Abstract
Objectives
During recent decades, there has been limited attention on the seasonal pattern of pertussis within a high vaccine coverage population. This study aimed to compare the seasonal patterns of clinical suspected pertussis cases with those of laboratory confirmed cases in Iran.
Methods
The current study was conducted using time series methods. Time variables included months and seasons during 2011–2013. The effects of seasons and months on the incidence of pertussis were estimated using analysis of variance or Kruskal–Wallis.
Results
The maximum average incidence of clinically confirmed pertussis was 23.3 in July (p = 0.04), but the maximum incidence of clinical suspected pertussis was 115.7 in May (p = 0.6). The maximum seasonal incidences of confirmed and clinical pertussis cases were reported in summer (average: 12, p = 0.004), and winter (average: 108.1; p = 0.4), respectively.
Conclusion
The present study showed that the seasonal pattern of laboratory confirmed pertussis cases is highly definite and different from the pattern of clinical suspected cases.
Keywords: epidemiologic, pertussis, seasonal, time series
1. Introduction
Pertussis is an acute communicable respiratory infection and a vaccine preventable disease which is one of the main public health concerns. This infection is caused by Bordetella pertussis and causes chronic cough in children, adults, and older people 1, 2, 3. Pertussis is a common infection in both children and adults, and is transmitted through respiratory droplets [2]. Parents and other family members are the main sources of infection for neonates. Newborns may also be accidentally infected by asymptomatic adults [4].
Pertussis is a mild to moderate disease among teenagers and adolescents, with rare serious complications, although, these age groups are considered as the main reservoirs within the community and the major factor of transmission particularly to the infants [5]. By contrast, the degree of severity, rate of hospitalization, pneumonia, apnea, epilepsy, encephalopathy, and death are more common among neonates in comparison with the other age groups. This infection is diagnosed by microbial culture or polymerase chain reaction (PCR) of nasopharyngeal swab samples, as well as serum anti-Bordetella antibody titration. Early detection and treatment during primary stages can shorten the clinical and transmission phase of the disease [6].
At the beginning of the 20th century and before the widespread implementation of vaccination programs, pertussis epidemics occurred every 2–3 years [7]. After introducing vaccines in 1950, remarkable reductions were observed in the incidence of the disease. In countries with high vaccination coverage, an outbreak of pertussis has occurred every 3–4 years [8]. Iran is a country with high vaccine coverage. The pertussis vaccination during the last decades has been increased so that the coverage for 1991, 1992, and 2010 was reported as of 87%, 95%, and 99%, respectively [6].
Seasonality is a general phenomenon among infectious diseases, but its mechanism has not been completely detected [9]. The main focuses in the seasonal pattern of respiratory pathogens are survival of the pathogen outside the host, host behavior, and its degree of immunization [10].
Many studies investigating the seasonal patterns of infectious diseases have reported that the incidences of most respiratory infections are changing seasonally. As a matter of fact, season may play a critical role in the mechanism of transmission and survival of the infectious agent by providing an optimal environment and host 11, 12, 13. The seasonal pattern of pertussis has been reported in a study by Greeff et al [7] during a low vaccine coverage period of time. The maximum incidence of pertussis was observed in the USA in August [11]. That was the case for all age groups except for people aged 13–18 in August in the Netherlands [7].
Understanding the seasonal pattern of pertussis can help us to predict future health problems, plan effective public health programs, determine goals and strategies, and finally, use the available resources more effectively [12]. In addition, seasonality signals the time of disease rising, and thus leads to an appropriate decision being made for the future 14, 15.
The above mentioned facts indicate that only limited investigations have been performed about the seasonal pattern of pertussis within a high vaccine coverage population, while study of the seasonal pattern of pertussis can reveal important modes of disease transmission and introduce a viewpoint for potential effects of vaccination strategies in the future. According to the role of the detection of seasonality in the awareness of the dynamics of infectious diseases, and particularly promotion in the management of a pertussis surveillance system, this study aims to compare the seasonal patterns of the laboratory confirmed and clinical suspected cases of pertussis in Iran.
2. Materials and methods
In this longitudinal time series approached study, we used pertussis surveillance system data provided from the Iranian Center for Diseases Control. Based on ethical considerations, names of all cases were deleted from the dataset. These data included all notified pertussis cases, laboratory confirmed cases, and clinically diagnosed cases. Nasopharyngeal swabs were taken from all notified pertussis cases and transferred to the national reference laboratory (Pasteur Institute) during 72 hours using the Bordetella-specific (Bordet-Gengou) transport environment to be confirmed by laboratory-based diagnostic methods. Isolation of samples was done using the gold standard method of microbial culture or PCR. Each patient with related clinical signs [a cough illness of ≥2 weeks duration and one of the following classical symptoms: (1) paroxysmal coughing; (2) inspiratory whooping; or (3) posttussive vomiting] and symptoms without laboratory confirmation was considered as a clinical suspected case 2, 16. A confirmed case was introduced as each clinical case whose diagnosis been laboratory confirmed [2].
This study was conducted from 2011 to 2013 including 36 monthly time points. A time variable was defined as each of the months and seasons of 2011–2013. To measure the cumulative occurrence of monthly cases, daily incident cases in each month were summed. Moreover, seasonal patterns of the total notified cases, confirmed cases, and clinical cases of different months were compared according to different age groups (<2 months, 2–11 months, 1–5 years, 6–10 years, >10 years). Seasons were defined as spring (April–June), summer (July–September), autumn (October–December), and winter (January–March).
We received all required data from the Iranian Ministry of Health and Medical Education within Excel spreadsheets and transferred them into SPSS 20 (SPSS Inc., Chicago IL) software after refining. The effects of season and month on the occurrence of notified, confirmed, and clinical cases were estimated using analysis of variance (when normality assumption was met) or Kruskal–Wallis (when normality assumption did not meet) tests. When these tests were statistically significant, LSD post hoc test was used to compare the results between different subgroups. A p value <0.05 was considered statistically significant.
3. Results
During 2011–2013, 3,629 suspected pertussis cases were reported; of these, 239 (6.6%) were laboratory confirmed and 3,390 (934%) were considered as cases with clinical diagnosis.
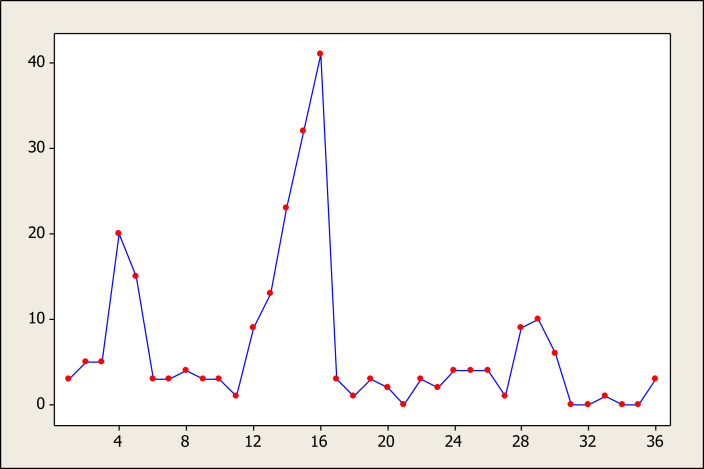
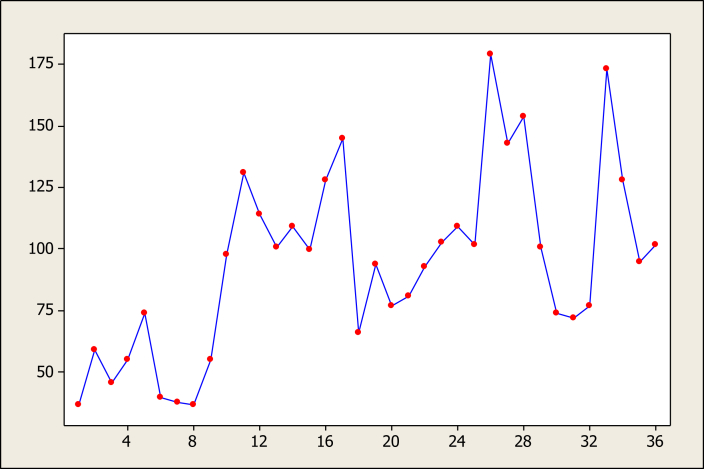
Figure 1, Figure 2 illustrate the time series data regarding laboratory confirmed and clinical suspected cases during the study period. These graphs show that laboratory confirmed data, in contrast to clinical suspected data, had regular patterns.
Figure 1.
Time series plot of raw data of confirmed cases.
Figure 2.
Time series plot of raw data of suspected cases.
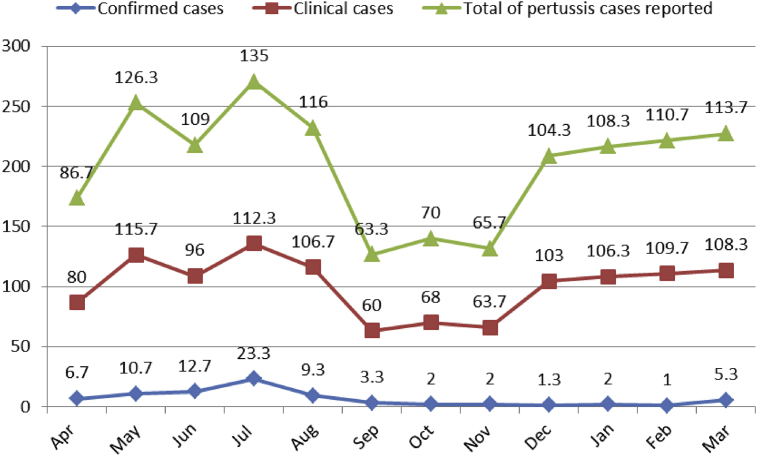
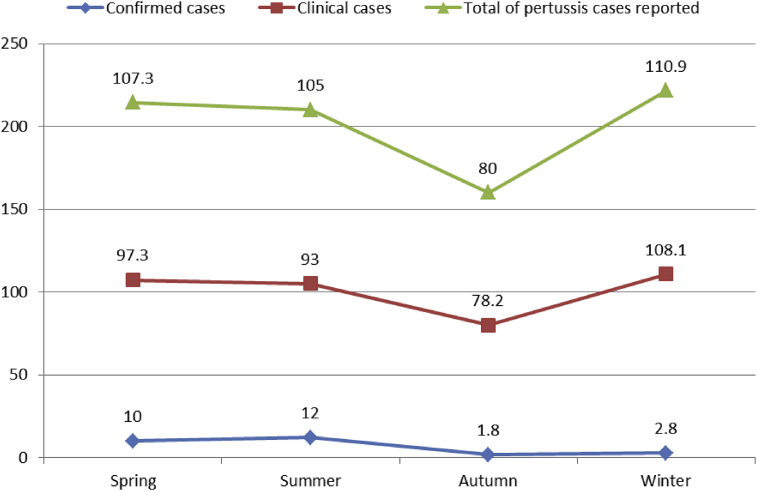
The average number of pertussis cases/month (total cases) differed from at least 63.3 (September) to at most 135 (July) (Figure 3). No significant differences were found between different months with regard to the total notified cases (p = 0.4). The minimum number of total cases were reported in autumn (mean = 80) and the maximum number of reported cases were in winter (mean = 110.9), without any significant difference between seasons (p = 0.3) (Figure 4).
Figure 3.
Monthly means of pertussis cases reported in Iran.
Figure 4.
Seasonal pattern of pertussis cases reported in Iran, 2011–2013.
The minimum number of cases of confirmed pertussis were reported in February (mean = 1/month) and most cases were reported in July (mean = 23.3) (Figure 3). The observed differences were statistically significant (p = 0.04). Figure 4 illustrates that the average seasonal distributions of confirmed cases varied between 1.8 and 12 cases for autumn and summer, respectively (p = 0.004).
Figure 3 shows that the minimum and maximum average incidences of clinical pertussis were 60 cases in September and 115.7 cases in May, respectively (p = 0.6). Most clinical cases of pertussis were notified in winter (108.1 cases). No statistically significant difference was observed (p = 0.4) between the average cases reported in winter and other seasons (Figure 4).
Table 1 shows that the highest seasonal incidences of clinical pertussis were observed among children aged <2 months (in average 32.9 cases) and 2–11 months (in average 41.8 cases) in winter, and among children aged 1–5 years (in average 13.7 cases), 6–10 years (in average 11.7 cases), and >10 years (in average 18.9 cases) in the summer. None of the differences were statistically significant (p > 0.05). In spring, children <2 months and >10 years had the most incidences of confirmed pertussis (in average 4.4 cases and 1.4 cases, respectively). The corresponding figures for summer were 4.3 cases (2–11 months), 1.57 cases (1–5 years), and one case (6–10 years). The seasonal differences for children aged 2–11 months (p = 0.04) and 6–10 years (p = 0.03) were statistically significant. According to a post hoc test, the average numbers of incident cases among children aged 2–11 years differed between autumn and summer (p = 0.009) and also differed between summer and winter (p = 0.045). In addition, statistically significant differences were observed between 6–10-year-olds incident cases in summer and autumn (p = 0.02) as well as summer and winter (p = 0.02).
Table 1.
Seasonal differences in incidence of pertussis cases reported by age group in Iran.
| Variable |
Spring |
Summer |
Autumn |
Winter |
ANOVA/Kruskal–Wallis |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | ||
| Total of pertussis cases reported | <2 mo | 28 | 17.4 | 24.1 | 13.7 | 18.1 | 15.4 | 34.1 | 10.4 | 0.142 |
| 2–11 mo | 41.6 | 24.3 | 33.3 | 19.3 | 30.3 | 21.1 | 43.1 | 5.3 | 0.420 | |
| 1–5 y | 11.1 | 3.3 | 15.2 | 6.7 | 13.1 | 5.2 | 12.4 | 3.04 | 0.345 | |
| 6–10 y | 9.8 | 6.6 | 12.7 | 8.05 | 7.6 | 2.9 | 8.7 | 2.4 | 0.257 | |
| >10 y | 16.9 | 12.5 | 19.7 | 11.8 | 10.9 | 6.2 | 12.6 | 5.4 | 0.212 | |
| Laboratory confirmed cases | <2 mo | 4.4 | 5.1 | 4.3 | 6.02 | 1 | 0.87 | 1.22 | 1.3 | 0.134 |
| 2–11 mo | 2.9 | 2.9 | 4.3 | 5.2 | 0.33 | 0.5 | 1.33 | 1.3 | 0.046 | |
| 1–5 y | 0.44 | 0.72 | 1.57 | 2.2 | 0.22 | 0.44 | 0.11 | 1.23 | 0.55 | |
| 6–10 y | 0.78 | 1.09 | 1 | 1.3 | 0 | – | 0 | – | 0.032 | |
| >10 y | 1.4 | 1.9 | 0.8 | 1.6 | 0.22 | 0.4 | 0.1 | 0.3 | 0.125 | |
| Clinically suspected cases | <2 mo | 23.6 | 16.9 | 19.8 | 11.7 | 17.1 | 15.6 | 32.9 | 10.9 | 0.111 |
| 2–11 mo | 38.7 | 24.7 | 29 | 16.9 | 30 | 21.3 | 41.8 | 5.02 | 0.384 | |
| 1–5 y | 10.7 | 3.2 | 13.7 | 5.4 | 12.9 | 5.3 | 12.3 | 3.1 | 0.533 | |
| 6–10 y | 9 | 5.6 | 11.7 | 7.8 | 7.6 | 2.9 | 8.7 | 2.4 | 0.397 | |
| >10 y | 15.4 | 11.2 | 18.9 | 11.6 | 10.7 | 6.2 | 12.4 | 5.2 | 0.250 | |
ANOVA = analysis of variance; SD = standard deviation.
4. Discussion
A study of the seasonal pattern of pertussis cases in Iran showed that most cases of clinical pertussis were reported in winter and spring seasons, as well as May and July months. The minimum number of cases were notified in autumn and September. These seasonal and monthly differences were not statistically significant, but the seasonal differences observed between the confirmed cases in summer and spring (maximum incidence) and autumn (minimum incidence) were statistically significant. That was the case for monthly differences between July (maximum) and February and December (minimum). Moreover, trends of confirmed cases and clinical cases differed with regard to age groups and the peak incidence of confirmed pertussis cases was spring and summer among children < 2 months. The differences between the optimum and the lowest seasonal means of confirmed incident cases were much more than those of clinical cases.
In an epidemiological study investigating the 125 years trend of pertussis in Italy, the incident rate of death decreased from 42.5/100,000 populations in 1890 to zero in 2002 and no cases have been reported until now. Moreover, the incidence of pertussis disease decreased from 86.3/100,000 in 1927 to 1/100,000 in 2008. The optimum annual periodicity in the occurrence of pertussis in Italy was between March and August (spring/summer), while the lowest incidence rate was reported between September and February (autumn/winter), which is similar to the seasonal patterns observed in the current study [17]. A study in Australia indicated that pertussis remains a seasonal illness, with annual peaks in the summer months [18].
In another study about the epidemiological aspects of pertussis among adults in South Korea, of 490 registered patients, 34 (6.9%) were PCR or culture positive. In that study, the peak incidences of confirmed cases occurred in February (15.8%) and August (15.9%), while the peak incidence of nonconfirmed cases was between March and June, which is in contrast to our results [16].
An investigation of the seasonal pattern of pertussis and its periodic trend in the Netherlands showed that the monthly incidence of notified cases was slightly increased from January 1996 to June 2006. The peak incidence was reported in August for all age groups, except for the 13–18 years age group, whose peak incidence was in November which is not in agreement with the observed pattern of the current study. In addition, no evidence of association between the increased incidence and the reopening of schools was found [7]. In the USA, Shah et al [11] found that the peak incidence of confirmed pertussis was in August. Most of the studies introduced the second month of summer as the time for peak incidence of confirmed cases, while this time in our study was the first month of summer. Although the month of the peak incidence in Iran differed from that of similar studies, the seasonal patterns of most of the above mentioned studies were in keeping with that observed in our study.
It has been reported that the reopening of schools and consequently crowding of susceptible students, is one of the main factors of the seasonal spread of measles. This factor may play a similar function in the seasonal increase in the incidence of pertussis [7]. However, no association was found between the seasonal peak of disease incidence and reopening of schools in the current and other similar studies. It should also be noted that the high coverage of vaccination against pertussis may increase the interepidemic interval by reducing the effective reproduction number [7]. In addition, the fluctuations in the incidence of pertussis among adults and neonates can indicate the transmissions within age groups.
There was a prominent seasonal pattern of confirmed pertussis cases, especially among children < 2 months – with no experience of vaccination – and the 2–11 months age group who received at most three doses of pertussis vaccine. There are several explanations for these reasons. First, newborns do not have any immunity against pertussis and three doses of vaccine cannot provide effective immunity during the 1st year of life and the incidence of the disease is higher in this age group compare to the others. Moreover, it may be due to different clinical signs and symptoms in neonates, adolescents, and adults, i.e., neonates have typical symptoms leading to better diagnosis in these groups. By contrast, chronic coughs in adults may be ignored leading the diagnosis being missed. In a study assessing the clinical and epidemiological aspects of pertussis among 1,047 patients, 102 (9.49%) was diagnosed using the PCR method. Of these, 94.2% of admitted patients and 42.8% of outpatients were < 6 months. The outcome was poor in 23.1% of children who did not experience vaccination (case fatality rate was 4.9%). Those results indicated that pertussis affects mainly children < 6 months, those received no vaccine, or less than three doses [2].
Low immunity against pertussis is another explanation of the high incidence of disease among neonates and children aged < 1 year. Plans et al [19] showed that antipertussis antibody is undetectable among 27% of Spanish newborns due to very low levels of maternal antibodies. They also reported that 1.8% of laborers had evidence of recent pertussis infection. These results are consistent with the current results which indicated high incidence of pertussis among newborns.
In the study conducted by Bellettini et al [20], of 222 investigated patients, 72.5% had developed confirmed pertussis, 60.9% of them aged < 1 month. Cyanosis was observed to be an independent predictor of pertussis (OR = 8; 95% CI = 1.8–36.3) among children < 6 months. This specific symptom in neonates caused more attention of physicians on the diagnosis of pertussis, leading to a greater case detection rate, while the symptoms are not specific among adults [20]. Eidlitz-Markus et al [21] studied 60 pertussis patients aged 7–18 years old and 20 infants < 6 months, and observed no differences between the two age groups during day and night with regard to cough and vomiting. Older children had been diagnosed later than the others. In addition, whooping cough, redness and flushing during cough, cyanosis, leukocytosis, lymphocytosis, and abnormal chest X-ray were seen in higher rates in neonates than in older children. These specific characteristics helped earlier diagnosis among newborns, explaining some differences in the incidence of disease among different age groups [21]. Little attention of physicians to the pertussis during chronic coughs in adults is another reason of underreporting pertussis among this age group and caused low detection of their seasonal pattern. Results of a serosurvey demonstrated that 20–30% of adults with chronic cough for > 2 weeks may develop pertussis [22].
Using antibiotics for some patients before sample collection, delay in sample transportation, and probable use of expired transport mediums are some limitations of our study which potentially cause false negative results. Although these limitations are due to random errors and may be similar in both confirmed and clinical groups, the power of the statistical tests for comparison of cases among seasons, months and age groups has been decreased because of reduction in laboratory confirmed cases.
In conclusion, our study provided clear evidence that the incidence of confirmed pertussis follows a definite seasonal pattern, different from the pattern of clinical pertussis. Moreover, seasonal incidence of confirmed cases is prominent in accordance with age. Therefore, it is highly advised that during the seasons with more cases, all parts of the surveillance system should be designed appropriate programs for active case finding and early treatment of cases in order to rapidly control the reservoirs of infection, as well as to limit the severity and complications within community, especially among newborns.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
M.M. contributed to the analysis, interpretation of data, and writing of this manuscript, G.R.G. and F.D. contributed to the acquisition of data, G.R.G. contributed to the design of the present manuscript, S.M.Z. contributed to the final approval of the version to be published, and M.A. contributed to the drafting of the manuscript. This research was supported by grant number 1497 from Mazandaran University of Medical Sciences of Iran.
References
- 1.Rumbo M., Hozbor D. Development of improved pertussis vaccine. Hum Vaccin Immunother. 2014 Jun;10(8):2450–2453. doi: 10.4161/hv.29253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusznierz G., Schmeling F., Cociglio R. Epidemiologic and clinical characteristics of children with disease due to Bordetella pertussis in Santa Fe, Argentina. Rev Chilena Infectol. 2014 Aug;31(4):385–392. doi: 10.4067/S0716-10182014000400002. [DOI] [PubMed] [Google Scholar]
- 3.Ochoa-Perez U.R., Hernández-Sierra J.F., Escalante-Padrón F.J. Epidemiology of Bordetella pertussis in San Luis Potosí, Mexico. Pediatr Infect Dis J. 2014 May;33(5):540–542. doi: 10.1097/INF.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 4.Turkoglu E., Sonmez C., Kurugol Z. Pertussis serosurveillance study in Izmir, Turkey. J Trop Pediatr. 2015 Feb;61(1):32–36. doi: 10.1093/tropej/fmu062. [DOI] [PubMed] [Google Scholar]
- 5.Wendelboe A.M., Njamkepo E., Bourillon A. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007 Apr;26(4):293–299. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 6.Shahcheraghi F., Nakhost Lotfi M., Parzadeh M. Isolation of Bordetella pertussis and Bordetella parapertussis from clinical specimens at different provinces. J Mazandaran Univ Med Sci. 2012 May;22(88):2–8. [Google Scholar]
- 7.De Greeff S.C., Dekkers A.L., Teunis P. Seasonal patterns in time series of pertussis. Epidemiol Infect. 2009 Oct;137(10):1388–1395. doi: 10.1017/S0950268809002489. [DOI] [PubMed] [Google Scholar]
- 8.Broutin H., Guegan J.F., Elguero E. Large-scale comparative analysis of pertussis population dynamics: periodicity, synchrony, and impact of vaccination. Am J Epidemiol. 2005 Jun;161(12):1159–1167. doi: 10.1093/aje/kwi141. [DOI] [PubMed] [Google Scholar]
- 9.Altizer S., Dobson A., Hosseini P. Seasonality and the dynamics of infectious diseases. Ecol Lett. 2006 Apr;9(4):467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 10.Grassly N.C., Fraser C. Seasonal infectious disease epidemiology. Proc Biol Sci. 2006 Oct;273(1600):2541–2550. doi: 10.1098/rspb.2006.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A.P., Smolensky M.H., Burau K.D. Seasonality of primarily childhood and young adult infectious diseases in the United States. Chronobiol Int. 2006 February;23(5):1065–1082. doi: 10.1080/07420520600920718. [DOI] [PubMed] [Google Scholar]
- 12.Moosazadeh M., Nasehi M., Bahrampour A. Forecasting tuberculosis incidence in Iran using Box–Jenkins models. Iran Red Crescent Med J. 2014 May;16(5):e11779. doi: 10.5812/ircmj.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willis M.D., Winston C.A., Heilig C.M. Seasonality of tuberculosis in the United States, 1993–2008. Clin Infect Dis. 2012 Jun;54(11):1553–1560. doi: 10.1093/cid/cis235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moosazadeh M., Khanjani N., Bahrampour A. Does tuberculosis have a seasonal pattern among migrant population entering Iran? Int J Health Policy Manag. 2014 May;2(4):181–185. doi: 10.15171/ijhpm.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moosazadeh M., Khanjani N., Bahrampoor A. Seasonality and temporal variations of tuberculosis in the North of Iran. Tanaffos. 2013 December;12(4):35–41. [PMC free article] [PubMed] [Google Scholar]
- 16.Park S., Lee S.H., Seo K.H. Epidemiological aspects of pertussis among adults and adolescents in a Korean outpatient setting: a multicenter, PCR-based study. J Korean Med Sci. 2014 Sep;29(9):1232–1239. doi: 10.3346/jkms.2014.29.9.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonfiantini M.V., Carloni E., Gesualdo F. Epidemiology of pertussis in Italy: disease trends over the last century. Euro Surveill. 2014 Oct;19(40):20921. doi: 10.2807/1560-7917.es2014.19.40.20921. [DOI] [PubMed] [Google Scholar]
- 18.Kaczmarek M.C., Ware R.S., Nimmo G.R., Robson J.M.B., Lambert S.B. Pertussis seasonality evident in polymerase chain reaction and serological testing data, Queensland, Australia. J Ped Infect Dis. 2015 doi: 10.1093/jpids/piu144. [DOI] [PubMed] [Google Scholar]
- 19.Plans P., Jansà J., Doshi N. Prevalence of pertussis antibodies in umbilical cord blood samples in Catalonia, Spain. Pediatr Infect Dis J. 2008 Nov;27(11):1023–1025. doi: 10.1097/inf.0b013e318179264b. [DOI] [PubMed] [Google Scholar]
- 20.Bellettini C.V., Oliveira A.W., Tusset C. Clinical, laboratorial and radiographic predictors of Bordetella pertussis infection. Rev Paul Pediatr. 2014 Dec;32(4):292–298. doi: 10.1016/j.rpped.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eidlitz-Markus T., Mimouni M., Zeharia A. Pertussis symptoms in adolescents and children versus infants: the influence of vaccination and age. Clin Pediatr (Phila) 2007 Oct;46(8):718–723. doi: 10.1177/0009922807302093. [DOI] [PubMed] [Google Scholar]
- 22.Von König C.H., Halperin S., Riffelmann M. Pertussis of adults and infants. Lancet Infect Dis. 2002 Dec;2(12):744–750. doi: 10.1016/s1473-3099(02)00452-8. [DOI] [PubMed] [Google Scholar]