Abstract
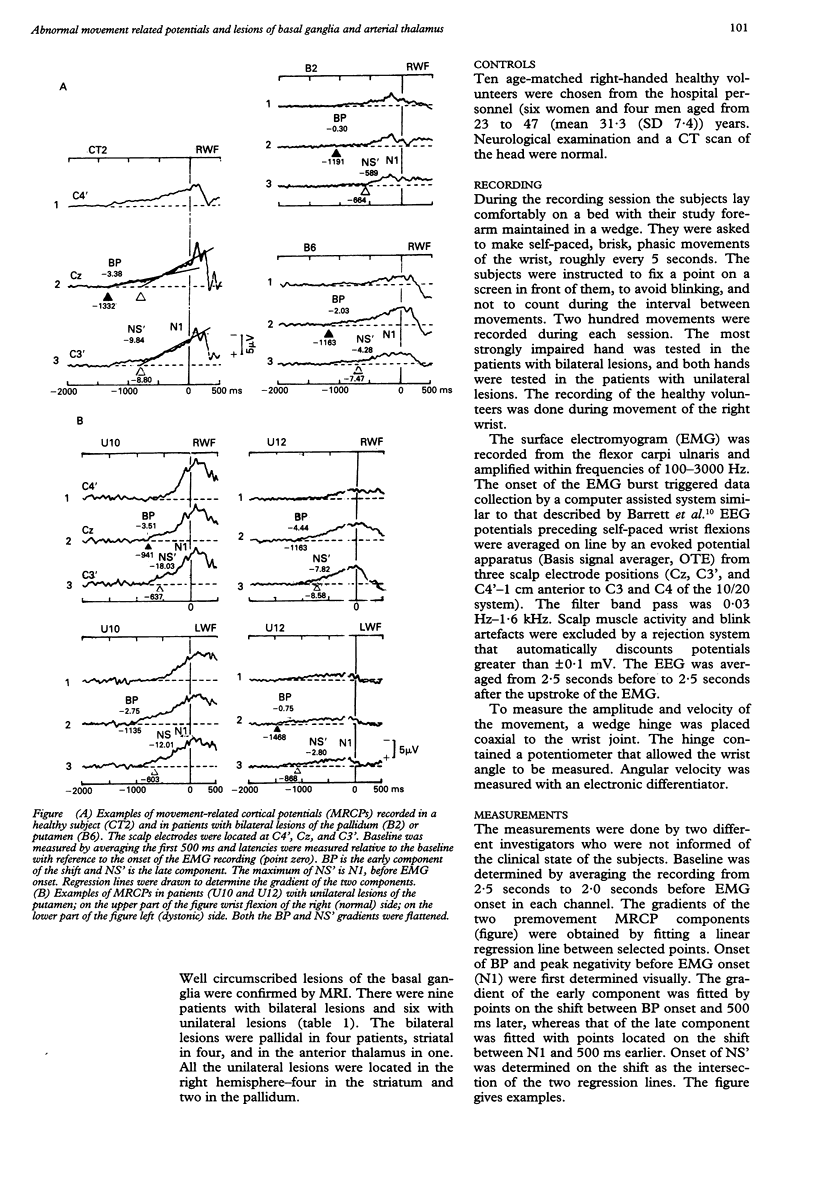
Movement-related cortical potentials (MRCPs) were recorded from scalp electrodes during wrist flexion in 15 dystonic patients with bilateral (nine) or unilateral (six) circumscribed lesions in the striatum (eight), pallidum (six), or anterior thalamus (one). The results were compared with those of 10 age-matched healthy volunteers. The early (BP) and late (NS') MRCP components were assessed in terms of their gradients and distribution on the scalp in Cz, C3', and C4'. The gradients of both BP and NS' components were significantly flatter in the patients with bilateral lesions than in the control subjects. Also, the BP gradient was maximum at Cz, and the NS' component was contralaterally predominant in the control subjects but not in the patients. In patients with unilateral lesions, the gradients were flatter (p < 0.05) during movement of the dystonic wrist than during movement of the normal wrist. This difference was significant for BP and NS', regardless of the location of the electrodes. Also, the normal topographic predominance of BP at Cz and of contralateral NS' disappeared. The BP and NS' components of the MRCPs are thought to reflect preparatory activity in the supplementary motor area and the primary motor cortex before movement. Reduced BP and NS' gradients in patients with both bilateral and unilateral lesions of the basal ganglia, which project towards the supplementary motor area, are consistent with this hypothesis. The bilateral nature of these reductions suggests that both the ipsilateral and the contralateral motor cortex are involved in the genesis of the MRCPs and that the dystonia in these patients is associated with impaired motor preparation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett G., Shibasaki H., Neshige R. A computer-assisted method for averaging movement-related cortical potentials with respect to EMG onset. Electroencephalogr Clin Neurophysiol. 1985 Mar;60(3):276–281. doi: 10.1016/0013-4694(85)90042-2. [DOI] [PubMed] [Google Scholar]
- Deecke L., Kornhuber H. H. An electrical sign of participation of the mesial 'supplementary' motor cortex in human voluntary finger movement. Brain Res. 1978 Dec 29;159(2):473–476. doi: 10.1016/0006-8993(78)90561-9. [DOI] [PubMed] [Google Scholar]
- Dick J. P., Cantello R., Buruma O., Gioux M., Benecke R., Day B. L., Rothwell J. C., Thompson P. D., Marsden C. D. The Bereitschaftspotential, L-DOPA and Parkinson's disease. Electroencephalogr Clin Neurophysiol. 1987 Mar;66(3):263–274. doi: 10.1016/0013-4694(87)90075-7. [DOI] [PubMed] [Google Scholar]
- Dick J. P., Rothwell J. C., Day B. L., Cantello R., Buruma O., Gioux M., Benecke R., Berardelli A., Thompson P. D., Marsden C. D. The Bereitschaftspotential is abnormal in Parkinson's disease. Brain. 1989 Feb;112(Pt 1):233–244. doi: 10.1093/brain/112.1.233. [DOI] [PubMed] [Google Scholar]
- Feve A. P., Bathien N., Rondot P. Chronic administration of L-dopa affects the movement-related cortical potentials of patients with Parkinson's disease. Clin Neuropharmacol. 1992 Apr;15(2):100–108. doi: 10.1097/00002826-199204000-00003. [DOI] [PubMed] [Google Scholar]
- Fève A. P., Fénelon G., Wallays C., Rémy P., Guillard A. Axial motor disturbances after hypoxic lesions of the globus pallidus. Mov Disord. 1993 Jul;8(3):321–326. doi: 10.1002/mds.870080311. [DOI] [PubMed] [Google Scholar]
- Hallett M., Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980 Jun;103(2):301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Ikeda A., Lüders H. O., Burgess R. C., Shibasaki H. Movement-related potentials recorded from supplementary motor area and primary motor area. Role of supplementary motor area in voluntary movements. Brain. 1992 Aug;115(Pt 4):1017–1043. doi: 10.1093/brain/115.4.1017. [DOI] [PubMed] [Google Scholar]
- KORNHUBER H. H., DEECKE L. HIRNPOTENTIALAENDERUNGEN BEI WILLKUERBEWEGUNGEN UND PASSIVEN BEWEGUNGEN DES MENSCHEN: BEREITSCHAFTSPOTENTIAL UND REAFFERENTE POTENTIALE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1965 May 10;284:1–17. [PubMed] [Google Scholar]
- Künzle H. An autoradiographic analysis of the efferent connections from premotor and adjacent prefrontal regions (areas 6 and 9) in macaca fascicularis. Brain Behav Evol. 1978;15(3):185–234. doi: 10.1159/000123779. [DOI] [PubMed] [Google Scholar]
- Leenders K. L., Palmer A. J., Quinn N., Clark J. C., Firnau G., Garnett E. S., Nahmias C., Jones T., Marsden C. D. Brain dopamine metabolism in patients with Parkinson's disease measured with positron emission tomography. J Neurol Neurosurg Psychiatry. 1986 Aug;49(8):853–860. doi: 10.1136/jnnp.49.8.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B., Gleason C. A., Wright E. W., Pearl D. K. Time of conscious intention to act in relation to onset of cerebral activity (readiness-potential). The unconscious initiation of a freely voluntary act. Brain. 1983 Sep;106(Pt 3):623–642. doi: 10.1093/brain/106.3.623. [DOI] [PubMed] [Google Scholar]
- Neshige R., Lüders H., Shibasaki H. Recording of movement-related potentials from scalp and cortex in man. Brain. 1988 Jun;111(Pt 3):719–736. [PubMed] [Google Scholar]
- Roland P. E., Larsen B., Lassen N. A., Skinhøj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980 Jan;43(1):118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- Schell G. R., Strick P. L. The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci. 1984 Feb;4(2):539–560. doi: 10.1523/JNEUROSCI.04-02-00539.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki H., Barrett G., Halliday E., Halliday A. M. Components of the movement-related cortical potential and their scalp topography. Electroencephalogr Clin Neurophysiol. 1980 Aug;49(3-4):213–226. doi: 10.1016/0013-4694(80)90216-3. [DOI] [PubMed] [Google Scholar]
- Vaughan H. G., Jr, Costa L. D., Ritter W. Topography of the human motor potential. Electroencephalogr Clin Neurophysiol. 1968 Jul;25(1):1–10. doi: 10.1016/0013-4694(68)90080-1. [DOI] [PubMed] [Google Scholar]
- Wolfson L. I., Leenders K. L., Brown L. L., Jones T. Alterations of regional cerebral blood flow and oxygen metabolism in Parkinson's disease. Neurology. 1985 Oct;35(10):1399–1405. doi: 10.1212/wnl.35.10.1399. [DOI] [PubMed] [Google Scholar]
- van der Kamp W., Berardelli A., Rothwell J. C., Thompson P. D., Day B. L., Marsden C. D. Rapid elbow movements in patients with torsion dystonia. J Neurol Neurosurg Psychiatry. 1989 Sep;52(9):1043–1049. doi: 10.1136/jnnp.52.9.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]



