Abstract
The aim of this study was to determine the specific microRNA (miRNA) that regulates expression of osteopontin (OPN) in osteoarthritis (OA). The potential regulatory miRNAs for OPN messenger RNA (mRNA) were predicted by miRNA prediction programs. Among eight potential regulatory miRNAs, miR-220b, miR-513a-3p and miR-548n increased, while miR-181a, miR-181b, miR-181c, miR-181d and miR-127-5p decreased in OA patients. miRNA-127-5p mimics suppressed OPN production as well as the activity of a reporter construct containing the 3′-UTR of human OPN mRNA. In addition, mutation of miR-127-5p binding site in the 3′-UTR of OPN mRNA abolished miR-127-5p-mediated repression of reporter activity. Conversely, treatment with miR-127-5p inhibitor increased reporter activity and OPN production. Interestingly, miR-127-5p inhibited proliferation of chondrocytes through OPN. In conclusion, miRNA-127-5p is an important regulator of OPN in human chondrocytes and may contribute to the development of OA.
Osteoarthritis (OA) is regarded as the most prevalent chronic joint disease, and is characterized by a group of mechanical abnormalities, such as degradation of articular cartilage, thickening of subchondral bone, and synovial inflammation1,2,3. There is a growing knowledge and understanding on the pathogenesis of OA3. Osteopontin (OPN) is a 44~75 KD multifunctional phosphoprotein, and is associated with the pathogenesis of OA4. OPN regulates expression of various factors associating with the pathogenesis of OA, including matrix metalloprotease 13 (MMP13)5, hypoxia-inducible factor-2α6, ADAMTS4 (a disintegrin and metalloproteinase with thrombospondin motifs)7, tissue inhibitors of metalloproteinases (TIMPs)8, interlukine-6 and 89, and even caveolin-110.
Although the etiology of OA is complex, recent evidence has made it apparent that epigenetic changes, altered expression of regulatory RNA and its consequent in gene expression modifications could also participate in the pathogenesis of OA11,12. MicroRNA (miRNA) is small noncoding RNA with the length of about 20–25 nucleotides (nt), and is transcribed in the nucleus by RNA polymerase II or III. miRNA is involved in regulation of post-transcriptional gene expression by translational suppression or direct degradation of the mRNA via targeting of the coding genes through complementary base pairing between the miRNA and the 3′-Untranslated region (UTR) of the messenger RNA (mRNA) target13.
Increasing investigations are evaluating the differential expression of miRNA in OA vs a normal condition. Early study has compared the miRNA profiling between OA patient-derived osteoarthritic cartilage and normal cartilage, and 16 microRNAs have been characterized as osteoarthritis gene signature14. Jones et al. have identified 17 differential expression miRNAs with more than 4-fold in OA cartilage, and 30 differential expression mRNAs with more than 4-fold in OA bone14. Further study has found 12 overexpressed miRNAs in the plasma of patients with primary OA by detecting the expression of 380 miRNAs in OA15. Subsequently, some specific miRNAs have shown important roles in OA; for example, miR-140 regulates ADAMTs-5 expression16, miR-27b regulates MMP13 expression17, and miR-146 is intensely expressed in low grade OA cartilage, and its expression is induced by stimulation of IL-1β, suggesting its involvement in OA pathogenesis18. These investigations are highlighting the importance of miRNA in the initiation and development of OA. However, the specific miRNA that regulates expression of OPN in OA is largely unknown. In this study, we have investigated the miRNA that targets expression of OPN.
Results
Identification of miRNAs targeting OPN
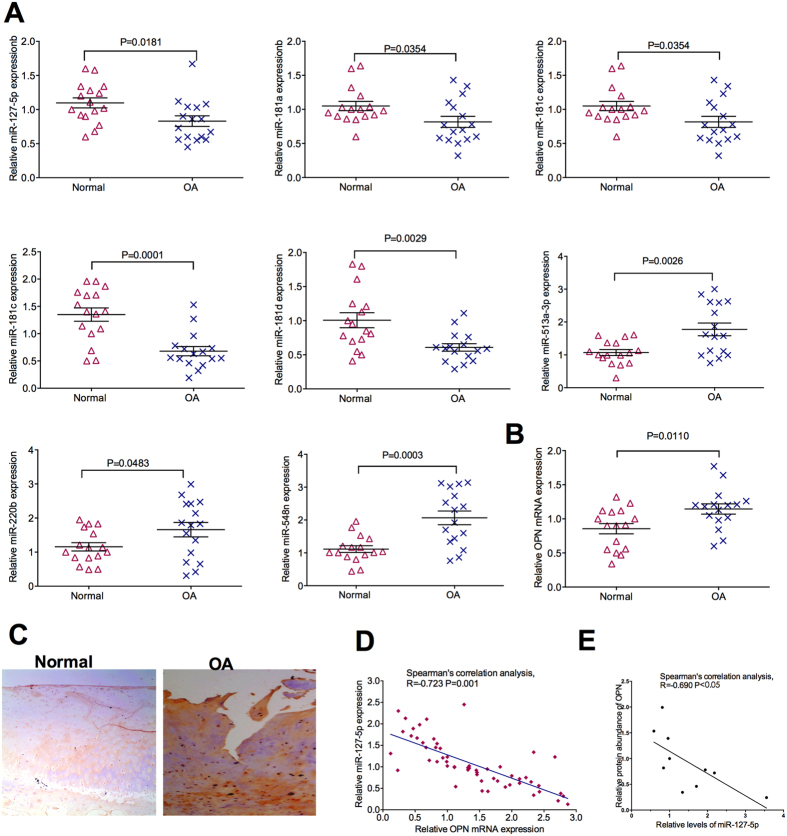
In total, eight potential regulatory miRNAs, including miR-220b, miR-513a-3p, miR-181a, miR-181b, miR-181c, miR-181d, miR-548n and miR-127-5p, were identified by the five algorithms. Next, expression of these miRNAs in OA patients and control were analyzed by RT-PCR. OA patients have higher expression of miR-220b, miR-513a-3p and miR-548n, but lower expression of miR-181a, miR-181b, miR-181c, miR-181d and miR-127-5p, compared to non-OA patients (Fig. 1A). Similar to previous study4, mRNA expression of OPN increased in OA patients compared to non-OA patients (Fig. 1B). Also, with analysis from immunohistochemistry, the protein abundance of OPN was higher in OA patients compared to non-OA patients (Fig. 1C). Notably, spearman’s correlation analysis shown that there was a significant negative correlation between expression of miR-127-5p and mRNA expression of OPN (r = −0.723, P = 0.001) (Fig. 1D). Similarly, spearman’s correlation analysis shown that there was a significant negative correlation between expression of miR-127-5p and protein abundance of OPN (r = −0.69, P < 0.05) (Fig. 1E). Summarily, OA patients have alterations in expression of miRNAs and OPN, and miR-127-5p may inhibit expression of OPN.
Figure 1. Identification of miRNAs targeting OPN.
(A) Expression of miR-220b, miR-513a-3p, miR-548n, miR-181a, miR-181b, miR-181c, miR-181d and miR-127-5p in OA patients (n = 16) and the non-OA patients (n = 16). (B) Expression of OPN in OA patients (n = 16) and non-OA patients (n = 16). (C) Expression of OPN proteins were analyzed using immunohistochemical analyses. Representative images from at least 10 patients for each group. (D) The correlation between the expression of miR-127-5p and mRNA expression of OPN. (E) The correlation between the expression of miR-127-5p and protein abundance of OPN. OA: osteoarthritis; OPN: osteopontin.
miR-127-5p inhibits OPN expression
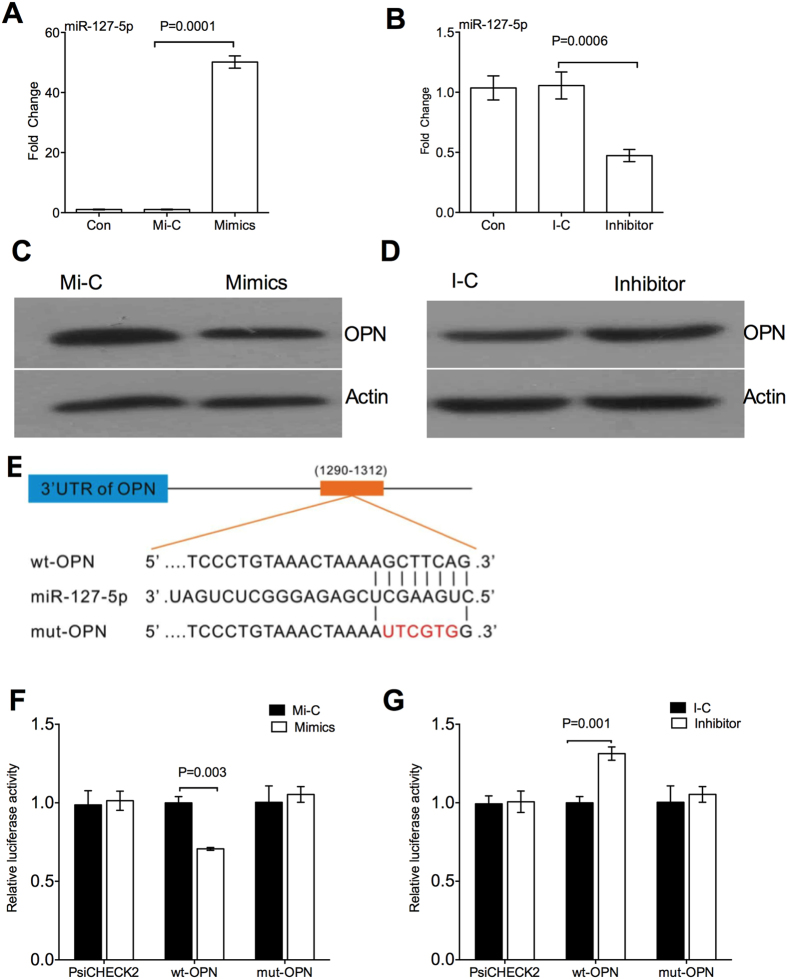
We tested the hypothesis that miR-127-5p directly influences OPN expression by transfecting a miR-127-5p mimics or inhibitor into the chondrocytes and then detecting protein abundance of OPN. miR-127-5p mimics transfection significantly promoted expression of miR-127-5p compared the controls (Fig. 2A), while miR-127-5p inhibitor inhibited expression of miR-127-5p (Fig. 2B). miR-127-5p mimics significantly reduced protein abundance of OPN in the chondrocytes (Fig. 2C), while miR-127-5p inhibitor increased protein abundance of OPN (Fig. 2D), compared to the controls. To directly test the hypothesis that OPN is a downstream target of miR-127-5p, OPN wild-type/mutant 3′-UTRs containing the putative miR-127-5p binding sites were cloned into the psi-CHECK2 reporter vector downstream of the Photinus pyralis/Renila reniformis dual luciferase reporter gene (Fig. 2E). Chondrocytes co-transfected with the wild-type 3′-UTR reporter vector and the miR-127-5p mimics showed a significant reduction in luciferase activity, whereas the luciferase activity in the cells transfected with the mutant-type 3′-UTR vector was unaffected by the miR-127-5p mimics (Fig. 2F). We co-transfected the miR-127-5p inhibitor and wild-type 3′-UTR reporter vector into chondrocytes, which demonstrated the luciferase activity was significantly increased in the presence of the miR-127-5p inhibitor (Fig. 2G). Taken together, miR-127-5p inhibits expression of OPN, and OPN mRNA is a downstream target of miR-127-5p.
Figure 2. miR-127-5p inhibits OPN expression.
(A,B) Expression of miR-127-5p in chondrocytes after miR-127-5p mimics (A) or miR-127-5p inhibitor (B) transfection. (C,D) Protein abundance of OPN in the chondrocytes after miR-127-5p mimics (C) or miR-127-5p inhibitor (D) transfection. (E) The illustration of OPN wild- type/mutant 3′-UTRs containing the putative miR-127-5p binding sites. (F) The luciferase activity in chondrocytes co-transfected with the wild-type or mutant 3′-UTR reporter vector and the miR-127-5p mimics. (G) The luciferase activity in chondrocytes co-transfected with the wild-type or mutant 3′-UTR reporter vector and the miR-127-5p inhibitor. Data are representative of three independent experiments with 4-6 repeats in each time. OPN: osteopontin. Mi-C: miR-127-5p mimics control; I-C: miR-127-5p inhibitor control.
miR-127-5p inhibits proliferation of chondrocytes though OPN
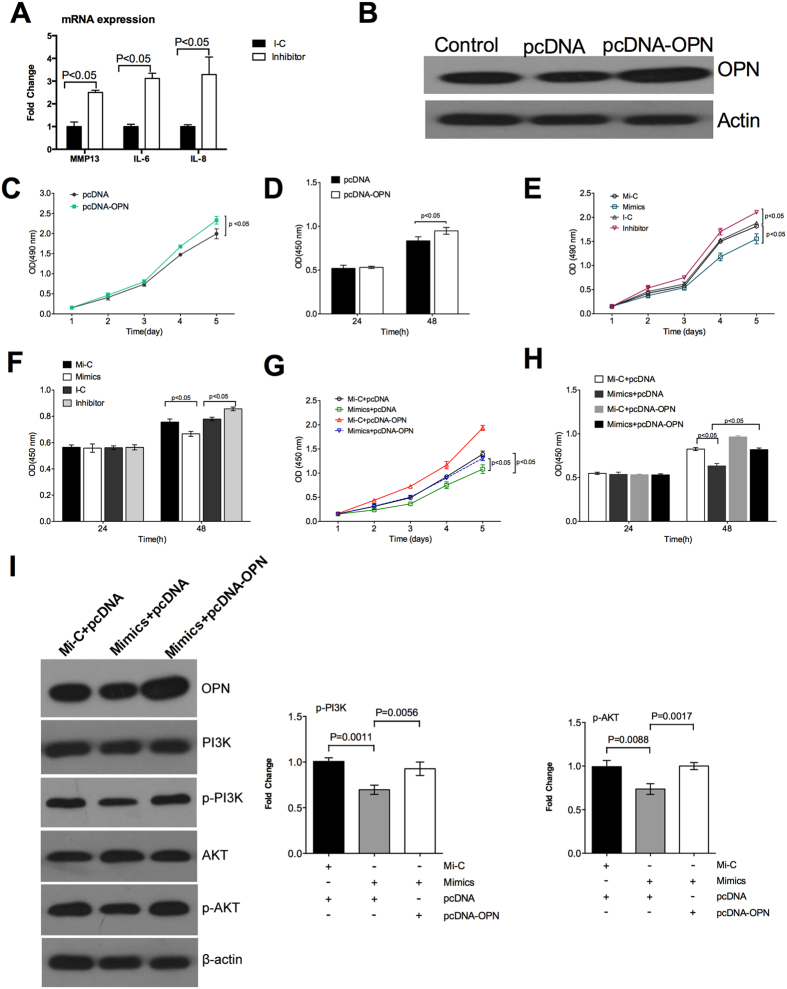
OPN promotes expression of various factors associated with the pathogenesis of OA, such as MMP135, interlukine-6 and 89. The miR-127-5p inhibitor significantly promoted the mRNA expression of MMP13, IL-6 and IL-8 in the chondrocytes (Fig. 3A). OPN has critical importance on the pathogenesis of OA, associating with the promotion in proliferation of chondrocytes (Fig. 3B–D). miR-127-5p mimics significantly reduced proliferation of chondrocytes, while miR-127-5p inhibitor promoted proliferation of chondrocytes (Fig. 3E,F). To further confirm that OPN is a functional target of miR-127-5p, we rescued the expression of OPN by the transfecting the pcDNA3.1-OPN in chondrocytes, which increased protein abundance of OPN in chondrocytes (Fig. 3B). Although miR-127-5p mimics significantly decreased proliferation of chondrocytes, pcDNA3.1-OPN rescued proliferation of chondrocytes (Fig. 3G,H). Furthermore, although miR-127-5p mimics significantly inhibited the activation of phosphatidylinositide 3-kinase (PI3K)-Akt pathway, pcDNA3.1-OPN rescued the activation of PI3K-Akt pathway (Fig. 3I). Collectively, miR-127-5p inhibits proliferation of chondrocytes by targeting expression of OPN.
Figure 3. miR-127-5p inhibits proliferation of chondrocytes though OPN.
(A) mRNA expression of MMP13, IL-6 and IL-8 in the chondrocytes after miR-127-5p inhibitor treatment. B. Protein abundance of OPN in the chondrocytes after pcDNA-OPN transfection. (C,D) Proliferation of chondrocytes after pcDNA-OPN transfection by MTT assay (C) and BrdU incorporation assays (D). (E,F) Proliferation of chondrocytes after indicted treatments by MTT assay (E) and BrdU incorporation assays (F). (G,H) Proliferation of chondrocytes after indicted treatments by MTT assay (G) and BrdU incorporation assays (H). (I) The activation of PI3K-Akt pathway by immunoblotting analysis. Data are representative of three independent experiments with 4–6 repeats in each time. OPN: osteopontin; PI3K: phosphatidylinositide 3-kinases; Mi-C: miR-127-5p mimics control; I-C: miR-127-5p inhibitor control.
Discussion
Many miRNAs are differentially expressed during OA14,19,20,21, including miR-9, miR-98, miR-146a, miR-483, miR-149, miR-582, miR-1227, miR-634, miR-576, miR-641, miR-27a and b, and miRNA-140. However, as far as we known, no study has reported the miRNA that regulates the expression of OPN in OA. OPN is known as early T cell activation gene-1 (Eta-1)22,23. OPN is secreted by many types of cells, including macrophages, lymphocytes, epithelial cells, vascular smooth muscle cells, and even chondrocytes as well as synoviocytes24,25,26,27. OPN is highly abundant in the extracellular fluids at sites of inflammation, extracellular matrix (ECM) of mineralised tissues and even in the bone24,26,28. In the bone, OPN regulates the interactions of cell-matrix and cell-cell, the transitions of cartilage-to-bone in fracture repair, the attachment of osteoclasts to the bone matrix23,29,30. Interestingly, mRNA expression and protein abundance of OPN are associated with the pathogenesis of OA. At the begin, a study found that mRNA expression of OPN isolated from human OA cartilage is higher than the normal cartilage31. Subsequently, increased abundance of OPN in the plasma, synovial fluid and articular cartilage in OA patients are found3,32,33, indicating expression of OPN is associated with progressive joint damage, and the severity and progression of OA.
In human breast cancer cell lines (MCF7, MCF10AT and MCF10DCIS.com), hsa-miR-299-5p has been reported to target OPN and regulate the expression of OPN34. miRNA 181a targets OPN and decreases OPN expression in hepatocellular cancer cell lines (Hep 3B and Hep G2)35, vascular smooth muscle cells36. Besides miRNA 181a, miR-220b, miR-513a-3p, miR-181b, miR-181c, miR-181d, miR-548n and miR-127-5p, are also predicted to target and regulate the expression of OPN. Further analysis found that expression of miR-220b, miR-513a-3p and miR-548n increase in OA patients compared to non-OA patients. miR-220b inhibits the autoimmune regulator (AIRE) gene translation through the 3′UTR region of AIRE gene, which is responsible for autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy37. miR-513a-3p has been reported to regulate expression of the luteinizing hormone/chorionic gonadotropin receptor (LHCGR), which is essential for normal male and female reproductive processes38. miRNA-548n regulates host antiviral response by direct targeting of Interferon (IFN)-λ139. Although the exact role of miR-220b, miR-513a-3p and miR-548n in the pathogenesis of OA is unknown, it is interesting to investigate the function of increased expression of miR-220b, miR-513a-3p and miR-548n in the establishment and development of OA.
As OPN increased in the pathogenesis of OA32, we focused on the down-expressed miRNA, including miR-181a, miR-181b, miR-181c, miR-181d and miR-127-5p. miR-miR181 family members have been reported to regulate the differentiation stages of chondrocyte and chondrocyte formation40. Hypertrophic mesenchymal stromal cells (MSC)-derived chondrocytes and non-hypertrophic articular chondrocytes show differential expression of miR-181a40. This compelling study is indicating miR181 family members have critical importance in the establishment and development of OA. Indeed, previous reports have shown miR181a directly target and regulate expression of OPN in hepatocellular cancer cell lines (Hep 3B and Hep G2)35, vascular smooth muscle cells36. Thus, this study mainly focused on the miR-127-5p. miR-127-5p targets the 3′UTR of β-F1-ATPase mRNA (β-mRNA), which is a catalytic subunit of mitochondrial H(+)-ATP synthase and functions in the provision of metabolic energy by oxidative phosphorylation41. Notably, miR-127-5p is an important regulator of MMP-13 in human chondrocytes and contributes to the development of OA42. This study also shows that miR-127-5p is down regulated in the OA patients, and miR-127-5p targets the 3′UTR of OPN mRNA to down-regulate the expression of OPN. More importantly, we have shown miR-127-5p regulates proliferation of chondrocytes through targeting expression of OPN. Thus, it is fruitful to use miR-127-5p to manipulate the establishment and development of OA.
In conclusion, this study identified that miR-127-5p targets the 3′UTR of OPN mRNA to down-regulate the expression of OPN. In OA, the down-expressed miR-127-5p allows the expression of OPN, which mediates the establishment and development of OA. As far as we known, this is the first study show miR-127-5p directly targets OPN to regulate expression of OPN in OA.
Materials and Methods
Cartilage acquisition and assessment
The study was approved by the institutional review board and ethics committee of Xiangya Hospital affiliated to Central South University, which conformed with the regulations of medical ethics. All experiments were conducted in accordance with the approved guidelines. The normal cartilage tissues from non-OA patients and degenerated cartilage tissues from OA patients were obtained in previous study5,6,7,32. The cartilage tissues were assessed with hematoxylin- eosin (HE) and safranin-O staining, and a modified Mankin grading system in previous study (Supplementary Figure 1)5,6,7,32. A written informed consent about this experiment was obtained from all subjects.
Cell isolation and culture conditions
The chondrocytes were isolated and cultured according to previous study5,6,7. Briefly, samples were minced into pieces of less than 1 mm3, followed by sequential digestion at 37 °C with 0.15% collagenase II (Invitrogen, Carlsbad, CA, USA) for 5–6 h with stirring every 20 min after 2 h. Chondrocytes were isolated after centrifugation and cultured in DMEM-F12 containing 10% fetal bovine serum (FBS) and antibiotics for 5–7 days before use.
miRNA prediction
To predict the miRNA targeting in the 3′UTR of OPN, five miRNA prediction programs, RNA22, TargetScan, miRDB, miRWalk and miRanda, were used to confirm the same target binding sites.
Recombinant plasmid construction
The 3′-UTR sequence of OPN (TCC CTG TAA ACT AAA AGC TTC AG) containing the putative miR-127-5p binding site were synthesized by Invitrogen (USA). The mutant sequence (TCC CTG TAA ACT AAA AUT CGT GG) by mutating the seed regions of the miR-193b binding sites was also synthesized by Invitrogen (USA). The synthesized products were cloned into the psiCHECK-2 vector (Invitrogen, USA). The recombinant plasmids were named as psiCHECK-2-OPN-wt and psiCHECK-2-OPN-mut. OPN expression vector was established for the “rescue” experiment, where the open reading frame of OPN was cloned into pcDNA3.1 (Invitrogen, USA). The recombinant plasmid was named as pcDNA3.1-OPN.
Cell transfection
The miR-127-5p mimics, inhibitors and their negative controls (NC) were purchased from Promega (USA). Chondrocytes were transfected with pcDNA3.1 (2 μg/mL), psiCHECK-2 reporter plasmid (2 μg/mL), miR-222 mimics (50 nM), inhibitor (50 nM) or their negative controls (50 nM) using Lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions.
Luciferase assay
Chondrocytes were transfected with psiCHECK-2-OPN-wt, psiCHECK-2-OPN-mut, miR-127-5p mimics, miR-127-5p inhibitor or its control using Lipofectamine 2000 reagent. After 48 h transfection, cells were lysed, and assays were performed using the Dual-Luciferase Reporter Assay System kit (Promega, USA) according to the manufacturer’s instructions.
BrdU incorporation assays
Chondrocytes were cultured for 16 h, and then pulsed with 5-Bromo-2-deoxyuridine (BrdU) for an additional 8 h. Cell proliferation was determined by BrdU incorporation assay according to the manufacturer’s instructions (Roche Diagnostics GmbH, Roche Applied Science, Germany).
MTT assay
Cell viability was assayed by using 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide (MTT). After treatment, 10 μL MTT (5 mg/mL) was added into cultured medium in each well for 2–4 hours until purple precipitate is visible. After the removal of culture medium, 75 μL dimethyl sulphoxide was added to each well, leaving the cells at room temperature in the dark for 2 hours. The absorbance at 570 nm was recorded.
RT-PCR
RT-PCR analysis was performed according to previous reports43,44,45. Briefly, total RNA was isolated from liquid nitrogen frozen samples using TRIZOL regent (Invitrogen, USA) and then treated with DNase I (Invitrogen, USA) according to the manufacturer’s instructions. Synthesis of the first strand (cDNA) was performed using oligo (dT) 20 and Superscript II reverse transcriptase (Invitrogen, USA). Primers used in this study were designed with Primer 5.0. Sequences of all primers used were: OPN-F: 5′-GTGGGA AGG ACA GTT ATG AA-3′; OPN-R: 5′-CTG ACT TTG GAA AGT TCC TG-3′; GAPDH-F: 5′-TGA CTT CAA CAG CGA CAC CCA-3′; GAPDH-R: 5′-CAC CCT GTT GCT GTA GCC AAA-3′; MMP13-F: 5′-CTT AGA GGT GAC TGG CAA AC-3′; MMP13-R: 5′- GCC CAT CAA ATG GGT AGA AG -3′. The primer pair for IL-6 was made to bp 42–61 (sense) and bp 334–354 (antisense) according to IL-6 cDNA sequence, and the IL-8 primer pair was made to the bp 147–174 (sense) and bp 342–366 (antisense). GAPDH was used as an internal control to normalize target gene transcript levels.
Expression of mature miRNA was quantified using a TaqMan miRNA assay kit (Applied Biosystems). Purified miRNA was reverse transcribed using a TaqMan miRNA RT kit (Applied Biosystems) and miRNA-specific stem-loop RT primers (Applied Biosystems). Real-time PCR was performed using a StepOnePlus Real-time PCR System (Applied Biosystems) in a 10 μL PCR mixture containing 2 μL RT product, 5 μLTaqMan Universal PCR Master Mix, 0.2 μM TaqMan probe, and 10 μM forward and reverse primers. RNU6B was used as an internal control for miRNA detection.
Immunoblotting
Western blot analysis was conducted according to previous study46,47,48. Equal amounts of proteins obtained from samples were separated by SDS-PAGE, transferred to PVDF membranes (Millipore, MA, USA), and blocked with 5% non-fat milk in Tris-Tween buffered saline buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 0.1% Tween-20) for 3 h. Antibodies against OPN (AP11567a, Abgent, CA, USA), PI3K (ab86714, Abcam, MA, USA), p- PI3K (ab182651, Abcam, MA, USA), Akt (ab8805, Abcam, MA, USA) or p-Akt (ab38449, Abcam, MA, USA) were incubated overnight at 4 °C and HRP-conjugated secondary antibodies were incubated for 1 h at room temperature before development and analysis using Alpha Imager 2200 software (Alpha Innotech Corporation, CA, USA). Signal intensity was digitally quantified and normalized to actin protein abundance.
Statistical analyses
Data shown are the means ± the standard error of the mean (SEM). All statistical analyses for data were performed using SPSS 16.0 software (Chicago, IL, USA). Data were analyzed between two groups using the Student’s t-test, while among more than two groups by the One-Way ANOVA method48,49,50,51. Differences of p < 0.05 were considered significant.
Additional Information
How to cite this article: Tu, M. et al. MicroRNA-127-5p regulates osteopontin expression and osteopontin-mediated proliferation of human chondrocytes. Sci. Rep. 6, 25032; doi: 10.1038/srep25032 (2016).
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos 81201420, 81272034, 81402224, 81472130), the Provincial Science Foundation of Hunan (No. 14JJ3032, No. 2015JJ3139), the Development and Reform Commission of Hunan Province ([2013]1199, [2014]658-8), the Science and Technology Office of Hunan Province (2012FJ6001,2013SK2018), the Science and Technology Office of Changsha City (K1203040-31), the Health and Family Planning Commission of Hunan Province (B2014-12), the Administration of Traditional Chinese Medicine of Hunan Province (No. 2015115) and the scientific and technical innovation committee of Shenzhen City (JCYJ20150403101028191).
Footnotes
Author Contributions Y.L. and G.L. designed the experiment. Y.L., M.T., Z.D., C.Z. and S.G. performed the experiment. Y.L., W.L., W.X. and M.T. analyzed the data. W.J. and L.L. helped in experiment. Y.L. and G.L. wrote the manuscript.
References
- Poole A. R. An introduction to the pathophysiology of osteoarthritis. Front Biosci 4, D662–670 (1999). [DOI] [PubMed] [Google Scholar]
- Laslett L. L. et al. A prospective study of the impact of musculoskeletal pain and radiographic osteoarthritis on health related quality of life in community dwelling older people. BMC Musculoskelet Disord 13, 168, doi: 10.1186/1471-2474-13-168 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C., Gao S. & Lei G. Association of osteopontin with osteoarthritis. Rheumatol Int 34, 1627–1631, doi: 10.1007/s00296-014-3036-9 (2014). [DOI] [PubMed] [Google Scholar]
- Gao S. G. et al. Usefulness of specific OA biomarkers, thrombin-cleaved osteopontin, in the posterior cruciate ligament OA rabbit model. Osteoarthritis Cartilage 21, 144–150, doi: 10.1016/j.joca.2012.09.006 (2013). [DOI] [PubMed] [Google Scholar]
- Xu M. et al. Phosphorylation of osteopontin in osteoarthritis degenerative cartilage and its effect on matrix metalloprotease 13. Rheumatol Int 33, 1313–1319, doi: 10.1007/s00296-012-2548-4 (2013). [DOI] [PubMed] [Google Scholar]
- Cheng C. et al. Osteopontin inhibits HIF-2alpha mRNA expression in osteoarthritic chondrocytes. Exp Ther Med 9, 2415–2419, doi: 10.3892/etm.2015.2434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S. G. et al. Effect of osteopontin on the mRNA expression of ADAMTS4 and ADAMTS5 in chondrocytes from patients with knee osteoarthritis. Exp Ther Med 9, 1979–1983, doi: 10.3892/etm.2015.2310 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. J. et al. Effect of osteopontin on TIMP-1 and TIMP-2 mRNA in chondrocytes of human knee osteoarthritis. Exp Ther Med 8, 391–394, doi: 10.3892/etm.2014.1750 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. et al. Effects of osteopontin on the expression of IL-6 and IL-8 inflammatory factors in human knee osteoarthritis chondrocytes. Eur Rev Med Pharmacol Sci 18, 3580–3586 (2014). [PubMed] [Google Scholar]
- Min T. U. et al. Correlation between osteopontin and caveolin-1 in the pathogenesis and progression of osteoarthritis. Exp Ther Med 9, 2059–2064, doi: 10.3892/etm.2015.2433 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. M. et al. MicroRNAs’ Involvement in Osteoarthritis and the Prospects for Treatments. Evid Based Complement Alternat Med 2015, 236179, doi: 10.1155/2015/236179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsezou A. Osteoarthritis year in review 2014: genetics and genomics. Osteoarthritis Cartilage 22, 2017–2024, doi: 10.1016/j.joca.2014.07.024 (2014). [DOI] [PubMed] [Google Scholar]
- Pillai R. S., Bhattacharyya S. N. & Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol 17, 118–126, doi: 10.1016/j.tcb.2006.12.007 (2007). [DOI] [PubMed] [Google Scholar]
- Iliopoulos D., Malizos K. N., Oikonomou P. & Tsezou A. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. PLoS ONE 3, e3740, doi: 10.1371/journal.pone.0003740 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgonio Cuadra V. M., Gonzalez-Huerta N. C., Romero-Cordoba S., Hidalgo-Miranda A. & Miranda-Duarte A. Altered expression of circulating microRNA in plasma of patients with primary osteoarthritis and in silico analysis of their pathways. PLoS ONE 9, e97690, doi: 10.1371/journal.pone.0097690 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaki S. et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev 24, 1173–1185, doi: 10.1101/gad.1915510 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N. et al. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum 62, 1361–1371, doi: 10.1002/art.27329 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki K. et al. Expression of MicroRNA-146a in osteoarthritis cartilage. Arthritis Rheum 60, 1035–1041, doi: 10.1002/art.24404 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. et al. The identification of differentially expressed microRNA in osteoarthritic tissue that modulate the production of TNF-alpha and MMP13. Osteoarthritis Cartilage 17, 464–472, doi: 10.1016/j.joca.2008.09.012 (2009). [DOI] [PubMed] [Google Scholar]
- Diaz-Prado S. et al. Characterization of microRNA expression profiles in normal and osteoarthritic human chondrocytes. BMC Musculoskelet Disord 13, 144, doi: 10.1186/1471-2474-13-144 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif G., Hum D., Pelletier J. P., Duval N. & Martel-Pelletier J. Regulation of the IGFBP-5 and MMP-13 genes by the microRNAs miR-140 and miR-27a in human osteoarthritic chondrocytes. BMC Musculoskelet Disord 10, 148, doi: 10.1186/1471-2474-10-148 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe M. A., Patarca R., Iregui M. V. & Cantor H. Polyclonal B cell activation by the Eta-1 cytokine and the development of systemic autoimmune disease. J Immunol 147, 2902–2906 (1991). [PubMed] [Google Scholar]
- O’Regan A. W., Nau G. J., Chupp G. L. & Berman J. S. Osteopontin (Eta-1) in cell-mediated immunity: teaching an old dog new tricks. Immunol Today 21, 475–478 (2000). [DOI] [PubMed] [Google Scholar]
- Gravallese E. M. Osteopontin: a bridge between bone and the immune system. J Clin Invest 112, 147–149, doi: 10.1172/JCI19190 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F. J. et al. The effect of hyaluronic acid on osteopontin and CD44 mRNA of fibroblast-like synoviocytes in patients with osteoarthritis of the knee. Rheumatol Int 33, 79–83, doi: 10.1007/s00296-011-2339-3 (2013). [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. & Noda M. Osteopontin expression and function: role in bone remodeling. J Cell Biochem Suppl 30-31, 92–102 (1998). [PubMed] [Google Scholar]
- Denhardt D. T. & Guo X. Osteopontin: a protein with diverse functions. FASEB J 7, 1475–1482 (1993). [PubMed] [Google Scholar]
- Sodek J. et al. Regulation of osteopontin expression in osteoblasts. Ann N Y Acad Sci 760, 223–241 (1995). [DOI] [PubMed] [Google Scholar]
- Standal T., Borset M. & Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol 26, 179–184 (2004). [PubMed] [Google Scholar]
- Chellaiah M. A. & Hruska K. A. The integrin alpha(v)beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int 72, 197–205, doi: 10.1007/s00223-002-1025-6 (2003). [DOI] [PubMed] [Google Scholar]
- Pullig O., Weseloh G., Gauer S. & Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: protein and mRNA analysis of normal and osteoarthritic cartilage. Matrix Biol 19, 245–255 (2000). [DOI] [PubMed] [Google Scholar]
- Gao S. G. et al. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis Cartilage 18, 82–87, doi: 10.1016/j.joca.2009.07.009 (2010). [DOI] [PubMed] [Google Scholar]
- Honsawek S., Tanavalee A., Sakdinakiattikoon M., Chayanupatkul M. & Yuktanandana P. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem 42, 808–812, doi: 10.1016/j.clinbiochem.2009.02.002 (2009). [DOI] [PubMed] [Google Scholar]
- Shevde L. A. et al. Spheroid-forming subpopulation of breast cancer cells demonstrates vasculogenic mimicry via hsa-miR-299-5p regulated de novo expression of osteopontin. J Cell Mol Med 14, 1693–1706, doi: 10.1111/j.1582-4934.2009.00821.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S. D. et al. Micro-RNA-181a regulates osteopontin-dependent metastatic function in hepatocellular cancer cell lines. Surgery 148, 291–297, doi: 10.1016/j.surg.2010.05.007 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus E. W. et al. miR181a protects against angiotensin II-induced osteopontin expression in vascular smooth muscle cells. Atherosclerosis 228, 168–174, doi: 10.1016/j.atherosclerosis.2013.01.037 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T. et al. Regulation of human autoimmune regulator (AIRE) gene translation by miR-220b. Gene 530, 19–25, doi: 10.1016/j.gene.2013.08.015 (2013). [DOI] [PubMed] [Google Scholar]
- Troppmann B., Kossack N., Nordhoff V., Schuring A. N. & Gromoll J. MicroRNA miR-513a-3p acts as a co-regulator of luteinizing hormone/chorionic gonadotropin receptor gene expression in human granulosa cells. Mol Cell Endocrinol 390, 65–72, doi: 10.1016/j.mce.2014.04.003 (2014). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. MicroRNA-548 down-regulates host antiviral response via direct targeting of IFN-lambda1. Protein Cell 4, 130–141, doi: 10.1007/s13238-012-2081-y (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabler J. et al. Stage-specific miRs in chondrocyte maturation: differentiation-dependent and hypertrophy-related miR clusters and the miR-181 family. Tissue Eng Part A, doi: 10.1089/ten.TEA.2015.0352 (2015). [DOI] [PubMed] [Google Scholar]
- Willers I. M., Martinez-Reyes I., Martinez-Diez M. & Cuezva J. M. miR-127-5p targets the 3’UTR of human beta-F1-ATPase mRNA and inhibits its translation. Biochim Biophys Acta 1817, 838–848, doi: 10.1016/j.bbabio.2012.03.005 (2012). [DOI] [PubMed] [Google Scholar]
- Park S. J., Cheon E. J., Lee M. H. & Kim H. A. MicroRNA-127-5p regulates matrix metalloproteinase 13 expression and interleukin-1beta-induced catabolic effects in human chondrocytes. Arthritis Rheum 65, 3141–3152, doi: 10.1002/art.38188 (2013). [DOI] [PubMed] [Google Scholar]
- Ren W. K. et al. Dietary L-glutamine supplementation increases Pasteurella multocida burden and the expression of its major virulence factors in mice. Amino Acids 45, 947–955, doi: Doi 10.1007/S00726-013-1551-8 (2013). [DOI] [PubMed] [Google Scholar]
- Ren W. K. et al. Dietary L-glutamine supplementation improves pregnancy outcome in mice infected with type-2 porcine circovirus. Amino Acids 45, 479–488, doi: Doi 10.1007/S00726-011-1134-5 (2013). [DOI] [PubMed] [Google Scholar]
- Ren W. K. et al. Glutamine modifies immune responses of mice infected with porcine circovirus type 2. British Journal of Nutrition 110, 1053–1060, doi: Doi 10.1017/S0007114512006101 (2013). [DOI] [PubMed] [Google Scholar]
- Ren W. et al. Dietary arginine supplementation of mice alters the microbial population and activates intestinal innate immunity. J Nutr 144, 988–995, doi: 10.3945/jn.114.192120 (2014). [DOI] [PubMed] [Google Scholar]
- Ren W. et al. Dietary L-glutamine supplementation modulates microbial community and activates innate immunity in the mouse intestine. Amino Acids, doi: 10.1007/s00726-014-1793-0 (2014). [DOI] [PubMed] [Google Scholar]
- Ren W. et al. Serum amino acids profile and the beneficial effects of L-arginine or L-glutamine supplementation in dextran sulfate sodium colitis. PLoS ONE 9, e88335, doi: 10.1371/journal.pone.0088335 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W. et al. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 42, 2089–2094, doi: 10.1007/s00726-011-0942-y (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W. et al. DNA vaccine encoding the major virulence factors of Shiga toxin type 2e (Stx2e)-expressing Escherichia coli induces protection in mice. Vaccine 31, 367–372, doi: 10.1016/j.vaccine.2012.10.107 (2013). [DOI] [PubMed] [Google Scholar]
- Ren W. et al. Dietary arginine supplementation enhances immune responses to inactivated Pasteurella multocida vaccination in mice. Br J Nutr 109, 867–872, doi: 10.1017/S0007114512002681 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.