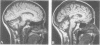Abstract
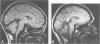
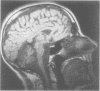
MRI of the brain was performed in 53 patients with a variety of degenerative ataxias and related disorders and 96 control subjects. Atrophy of intracranial structures was not seen in patients with the pure type of hereditary spastic paraplegia, or in early cases of Friedreich's ataxia. In advanced Friedreich's ataxia there was atrophy of the vermis and medulla. The MRI features of early onset cerebellar ataxia with retained reflexes were variable, and suggest heterogeneity. In autosomal dominant cerebellar ataxias, most patients had cerebellar and brainstem atrophy, probably reflecting the pathological process of olivopontocerebellar atrophy; there was no clearly defined group with both clinical and imaging features of isolated cerebellar involvement. The MRI abnormalities in idiopathic late onset cerebellar ataxia were predominantly those of cerebellar and brainstem atrophy or pure cerebellar atrophy. The clinical and imaging features of brainstem abnormalities were discordant in several patients. Pure cerebellar atrophy was associated with slower progression of disability. Cerebral atrophy was common in the late onset ataxias. Cerebral white matter lesions, although usually few in number, were observed in significantly more patients than controls, particularly those aged over 50 years.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awad I. A., Johnson P. C., Spetzler R. F., Hodak J. A. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986 Nov-Dec;17(6):1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- Behan W. M., Maia M. Strümpell's familial spastic paraplegia: genetics and neuropathology. J Neurol Neurosurg Psychiatry. 1974 Jan;37(1):8–20. doi: 10.1136/jnnp.37.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosley T. M., Cohen D. A., Schatz N. J., Zimmerman R. A., Bilaniuk L. T., Savino P. J., Sergott R. S. Comparison of metrizamide computed tomography and magnetic resonance imaging in the evaluation of lesions at the cervicomedullary junction. Neurology. 1985 Apr;35(4):485–492. doi: 10.1212/wnl.35.4.485. [DOI] [PubMed] [Google Scholar]
- Brant-Zawadzki M., Fein G., Van Dyke C., Kiernan R., Davenport L., de Groot J. MR imaging of the aging brain: patchy white-matter lesions and dementia. AJNR Am J Neuroradiol. 1985 Sep-Oct;6(5):675–682. [PMC free article] [PubMed] [Google Scholar]
- Bydder G. M., Steiner R. E., Thomas D. J., Marshall J., Gilderdale D. J., Young I. R. Nuclear magnetic resonance imaging of the posterior fossa: 50 cases. Clin Radiol. 1983 Mar;34(2):173–188. doi: 10.1016/s0009-9260(83)80302-x. [DOI] [PubMed] [Google Scholar]
- Chida K., Tamura M., Kamikura I., Takasu T., Goto N. A quantitative evaluation of pontine volume by computed tomography in patients with cerebellar degeneration. Neurology. 1990 Aug;40(8):1241–1245. doi: 10.1212/wnl.40.8.1241. [DOI] [PubMed] [Google Scholar]
- Claus D., Aschoff J. C. Cranial computerized tomography in spinocerebellar atrophies. Ann N Y Acad Sci. 1981;374:831–838. doi: 10.1111/j.1749-6632.1981.tb30923.x. [DOI] [PubMed] [Google Scholar]
- Flannigan B. D., Bradley W. G., Jr, Mazziotta J. C., Rauschning W., Bentson J. R., Lufkin R. B., Hieshima G. B. Magnetic resonance imaging of the brainstem: normal structure and basic functional anatomy. Radiology. 1985 Feb;154(2):375–383. doi: 10.1148/radiology.154.2.3966125. [DOI] [PubMed] [Google Scholar]
- Gerard G., Weisberg L. A. MRI periventricular lesions in adults. Neurology. 1986 Jul;36(7):998–1001. doi: 10.1212/wnl.36.7.998. [DOI] [PubMed] [Google Scholar]
- Han J. S., Bonstelle C. T., Kaufman B., Benson J. E., Alfidi R. J., Clampitt M., Van Dyke C., Huss R. G. Magnetic resonance imaging in the evaluation of the brainstem. Radiology. 1984 Mar;150(3):705–712. doi: 10.1148/radiology.150.3.6695071. [DOI] [PubMed] [Google Scholar]
- Harding A. E. "Idiopathic" late onset cerebellar ataxia. A clinical and genetic study of 36 cases. J Neurol Sci. 1981 Aug;51(2):259–271. doi: 10.1016/0022-510x(81)90104-0. [DOI] [PubMed] [Google Scholar]
- Harding A. E. Early onset cerebellar ataxia with retained tendon reflexes: a clinical and genetic study of a disorder distinct from Friedreich's ataxia. J Neurol Neurosurg Psychiatry. 1981 Jun;44(6):503–508. doi: 10.1136/jnnp.44.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A. E. Friedreich's ataxia: a clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain. 1981 Sep;104(3):589–620. doi: 10.1093/brain/104.3.589. [DOI] [PubMed] [Google Scholar]
- Harding A. E. Hereditary "pure" spastic paraplegia: a clinical and genetic study of 22 families. J Neurol Neurosurg Psychiatry. 1981 Oct;44(10):871–883. doi: 10.1136/jnnp.44.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A. E. The clinical features and classification of the late onset autosomal dominant cerebellar ataxias. A study of 11 families, including descendants of the 'the Drew family of Walworth'. Brain. 1982 Mar;105(Pt 1):1–28. doi: 10.1093/brain/105.1.1. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick J. B., Hayman L. A. White-matter lesions in MR imaging of clinically healthy brains of elderly subjects: possible pathologic basis. Radiology. 1987 Feb;162(2):509–511. doi: 10.1148/radiology.162.2.3797666. [DOI] [PubMed] [Google Scholar]
- Klockgether T., Petersen D., Grodd W., Dichgans J. Early onset cerebellar ataxia with retained tendon reflexes. Clinical, electrophysiological and MRI observations in comparison with Friedreich's ataxia. Brain. 1991 Aug;114(Pt 4):1559–1573. doi: 10.1093/brain/114.4.1559. [DOI] [PubMed] [Google Scholar]
- Klockgether T., Schroth G., Diener H. C., Dichgans J. Idiopathic cerebellar ataxia of late onset: natural history and MRI morphology. J Neurol Neurosurg Psychiatry. 1990 Apr;53(4):297–305. doi: 10.1136/jnnp.53.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabatame H., Fukuyama H., Akiguchi I., Kameyama M., Nishimura K., Nakano Y. Spinocerebellar degeneration: qualitative and quantitative MR analysis of atrophy. J Comput Assist Tomogr. 1988 Mar-Apr;12(2):298–303. [PubMed] [Google Scholar]
- Oppenheimer D. R. Brain lesions in Friedreich's ataxia. Can J Neurol Sci. 1979 May;6(2):173–176. doi: 10.1017/s0317167100119596. [DOI] [PubMed] [Google Scholar]
- Ormerod I. E., Bronstein A., Rudge P., Johnson G., Macmanus D., Halliday A. M., Barratt H., Du Boulay E. P., Kendal B. E., Moseley I. F. Magnetic resonance imaging in clinically isolated lesions of the brain stem. J Neurol Neurosurg Psychiatry. 1986 Jul;49(7):737–743. doi: 10.1136/jnnp.49.7.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormerod I. E., Miller D. H., McDonald W. I., du Boulay E. P., Rudge P., Kendall B. E., Moseley I. F., Johnson G., Tofts P. S., Halliday A. M. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quantitative study. Brain. 1987 Dec;110(Pt 6):1579–1616. doi: 10.1093/brain/110.6.1579. [DOI] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Quinn N. Multiple system atrophy--the nature of the beast. J Neurol Neurosurg Psychiatry. 1989 Jun;Suppl:78–89. doi: 10.1136/jnnp.52.suppl.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoiardo M., Strada L., Girotti F., Zimmerman R. A., Grisoli M., Testa D., Petrillo R. Olivopontocerebellar atrophy: MR diagnosis and relationship to multisystem atrophy. Radiology. 1990 Mar;174(3 Pt 1):693–696. doi: 10.1148/radiology.174.3.2305051. [DOI] [PubMed] [Google Scholar]
- Scheltens P., Barkhof F., Valk J., Algra P. R., van der Hoop R. G., Nauta J., Wolters E. C. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer's disease. Evidence for heterogeneity. Brain. 1992 Jun;115(Pt 3):735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- Wicks D. A., Tofts P. S., Miller D. H., du Boulay G. H., Feinstein A., Sacares R. P., Harvey I., Brenner R., McDonald W. I. Volume measurement of multiple sclerosis lesions with magnetic resonance images. A preliminary study. Neuroradiology. 1992;34(6):475–479. doi: 10.1007/BF00598953. [DOI] [PubMed] [Google Scholar]