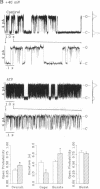
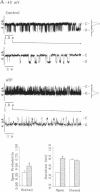
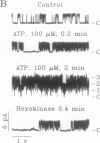
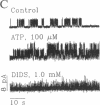
Abstract
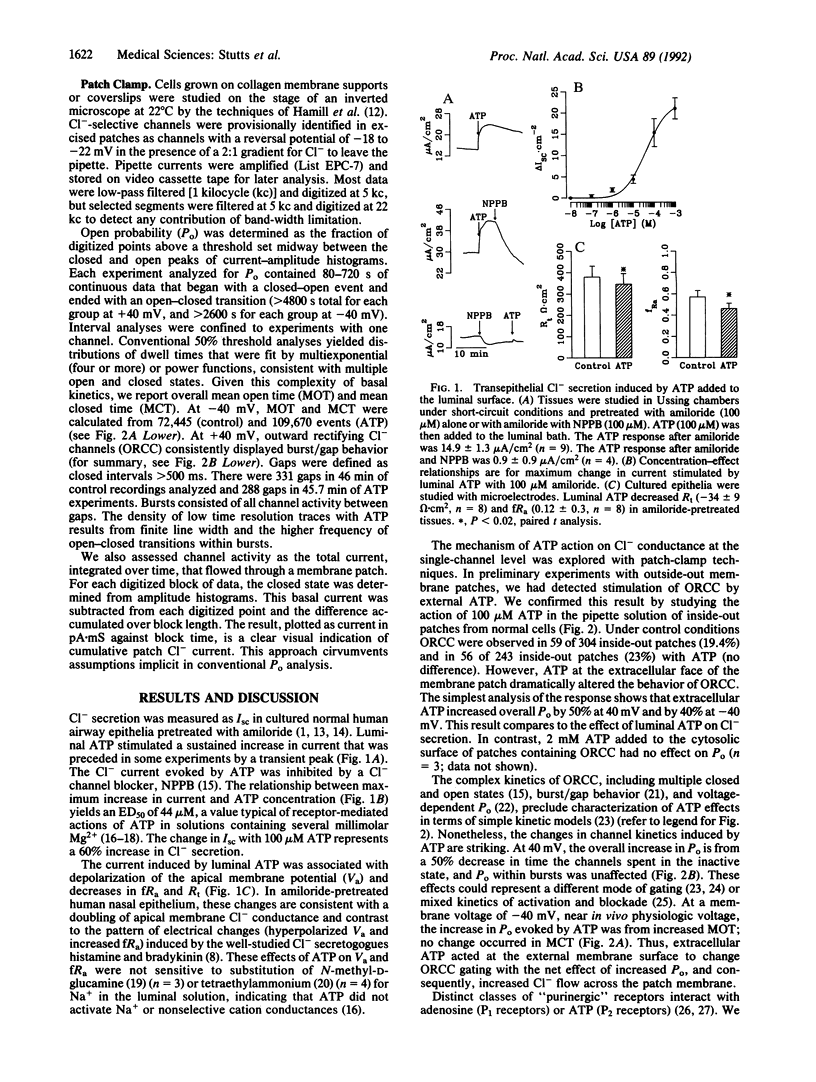
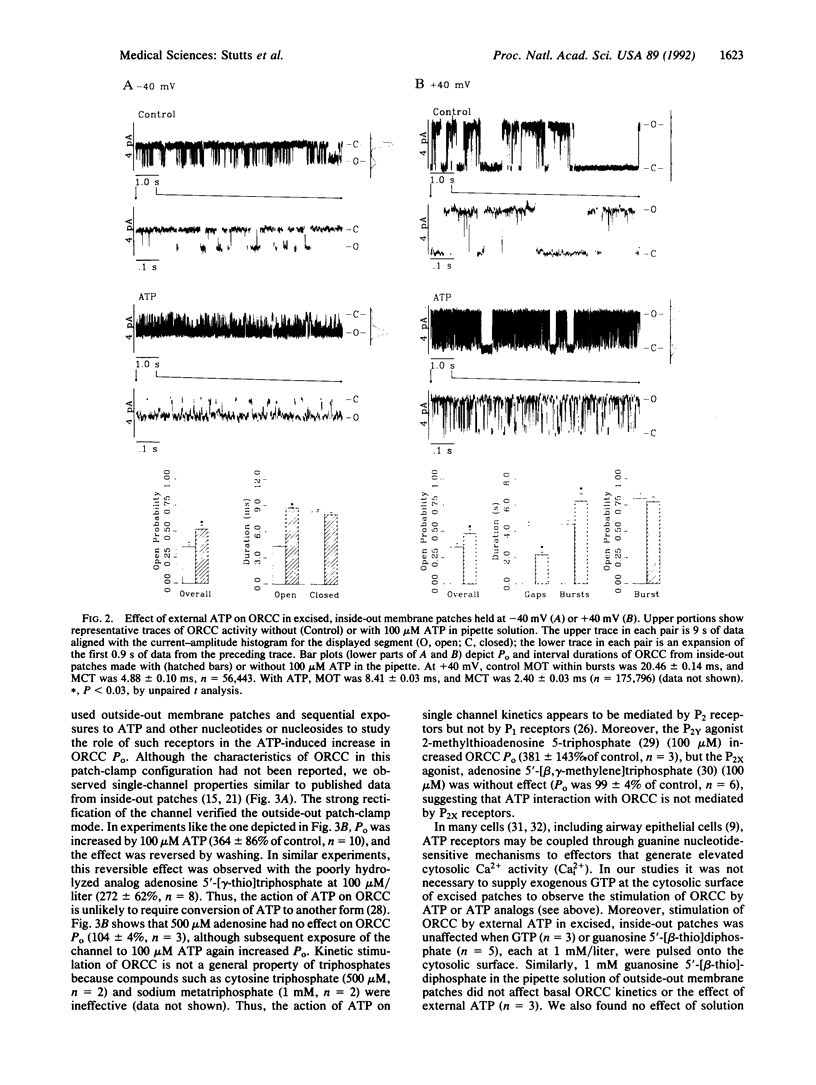
The rate of Cl- secretion by human airway epithelium is determined, in part, by apical cell membrane Cl- conductance. In cystic fibrosis airway epithelia, defective regulation of Cl- conductance decreases the capability to secrete Cl-. Here we report that extracytosolic ATP in the luminal bath of cultured human airway epithelia increased transepithelial Cl- secretion and apical membrane Cl- permeability. Single-channel studies in excised membrane patches revealed that ATP increased the open probability of outward rectifying Cl- channels. The latter effect occurs through a receptor mechanism that requires no identified soluble second messengers and is insensitive to probes of G protein function. These results demonstrate a mode of regulation of anion channels by binding ATP at the extracellular surface. Regulation of Cl- conductance by external ATP is preserved in cystic fibrosis airway epithelia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. P., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Generation of cAMP-activated chloride currents by expression of CFTR. Science. 1991 Feb 8;251(4994):679–682. doi: 10.1126/science.1704151. [DOI] [PubMed] [Google Scholar]
- Bailey S. J., Hourani S. M. A study of the purinoceptors mediating contraction in the rat colon. Br J Pharmacol. 1990 Aug;100(4):753–756. doi: 10.1111/j.1476-5381.1990.tb14087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett T. B., Knowles M. R. Diffuse bacterial bronchiolitis with bronchiolar pneumonia in adults. South Med J. 1987 Jan;80(1):10–15. doi: 10.1097/00007611-198701000-00003. [DOI] [PubMed] [Google Scholar]
- Bean B. P. ATP-activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. J Neurosci. 1990 Jan;10(1):1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Friel D. D. ATP-activated channels in excitable cells. Ion Channels. 1990;2:169–203. doi: 10.1007/978-1-4615-7305-0_5. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Williams C. A., Ceelen P. W. ATP-activated channels in rat and bullfrog sensory neurons: current-voltage relation and single-channel behavior. J Neurosci. 1990 Jan;10(1):11–19. doi: 10.1523/JNEUROSCI.10-01-00011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björnsson O. G., Monck J. R., Williamson J. R. Identification of P2Y purinoceptors associated with voltage-activated cation channels in cardiac ventricular myocytes of the rat. Eur J Biochem. 1989 Dec 8;186(1-2):395–404. doi: 10.1111/j.1432-1033.1989.tb15222.x. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Quantitative description of three modes of activity of fast chloride channels from rat skeletal muscle. J Physiol. 1986 Sep;378:141–174. doi: 10.1113/jphysiol.1986.sp016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. C., Cheng E. H., Paradiso A. M., Stutts M. J., Knowles M. R., Earp H. S. Chloride secretory response of cystic fibrosis human airway epithelia. Preservation of calcium but not protein kinase C- and A-dependent mechanisms. J Clin Invest. 1989 Nov;84(5):1424–1431. doi: 10.1172/JCI114316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHERNICK W. S., BARBERO G. J. Composition of tracheobronchial secretions in cystic fibrosis of the pancreas and bronchiectasis. Pediatrics. 1959 Nov;24:739–745. [PubMed] [Google Scholar]
- Cotton C. U., Boucher R. C., Gatzy J. T. Paths of ion transport across canine fetal tracheal epithelium. J Appl Physiol (1985) 1988 Dec;65(6):2376–2382. doi: 10.1152/jappl.1988.65.6.2376. [DOI] [PubMed] [Google Scholar]
- Cotton C. U., Stutts M. J., Knowles M. R., Gatzy J. T., Boucher R. C. Abnormal apical cell membrane in cystic fibrosis respiratory epithelium. An in vitro electrophysiologic analysis. J Clin Invest. 1987 Jan;79(1):80–85. doi: 10.1172/JCI112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreinhöfer J., Gögelein H., Greger R. Blocking kinetics of Cl- channels in colonic carcinoma cells (HT29) as revealed by 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB). Biochim Biophys Acta. 1988 Dec 8;946(1):135–142. doi: 10.1016/0005-2736(88)90466-x. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Gonzalez F. A., Alfonzo R. G., Toro J. R., Heppel L. A. Receptor specific for certain nucleotides stimulates inositol phosphate metabolism and Ca2+ fluxes in A431 cells. J Cell Physiol. 1989 Dec;141(3):606–617. doi: 10.1002/jcp.1041410320. [DOI] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halm D. R., Rechkemmer G., Schoumacher R. A., Frizzell R. A. Biophysical properties of a chloride channel in the apical membrane of a secretory epithelial cell. Comp Biochem Physiol A Comp Physiol. 1988;90(4):597–601. doi: 10.1016/0300-9629(88)90673-1. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Higashi K., Ogawara H. ATP-dependent regulation of phospholipase C in permeabilized 3T3 cells. FEBS Lett. 1990 Jul 2;267(1):51–54. doi: 10.1016/0014-5793(90)80285-q. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Lu L., Zeitlin P. L., Gruenert D. C., Huganir R., Guggino W. B. Cl- channels in CF: lack of activation by protein kinase C and cAMP-dependent protein kinase. Science. 1989 Jun 16;244(4910):1351–1353. doi: 10.1126/science.2472005. [DOI] [PubMed] [Google Scholar]
- Kartner N., Hanrahan J. W., Jensen T. J., Naismith A. L., Sun S. Z., Ackerley C. A., Reyes E. F., Tsui L. C., Rommens J. M., Bear C. E. Expression of the cystic fibrosis gene in non-epithelial invertebrate cells produces a regulated anion conductance. Cell. 1991 Feb 22;64(4):681–691. doi: 10.1016/0092-8674(91)90498-n. [DOI] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Knowles M. R., Clarke L. L., Boucher R. C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N Engl J Med. 1991 Aug 22;325(8):533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Knowles M., Gatzy J., Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983 May;71(5):1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M., Murray G., Shallal J., Askin F., Ranga V., Gatzy J., Boucher R. Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol Respir Environ Exerc Physiol. 1984 Apr;56(4):868–877. doi: 10.1152/jappl.1984.56.4.868. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A., Marchenko S. M., Obukhov A. G. Cationic channels activated by extracellular ATP in rat sensory neurons. Neuroscience. 1988 Dec;27(3):995–1000. doi: 10.1016/0306-4522(88)90203-5. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Anderson M. P., Clancy J. P., Liedtke C. M., Nairn A. C., Greengard P., Welsch M. J. Regulation of chloride channels by protein kinase C in normal and cystic fibrosis airway epithelia. Science. 1989 Jun 16;244(4910):1353–1356. doi: 10.1126/science.2472006. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Mason S. J., Paradiso A. M., Boucher R. C. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991 Jul;103(3):1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillian M. K., Soltoff S. P., Lechleiter J. D., Cantley L. C., Talamo B. R. Extracellular ATP increases free cytosolic calcium in rat parotid acinar cells. Differences from phospholipase C-linked receptor agonists. Biochem J. 1988 Oct 1;255(1):291–300. [PMC free article] [PubMed] [Google Scholar]
- Moczydlowski E., Latorre R. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J Gen Physiol. 1983 Oct;82(4):511–542. doi: 10.1085/jgp.82.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. L., Matthews L. W., Spector S., Lemm J. Studies on pulmonary secretions. II. Osmolality and the ionic environment of pulmonary secretions from patients with cystic fibrosis, bronchiectasis, and laryngectomy. Am Rev Respir Dis. 1967 Jul;96(1):83–87. doi: 10.1164/arrd.1967.96.1.83. [DOI] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Low W., Elie D., Hanrahan J. W. Low-conductance chloride channel activated by cAMP in the epithelial cell line T84. FEBS Lett. 1990 Sep 17;270(1-2):157–164. doi: 10.1016/0014-5793(90)81257-o. [DOI] [PubMed] [Google Scholar]
- Thomas S. A., Hume R. I. Permeation of both cations and anions through a single class of ATP-activated ion channels in developing chick skeletal muscle. J Gen Physiol. 1990 Apr;95(4):569–590. doi: 10.1085/jgp.95.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A., Cozens A. L., Schulman H., Gruenert D. C., Stryer L., Gardner P. Activation of chloride channels in normal and cystic fibrosis airway epithelial cells by multifunctional calcium/calmodulin-dependent protein kinase. Nature. 1991 Feb 28;349(6312):793–796. doi: 10.1038/349793a0. [DOI] [PubMed] [Google Scholar]
- Ward C. L., Krouse M. E., Gruenert D. C., Kopito R. R., Wine J. J. Cystic fibrosis gene expression is not correlated with rectifying Cl- channels. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5277–5281. doi: 10.1073/pnas.88.12.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welford L. A., Cusack N. J., Hourani S. M. ATP analogues and the guinea-pig taenia coli: a comparison of the structure-activity relationships of ectonucleotidases with those of the P2-purinoceptor. Eur J Pharmacol. 1986 Oct 7;129(3):217–224. doi: 10.1016/0014-2999(86)90431-0. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Li M., McCann J. D. Activation of normal and cystic fibrosis Cl- channels by voltage, temperature, and trypsin. J Clin Invest. 1989 Dec;84(6):2002–2007. doi: 10.1172/JCI114391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Shunt resistance and ion permeabilities in normal and cystic fibrosis airway epithelia. Am J Physiol. 1989 May;256(5 Pt 1):C1054–C1063. doi: 10.1152/ajpcell.1989.256.5.C1054. [DOI] [PubMed] [Google Scholar]
- Yankaskas J. R., Cotton C. U., Knowles M. R., Gatzy J. T., Boucher R. C. Culture of human nasal epithelial cells on collagen matrix supports. A comparison of bioelectric properties of normal and cystic fibrosis epithelia. Am Rev Respir Dis. 1985 Dec;132(6):1281–1287. doi: 10.1164/arrd.1985.132.6.1281. [DOI] [PubMed] [Google Scholar]