Abstract
Objectives
Vaginitis still remains as a health issue in women. It is notable that Candida albicans producing biofilm is considered a microorganism responsible for vaginitis with hard to treat. Also, Peganum harmala was applied as an anti fungal in treatment for many infections in Iran. Therefore, this study goal to investigate the role of P. harmala in inhibition of biofilm formation in C. albicans.
Methods
So, 27 C. albicans collected from women with Vaginitis, then subjected for biofilm formation assay. P. harmala was applied as antibiofilm formation in C. albicans.
Results
Our results demonstrated that P. harmala in concentration of 12 μg/ml easily inhibited strong biofilm formation; while the concentrations of 10 and 6 μg/ml inhibited biofilm formation in moderate and weak biofilm formation C. albicans strains, respectively.
Conclusion
Hence, the current study presented P. harmala as antibiofilm herbal medicine for C. albicans; but in vivo study suggested to be performed to confirm its effectiveness.
Keywords: biofilm formation, Candida albicans, Peganum harmala
1. Introduction
Unfortunately, vaginitis remains a health issue in women. It has been estimated that there are around 10 million physician visits for vaginitis annually [1]. However, biofilm formation is known as a major worldwide concern [2]. Despite this, there are a vast majority of microbiology studies focusing on bacterial biofilm, but there is less consideration to medically important fungal biofilms. Among fungi, the genus Candida, especially Candida albicans, is responsible for around 15–20% of vaginitis cases in women [3]. Studies have demonstrated that the majority of biofilm formation by C. albicans occurred in the oral cavities, environment, and vaginas of patients [4].
New drug discovery could also be useful for the eradication of potent fungi in biofilm formation. Herbal medicines are defined as natural products, which are used in traditional medicine. The use of medicinal plants goes back 60,000 years ago [5]. Peganum harmala, usually called Esfand, is a plant of the family of Nitrariaceae. It is applied as a traditional medicine for so many infections in Iran [6]. Hence, the current study aimed to investigate the role of P. harmala in the inhibition of biofilm formation produced by C. albicans.
2. Materials and methods
2.1. Organisms and identification
C. albicans were collected from vulvovaginal candidacies women in the Ilam province in the west of Iran. The isolates were cultured on Sabouraud dextrose agar and then re-identified with a germ tube test [7].
2.2. Cell culture
The P. harmala extract was tested for its cytotoxicity effect on a Vero cell line. The percentage viability of the cell line was carried out by using the MTT assay (Sigma, United States).
2.3. Toxicity assay
The cells were coated in a 96-well flat bottom plates at a density of 5,000–10,000 cells per well. Following this, the cells were treated with different concentrations of P. harmala ethanolic extract (1–100 μg/mL). After 24 hours, the MTT assay was applied to determine the toxicity concentration of P. harmala. The absorbance of the converted dye was measured at a wavelength of 570 nm with a background subtraction at 600 nm.
2.4. Biofilm formation assay
A 0.5 McFarland solution of C. albicans inoculated to 200 uL lurian broth (LB broth) in 96 micro plates for evaluation of biofilm formation. One colony from each C. albicans was applied to inoculate 5 mL LB broth. Then, the culture incubated for 24 hours at 35°C with aeration at 4 ×g. Then, 0.5 McFarland from each C. albicans was prepared. Following this, 200 μL of each C. albicans was transferred to a 96-well micro plate. The experiment was performed in duplicate. LB broth without C. albicans was considered as a negative control. The plates were incubated for 48 hours at 35oC.
2.5. Semiquantification of biofilm biomass
Biofilm biomass was quantified using a methodology described by Mowat et al [8].
2.6. Analysis of biofilm formation
The capacity of each strain to form a biofilm was compared with that of the confluent biofilm-forming C. albicans control by analyzing the absorbance of the crystal violet stain obtained for each biofilm. This allowed each isolate to be assigned a percentage value depending on the proportion of biofilm biomass it was able to establish after 48 hours in comparison with the control (taken as 100%).
Isolates were also divided into three groups depending on whether they formed fully established biofilms with 75% of the biomass of the positive control, moderately adherent biofilms with 25–75% biomass, or weak biofilms with 25% of the biomass of the positive control.
2.7. Antibiofilm formation activity determination of P. harmala
Different concentrations (1–15 μg/mL) of P. harmala were applied on the positive biofilm formation strains. Then, the biofilm formation assay, as described above, was performed to evaluate the efficacy of P. harmala on biofilm formation by C. albicans isolates.
3. Results
3.1. Biofilm formation in C. albicans
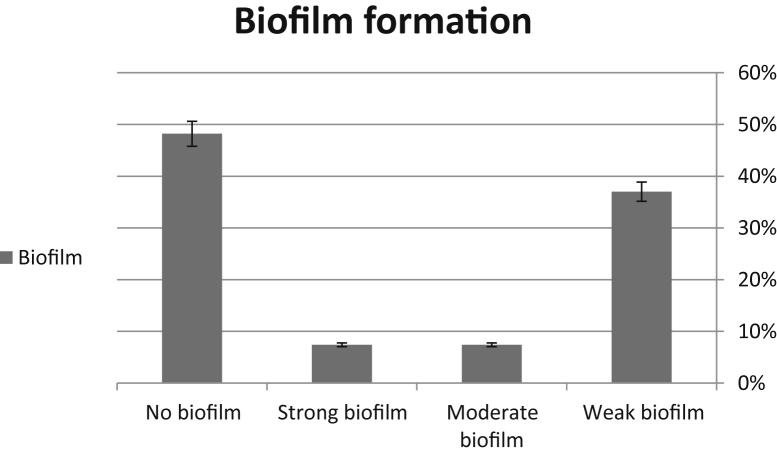
Our results demonstrated that among 100 yeasts responsible for vaginitis, 27% (n = 27) of them were C. albicans. Our analysis demonstrated that all types of biofilm formation were observed; while the most biofilm formation was observed as weak biofilms (37%, n = 10). The lowest biofilm formation was observed for both moderate and strong biofilm formation (each, 7.4%, n = 2). The remaining strains (48.2%, n = 13) showed no biofilm formation (Figure 1).
Figure 1.
Biofilm formation among Candida albicans collected from patients with vaginitis.
3.2. P. harmala as a candidate for inhibition of biofilm formation in C. albicans
The IC50 for P. harmala was 15 μg/mL. P. harmala, in a concentration of 12 μg/mL, easily inhibited biofilm formation on strong biofilm formation strains; while concentrations of 6 μg/mL and 10 μg/mL inhibited biofilm formation in weak and moderate biofilm formation C. albicans strains, respectively.
4. Discussion
C. albicans is considered as a human pathogen and causes many infections ranging from severe to mild candidacies. Many factors are involved in the pathogenicity of C. albicans, including the production of extracellular enzymes, biofilm formation, and surface adherence 9, 10. Also, C. albicans is settled in the vaginal cavity as a microbiota that causes vulvovaginal candidiasis. Our finding demonstrated that there is variation in biofilm formation in C. albicans, which is consistent with obtained results by Sherry et al [11]. It seems to be necessary to investigate traditional herbs against pathogenic microorganisms. Our findings demonstrated the antibiofilm effectiveness of P. harmala against C. albicans. However, more investigations, including in vivo studies, are needed to study P. harmala as an antibiofilm in C. albicans.
Conflicts of interest
The author has no conflicts of interest to declare.
Contributor Information
Sobhan Ghafourian, Email: sobhan.ghafurian@gmail.com.
Iraj Pakzad, Email: pakzadi2006@gmail.com.
References
- 1.Kent H.L. Epidemiology of vaginitis. Am J Obstet Gynecol. 1991 Oct;165(4 Pt 2):1168–1176. doi: 10.1016/s0002-9378(12)90722-x. [DOI] [PubMed] [Google Scholar]
- 2.Naparstek L., Carmeli Y., Navon-Venezia S.H. Biofilm formation and susceptibility to gentamicin and colistin of extremely drug-resistant KPC-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2014 Apr;69(4):1027–1034. doi: 10.1093/jac/dkt487. [DOI] [PubMed] [Google Scholar]
- 3.Sobel J.D., Faro S., Force R.W. Vulvovaginal candidiasis: epidemiologic, diagnostic, and therapeutic considerations. Am J Obstet Gynecol. 1998 Feb;178(2):203–211. doi: 10.1016/s0002-9378(98)80001-x. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Yan Z., Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003 Feb;149(Pt 2):353–362. doi: 10.1099/mic.0.25932-0. [DOI] [PubMed] [Google Scholar]
- 5.Thompson J.D., Manicacci D., Tarayre M. Thirty-five years of thyme: a tale of two polymorphisms. Why so many females? Why so many chemotypes? Bioscience. 1998 Oct;48(10):805–815. [Google Scholar]
- 6.Moloudizargari M., Mikaili P., Aghajanshakeri S.H. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn Rev. 2013 Jul–Dec;7(14):199–212. doi: 10.4103/0973-7847.120524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmadzadeh A., Valavi E., Shamsizadeh A. Fungal urinary tract infection in an infant with posterior urethral valves. Jundishapur J Microbiol. 2011;4(Suppl. 1):S71–S76. [Google Scholar]
- 8.Mowat E., Rajendran R., Williams C. Pseudomonas aeruginosa and their small diffusible extracellular molecules inhibit Aspergillus fumigatus biofilm formation. FEMS Microbiol Lett. 2010 Dec;313(2):96–102. doi: 10.1111/j.1574-6968.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 9.Calderone R.A., Fonzi W.A. Virulence factors of Candida albicans. Trends Microbiol. 2001 Jul;9(7):327–335. doi: 10.1016/s0966-842x(01)02094-7. [DOI] [PubMed] [Google Scholar]
- 10.Gokce G., Cerikcioglu N., Yagci A. Acid proteinase, phospholipase, and biofim production of Candida species isolated from blood cultures. Mycopathologia. 2007 Dec;164(6):265–269. doi: 10.1007/s11046-007-9053-4. [DOI] [PubMed] [Google Scholar]
- 11.Sherry L., Rajendran R., Lappin D.F. Biofilm formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014 Jul;14:182. doi: 10.1186/1471-2180-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]