lai J.S., Zhao C., Warsh J.J., Li P.P. Cytoprotection by lithium and valproate varies between cell types and cellular stresses. Eur. J. Pharmacol. 2006;539:18–26. doi: 10.1016/j.ejphar.2006.03.076. [DOI] [PubMed] [Google Scholar]
- Lamming D.W., Ye L., Sabatini D.M., Baur J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Invest. 2013;123:980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.B., Kim S., Lee J., Park J., Lee G., Kim Y., Kim J.-M., Chung J. ATG1, an autophagy regulator, inhibits cell growth by negatively regulating S6 kinase. EMBO Rep. 2007;8:360–365. doi: 10.1038/sj.embor.7400917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.-M., Lin S.-Z., Chang N.-C. Antiarrhythmic effect of lithium in rats after myocardial infarction by activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014;77:71–81. doi: 10.1016/j.freeradbiomed.2014.08.022. [DOI] [PubMed] [Google Scholar]
- Longo V.D., Antebi A., Bartke A., Barzilai N., Brown-Borg H.M., Caruso C., Curiel T.J., de Cabo R., Franceschi C., Gems D. Interventions to slow aging in humans: are we ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F., Pietrocola F., Eisenberg T., Kroemer G. Caloric restriction mimetics: towards a molecular definition. Nat. Rev. Drug Discov. 2014;13:727–740. doi: 10.1038/nrd4391. [DOI] [PubMed] [Google Scholar]
- Maher J., Yamamoto M. The rise of antioxidant signaling--the evolution and hormetic actions of Nrf2. Toxicol. Appl. Pharmacol. 2010;244:4–15. doi: 10.1016/j.taap.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Tanious M. Optimal frequency of lithium administration in the treatment of bipolar disorder: clinical and dosing considerations. CNS Drugs. 2011;25:289–298. doi: 10.2165/11586970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M., Gomes A.P., Ward T.M., Minor R.K., Blouin M.-J. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Gil C., Perez D.I. Glycogen synthase kinase 3 inhibitors in the next horizon for Alzheimer’s disease treatment. Int. J. Alzheimers Dis. 2011;2011:280502. doi: 10.4061/2011/280502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsagas K., Lim D.B., Horwitz M., Rizza C.L., Mueller L.D., Villeponteau B., Rose M.R. Long-term functional side-effects of stimulants and sedatives in Drosophila melanogaster. PLoS ONE. 2009;4:e6578. doi: 10.1371/journal.pone.0006578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P. Hormesis defined. Ageing Res. Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G., Killilea D.W., Hubbard A.E., Vantipalli M.C., Melov S., Lithgow G.J. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J. Biol. Chem. 2008;283:350–357. doi: 10.1074/jbc.M705028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee J.J., Schuster E., Blanc E., Piper M.D., Thomas J.H., Patel D.S., Selman C., Withers D.J., Thornton J.M., Partridge L., Gems D. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8:R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Flajolet M., Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Minois N. Longevity and aging: beneficial effects of exposure to mild stress. Biogerontology. 2000;1:15–29. doi: 10.1023/a:1010085823990. [DOI] [PubMed] [Google Scholar]
- Misra J.R., Horner M.A., Lam G., Thummel C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011;25:1796–1806. doi: 10.1101/gad.17280911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S., Lynn D.A., Lo J.Y., Paek J., Curran S.P. SKN-1 and Nrf2 couples proline catabolism with lipid metabolism during nutrient deprivation. Nat. Commun. 2014;5:5048. doi: 10.1038/ncomms6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel C.J., Klein P.S. Molecular targets of lithium action. Annu. Rev. Pharmacol. Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- Pitoniak A., Bohmann D. Mechanisms and functions of Nrf2 signaling in Drosophila. Free Radic. Biol. Med. 2015;88(Pt B):302–313. doi: 10.1016/j.freeradbiomed.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P., Rojo A.I., Evrard-Todeschi N., Innamorato N.G., Cotte A., Jaworski T., Tobón-Velasco J.C., Devijver H., García-Mayoral M.F., Van Leuven F. Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol. Cell. Biol. 2012;32:3486–3499. doi: 10.1128/MCB.00180-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattan S.I.S. Hormesis in aging. Ageing Res. Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Riera C.E., Dillin A. Can aging be ‘drugged’? Nat. Med. 2015;21:1400–1405. doi: 10.1038/nm.4005. [DOI] [PubMed] [Google Scholar]
- Rizak J., Tan H., Zhu H., Wang J.-F. Chronic treatment with the mood-stabilizing drug lithium up-regulates nuclear factor E2-related factor 2 in rat pheochromocytoma PC12 cells in vitro. Neuroscience. 2014;256:223–229. doi: 10.1016/j.neuroscience.2013.10.036. [DOI] [PubMed] [Google Scholar]
- Rojo A.I., Rada P., Egea J., Rosa A.O., López M.G., Cuadrado A. Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol. Cell. Neurosci. 2008;39:125–132. doi: 10.1016/j.mcn.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- Salih D.A.M., Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008;20:126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Floto R.A., Berger Z., Imarisio S., Cordenier A., Pasco M., Cook L.J., Rubinsztein D.C. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 2005;170:1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer M., Goodenough S., Moosmann B., Behl C. Inhibition of glycogen synthase kinase 3 beta is involved in the resistance to oxidative stress in neuronal HT22 cells. Brain Res. 2004;1005:84–89. doi: 10.1016/j.brainres.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Skorupa D.A., Dervisefendic A., Zwiener J., Pletcher S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C., Alic N., Foley A., Cabecinha M., Hoddinott M.P., Partridge L. The Ras-Erk-ETS-signaling pathway is a drug target for longevity. Cell. 2015;162:72–83. doi: 10.1016/j.cell.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofola-Adesakin O., Castillo-Quan J.I., Rallis C., Tain L.S., Bjedov I., Rogers I., Li L., Martinez P., Khericha M., Cabecinha M. Lithium suppresses Aβ pathology by inhibiting translation in an adult Drosophila model of Alzheimer’s disease. Front. Aging Neurosci. 2014;6:190. doi: 10.3389/fnagi.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh M.J., Sun L.Y., Bartke A., Miller R.A. Activation of genes involved in xenobiotic metabolism is a shared signature of mouse models with extended lifespan. Am. J. Physiol. Endocrinol. Metab. 2012;303:E488–E495. doi: 10.1152/ajpendo.00110.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh M.J., Narasimhan S.D., Robida-Stubbs S., Moronetti Mazzeo L.E., Dreyfuss J.M., Hourihan J.M., Raghavan P., Operaña T.N., Esmaillie R., Blackwell T.K. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife. 2015;4 doi: 10.7554/eLife.07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganthi M., Sangeetha G., Gayathri G., Ravi Sankar B. Biphasic dose-dependent effect of lithium chloride on survival of human hormone-dependent breast cancer cells (MCF-7) Biol. Trace Elem. Res. 2012;150:477–486. doi: 10.1007/s12011-012-9510-x. [DOI] [PubMed] [Google Scholar]
- Sykiotis G.P., Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell. 2008;14:76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G.P., Bohmann D. Stress-activated cap’n’collar transcription factors in aging and human disease. Sci. Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G.P., Habeos I.G., Samuelson A.V., Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi-Yanaga F. Activator or inhibitor? GSK-3 as a new drug target. Biochem. Pharmacol. 2013;86:191–199. doi: 10.1016/j.bcp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Tam Z.Y., Gruber J., Ng L.F., Halliwell B., Gunawan R. Effects of lithium on age-related decline in mitochondrial turnover and function in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:810–820. doi: 10.1093/gerona/glt210. [DOI] [PubMed] [Google Scholar]
- Taylor R.C., Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153:1435–1447. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet J.M.A. DAF-16 target identification in C. elegans: past, present and future. Biogerontology. 2015;16:221–234. doi: 10.1007/s10522-014-9527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet J.M.A., Hertweck M., An J.H., Baker J., Hwang J.Y., Liu S., Oliveira R.P., Baumeister R., Blackwell T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulgherait M., Rana A., Rera M., Graniel J., Walker D.W. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8:1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Karpac J., Jasper H. Promoting longevity by maintaining metabolic and proliferative homeostasis. J. Exp. Biol. 2014;217:109–118. doi: 10.1242/jeb.089920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.S., Harwood A.J. Lithium therapy and signal transduction. Trends Pharmacol. Sci. 2000;21:61–64. doi: 10.1016/s0165-6147(99)01428-5. [DOI] [PubMed] [Google Scholar]
- Zarse K., Terao T., Tian J., Iwata N., Ishii N., Ristow M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur. J. Nutr. 2011;50:387–389. doi: 10.1007/s00394-011-0171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Li Q., Zhang F., Sun X., Cai G., Zhang W., Chen X. Chronic lithium treatment diminishes the female advantage in lifespan in Drosophila melanogaster. Clin. Exp. Pharmacol. Physiol. 2015;42:617–621. doi: 10.1111/1440-1681.12393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.