Abstract
Maternal obesity increases the risk of obesity and/or obesity-related diseases in the offspring of animal models. The aim of this study was to identify metabolic dysfunctions that could represent an enhanced risk for human obesity or obesity-related diseases in newborn or in adult life, similar to what occurs in animal models. To this aim, we studied the proteome of 12 obese (Ob-) and 6 non-obese (Co-) human amniotic mesenchymal stem cells (hA-MSCs) obtained from women at delivery by cesarean section (pre-pregnancy body mass index [mean ± SD]: 42.7 ± 7.7 and 21.3 ± 3.3 kg/m2, respectively). The proteome, investigated by two-dimensional fluorescence difference gel electrophoresis/mass spectrometry, revealed 62 differently expressed proteins in Ob- vs Co-hA-MSCs (P < 0.05), nine of which were confirmed by western blotting. Bioinformatics analysis showed that these 62 proteins are involved in several statistically significant pathways (P < 0.05), including the stress response, cytoskeleton and metabolic pathways. Oxidative stress was shown to be an early triggering factor of tissue fat accumulation and obesity-related disorders in the offspring of obese animal models. Our finding of a reduced stress response in Ob-hA-MSCs suggests that a similar mechanism could occur also in humans. Long-term follow-up studies of newborns of obese mothers are required to verify this hypothesis.
Obesity is a worldwide epidemic health problem, and up to 60% of pregnant women are obese/overweight before pregnancy1. Clinical studies and findings obtained in animal models suggest that developmental programming in the presence of maternal obesity and nutrient excess in utero increases the risk of offspring to develop obesity and/or obesity-associated metabolic diseases later in life2,3. In humans, amnion from placental tissue is the main interface between the fetus and mother: it regulates intrauterine development and modulates adaptive responses to suboptimal in utero conditions such as obesity and/or an obesogenic diet4. Accordingly, during obesity, placental tissues undergo epigenetic and proteome alterations that involve pathways that are crucial for placental function and fetal growth5,6. Amnion is a source of fetal mesenchymal stem cells (hA-MSCs) that have a close ontogenic relationship with embryonic stem cells, and unlike the latter, they are accessible without ethical problems because the placenta is usually discarded at birth7. Furthermore, hA-MSCs have multipotent differentiation potential, including the potential to differentiate in adipocytes, and are thus a useful cellular model with which to investigate dysfunctions in adipose tissue during obesity7. In particular, studies on hA-MSCs could reveal the metabolic alterations that, if present at perinatal level, could impact on the fetal developmental program.
We recently reported that the adipogenic potential of hA-MSCs isolated from obese women (Ob-hA-MSCs) was higher than that of hA-MSCs from lean control women (Co-hA-MSCs)8. In detail, we demonstrated that high levels of CD13/aminopeptidase N on the Ob-hA-MSC surface, measured by immunophenotyping, resulted in enhanced adipogenesis of these cells, which, in turn, could be related to the pathogenesis of obesity8. Based on these encouraging results, we analyzed the entire proteome of Ob-hA-MSCs in the attempt to identify metabolic dysfunctions that, being present at perinatal level, could represent an enhanced risk for obesity or for obesity-related diseases in the newborn or in adult life, similar to what occurs in animal models3.
Results
Several anamnestic and biochemical characteristics (Supplementary Table S1), recorded immediately before delivery, were similar in the two groups of enrolled pregnant women, whereas serum leptin (P = 0.002) and the L/A ratio (P = 0.01) were significantly higher, and serum adiponectin lower, albeit not significantly so, in obese women than in non-obese women. The mean (±SD) pre-pregnancy body mass index (BMI, kg/m2) was higher [42.7(7.7) vs 21.3(3.3), P < 0.0001], and the weight gain in pregnancy was lower (9.5 vs 14.0) in obese than in non-obese women, as recommended by guidelines9 (Supplementary Table S1). Birth weight and length, head circumference, and the 1 and 5 min Apgar indexes did not differ between Ob- and Co-newborns (Supplementary Table S1).
Protein profile of hA-MSCs
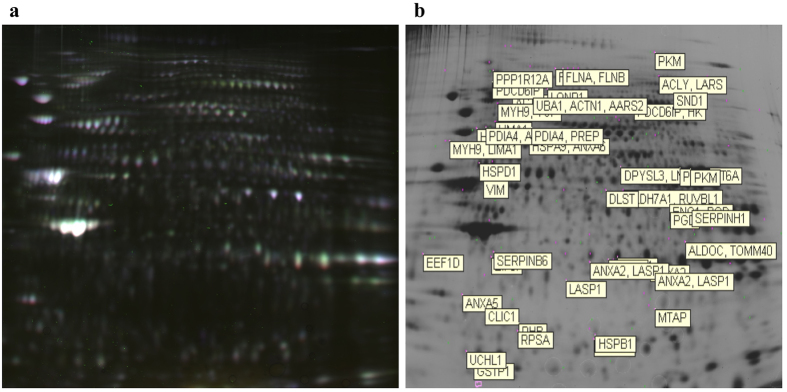
The master gel used to match the whole set of two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) profiles obtained in Ob-hA-MSCs and Co-hA-MSCs is shown in Fig. 1a,b. The analysis performed with DeCyder Software revealed approximately 7000 protein spots per gel in the 3–10 pH range. Approximately 2384 spots were matched in all six analytical gels. Spots at the extreme right and left sides of the gel were excluded from the evaluation. The DeCyder statistical analysis revealed 159 spots differentially expressed at a statistical significant level (P < 0.05), with an average ratio ≥1.2 for up-expressed and ≤−1.2 for down-expressed protein spots, in Ob-hA-MSCs versus Co-hA-MSCs. The preparative gel was stained with Comassie Brilliant-blue and used for automated spot picking. After excluding 43 spots for their low abundance, we focused on 116 spots that were very abundant on the analytical gel or on the preparative gel. The isolated spots were digested with trypsin and analyzed with mass spectrometry followed by database search. Sixty protein spots were considered informative.
Figure 1. Two-Dimensional Fluorescence Difference Gel Electrophoresis of hA-MSC proteins.
(a) Scan of the master gel used to match protein spots from the six analytical gels used for image analysis. This gel is constituted by three overlapping images: (1) proteins from obese hA-MSCs labeled with Cy3 (green); (2) proteins from non-obese hA-MSCs labeled with Cy5 (red); and (3) proteins from a pool of all samples labeled with Cy2 (blue) (used for normalization). (b) The NCBI gene symbol for each differently expressed protein is reported as indicated in Table 1.
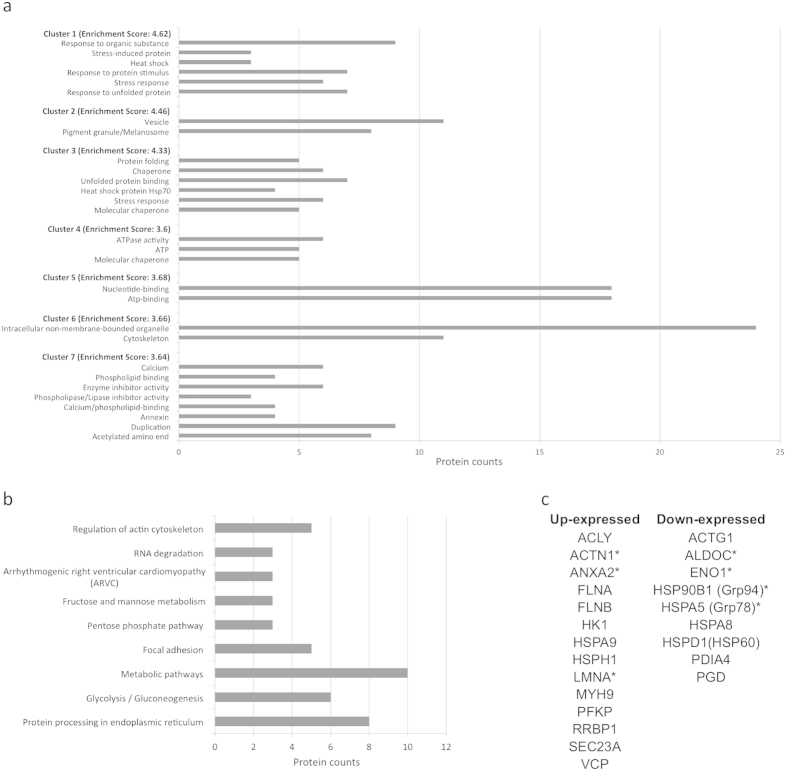
The relative expression ratios of each informative spot (Ob- vs Co-hA-MSCs), their statistical significant values (P < 0.05) and general characteristics of the proteins identified in each spot are listed in Table 1. Sixty-two per cent (37/60) of the spots were up-expressed, and 38% (23/60) were down-expressed. A total of 62 proteins was identified. We used the DAVID and KEGG databases to identify the statistically significant pathways (P < 0.001) containing the differentially expressed proteins (Supplementary Table S2). Among the pathways predicted by DAVID we arbitrarily considered only those grouped in highly scored clusters (4.62–3.64 enrichment score): cluster 1 (response to unfolded protein; stress response; response to protein stimulus; heat shock; stress-induced protein; response to organic substance); cluster 2 (pigment granule/melanosome; vesicle); cluster 3 (molecular chaperone; stress response; heat shock protein 70; unfolded protein binding; Chaperone; protein folding); cluster 4 (molecular chaperone; ATP; ATPase activity); cluster 5 (ATP-binding; nucleotide-binding); cluster 6 (cytoskeleton; intracellular non-membrane-bounded organelle); and cluster 7 (acetylated amino end; duplication; annexin; calcium binding; phospholipase/lipase inhibitor activity; enzyme inhibitor activity; phospholipid binding; calcium) (Fig. 2a). Figure 2b shows the statistical significant pathways (P < 0.05) predicted by KEGG: protein processing in the endoplasmic reticulum; glycolysis/gluconeogenesis; metabolic pathways; focal adhesion; the pentose phosphate pathway; fructose and mannose metabolism; arrhythmogenic right ventricular cardiomyopathy; RNA degradation; regulation of actin cytoskeleton. The proteins involved in statistical significant pathways predicted by both tools are listed in Fig. 2c.
Table 1. Identification and characterization of proteins differently expressed in Obese hA-MSCs vs Non-obese hA-MSCs.
| Spot no.a | DIGE Obese/Non-obese hAMSCsb | DIGE (P-value)c | Protein name | Gene Symbold | MW (Da)e | PIf | Gene IDg | Protein codeh |
|---|---|---|---|---|---|---|---|---|
| 3114 | 1.96 | 0.029 | Annexin A5 | ANXA5 | 35936 | 4.93 | 308 | P08758 |
| 873 | 1.86 | 0.024 | Alpha-actinin-1 | ACTN1 | 103057 | 5.25 | 87 | P12814 |
| 2710 | 1.77 | 0.031 | Serpin B6 | SERPINB6 | 42621 | 5.18 | 5269 | P35237 |
| 2748 | 1.77 | 0.031 | Annexin A1 | ANXA1 | 38714 | 6.57 | 301 | P04083 |
| 3711 | 1.66 | 0.0037 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | UCHL1 | 24824 | 5.33 | 7345 | P09936 |
| 1536 | 1.63 | 0.026 | Myosin-9 | MYH9 | 226532 | 5.50 | 4627 | P35579 |
| LIM domain and actin-binding protein 1 | LIMA1 | 85225 | 6.41 | 51474 | Q9UHB6 | |||
| 489 | 1.59 | 0.022 | Filamin-A | FLNA | 280739 | 5.70 | 2316 | P21333 |
| Filamin-B | FLNB | 278164 | 5.47 | 2317 | O75369 | |||
| 662 | 1.49 | 0.00067 | Heat shock protein 105 kDa | HSPH1 | 96864 | 5.27 | 10808 | Q92598 |
| 3335 | 1.49 | 0.041 | Prohibitin | PHB | 29804 | 5.57 | 5245 | P35232 |
| 660 | 1.47 | 0.0099 | Programmed cell death 6-interacting protein | PDCD6IP | 96023 | 6.13 | 10015 | Q8WUM4 |
| 2879 | 1.47 | 0,0042 | Elongation factor 1-delta | EEF1D | 31121 | 4.90 | 1936 | P29692 |
| 2730 | 1.47 | 0.049 | Heterogeneous nuclear ribonucleoprotein A1 | HNRNPA1 | 38746 | 9.17 | 3178 | P09651 |
| 522 | 1.44 | 0.0018 | Protein phosphatase 1 regulatory subunit 12A | PPP1R12A | 115281 | 5.31 | 4659 | O14974 |
| 1823 | 1.41 | 0.036 | Dihydropyrimidinase-related protein 3 | DPYSL3 | 61963 | 6.04 | 1809 | Q14195 |
| Prelamin-A/C | LMNA | 74139 | 6.57 | 4000 | P02545 | |||
| T-complex protein 1 subunit zeta | CCT6A | 58024 | 6.24 | 908 | P40227 | |||
| 2813 | 1.40 | 0.022 | Annexin A2 | ANXA2 | 38604 | 7.57 | 302 | P07355 |
| LIM and SH3 domain protein 1 | LASP1 | 29717 | 6.61 | 3927 | Q14847 | |||
| 521 | 1.39 | 0.0012 | Protein phosphatase 1 regulatory subunit 12A | PPP1R12A | 115281 | 5.31 | 4659 | O14974 |
| 1047 | 1.38 | 0.0056 | Myosin-9 | MYH9 | 226532 | 5.50 | 4627 | P35579 |
| Transitional endoplasmic reticulum ATPase | VCP | 89265 | 5.14 | 7415 | P55072 | |||
| 492 | 1.37 | 0.0056 | Filamin-A | FLNA | 280739 | 5.70 | 2316 | P21333 |
| Filamin-B | FLNB | 278164 | 5.47 | 2317 | O75369 | |||
| 2925 | 1.37 | 0.053 | Annexin A2 | ANXA2 | 38604 | 7.57 | 302 | P07355 |
| LIM and SH3 domain protein 1 | LASP1 | 29717 | 6.61 | 3927 | Q14847 | |||
| 574 | 1.33 | 0.040 | Ribosome-binding protein 1 | RRBP1 | 152472 | 8.69 | 6238 | Q9P2E9 |
| 1996 | 1.32 | 0.049 | Vimentin | VIM | 53651 | 5.05 | 7431 | P08670 |
| 452 | 1.28 | 0.0017 | Nodal modulator 1 | NOMO1 | 134324 | 5.54 | 23420 | Q15155 |
| 918 | 1.27 | 6.4e–005 | Ubiquitin-like modifier-activating enzyme 1 | UBA1 | 117849 | 5.49 | 7317 | P22314 |
| Alpha-actinin-1 | ACTN1 | 103057 | 5.25 | 87 | P12814 | |||
| Alanine-tRNA ligase, mitochondrial | AARS2 | 107340 | 5.87 | 57505 | Q5JTZ9 | |||
| 1395 | 1.27 | 0.0018 | ATP-dependent 6-phosphofructokinase, platelet type | PFKP | 85596 | 7.50 | 5214 | Q01813 |
| Protein transport protein Sec23A | SEC23A | 86160 | 6.64 | 10484 | Q15436 | |||
| 3408 | 1.26 | 0.0013 | 40S ribosomal protein SA | RPSA | 32854 | 4.79 | 3921 | P08865 |
| 1866 | 1.26 | 0.022 | Pyruvate kinase PKM | PKM | 57937 | 7.96 | 5315 | P14618 |
| 2861 | 1.26 | 0.049 | Annexin A2 | ANXA2 | 38604 | 7.57 | 302 | P07355 |
| 2955 | 1.25 | 0.038 | LIM and SH3 domain protein 1 | LASP1 | 29717 | 6.61 | 3927 | Q14847 |
| 1476 | 1.23 | 0.0029 | Stress-70 protein, mitochondrial | HSPA9 | 73680 | 5.87 | 3313 | P38646 |
| Annexin A6 | ANXA6 | 75873 | 5.41 | 309 | P08133 | |||
| 604 | 1.23 | 0.0032 | ATP-citrate syntase | ACLY | 120839 | 6.95 | 47 | P53396 |
| Leucine—tRNA ligase,cytoplasmic | LARS | 134466 | 6.95 | 51520 | Q9P2J5 | |||
| 279 | 1.23 | 0.014 | Pyruvate kinase PKM | PKM | 57937 | 7.96 | 5315 | P14618 |
| 903 | 1.22 | 0.0011 | Staphylococcal nuclease domain-containing protein 1 | SND1 | 101996 | 6.74 | 27044 | Q7KZF4 |
| 2769 | 1.22 | 0.016 | Eukaryotic translation initiation factor 3, subunit I | EIF3I | 36501 | 5.38 | 8668 | Q13347 |
| 456 | 1.21 | 0.0005 | Leucine-rich PPR motif-containing protein, mitochondrial | LRPRRC | 157905 | 5.81 | 10128 | P42704 |
| 1874 | 1.21 | 0.018 | Pyruvate kinase PKM | PKM | 57937 | 7.96 | 5315 | P14618 |
| 1236 | 1.20 | 0.0079 | LIM domainand actin-binding protein 1 | LIMA1 | 85225 | 6.41 | 51474 | Q9UHB6 |
| 1017 | 1.20 | 0.019 | Programmed cell death 6-interacting protein | PDCD6IP | 96023 | 6.13 | 10015 | Q8WUM4 |
| Hexokinase-1 | HK1 | 102485 | 6.36 | 3098 | P19367 | |||
| 2770 | −6.13 | 0.041 | Annexin A1 | ANXA1 | 38714 | 6.57 | 301 | P04083 |
| 3162 | −3.34 | 0.041 | Chloride intracellular channel protein 1 | CLIC1 | 26922 | 5.09 | 1192 | O00299 |
| 3248 | −2.21 | 0.051 | S-methyl-5′-thioadenosine phosphorylase | MTAP | 31236 | 6.75 | 4507 | Q13126 |
| 1385 | −2.05 | 0.019 | 78 kDa glucose-regulated protein | HSPA5 (Grp78) | 72332 | 5.07 | 3309 | P11021 |
| 3412 | −1.93 | 0.0030 | Heat shock protein beta-1 | HSPB1 (HSP27) | 22782 | 5.98 | 3315 | P04792 |
| 3452 | −1.70 | 0.00024 | Heat shock protein beta-1 | HSPB1 (HSP27) | 22782 | 5.98 | 3315 | P04792 |
| 3794 | −1.59 | 0.031 | Chain A, Three-Dimensional Structure Of Class Pi Glutathione S-Transferase From Human Placenta (Glutathione S-transferase P) | GSTP1 | 23355 | 5.43 | 2950 | P09211 |
| 2345 | −1.55 | 0.048 | 6-phosphogluconate dehydrogenase, decarboxylating | PGD | 53139 | 6.8 | 5226 | P52209 |
| 2606 | −1.42 | 0.029 | Fructose-bisphosphate aldolase C | ALDOC | 39455 | 6.41 | 230 | P09972 |
| Mitochondrial import receptor subunit TOM40 homolog | TOMM40 | 37893 | 6.79 | 10452 | O96008 | |||
| 2326 | −1.41 | 0.0093 | Serpin H1 | SERPINH1 | 46440 | 8.75 | 871 | P50454 |
| 798 | −1.39 | 0.046 | Lon protease homolog, mitochondrial | LONP1 | 106489 | 6.01 | 9361 | P36776 |
| 1243 | −1.37 | 0.0038 | Ran GTPase-activating protein 1 | RANGAP1 | 63541 | 4.63 | 5905 | P46060 |
| 2090 | −1.36 | 0.041 | Alpha-aminoadipic semialdehyde dehydrogenase | ALDH7A1 | 58487 | 8.21 | 501 | P49419 |
| RuvB-like 1 | RUVBL1 | 50228 | 6.02 | 8607 | Q9Y265 | |||
| 2125 | −1.35 | 0.017 | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex, mitochondrial | DLST | 48755 | 9.10 | 1743 | P36957 |
| 1245 | −1.35 | 0.023 | Caldesmon | CALD1 | 93231 | 5.62 | 800 | Q05682 |
| 1310 | −1.31 | 0.035 | Protein disulfide-isomerase A4 | PDIA4 | 72932 | 4.96 | 9601 | P13667 |
| Acylamino-acid-relasing enzyme | APEH | 81224 | 5.29 | 327 | P13798 | |||
| 829 | −1.30 | 0.028 | Endoplasmin | HSP90B1 (Grp94) | 92468 | 4.76 | 7184 | P14625 |
| 1408 | −1.28 | 0.00018 | 79 kDa glucose-regulated protein | HSPA5 (Grp78) | 72332 | 5.07 | 3309 | P11022 |
| 2258 | −1.26 | 0.028 | Alpha-enolase | ENO1 | 47168 | 7.01 | 2023 | P06733 |
| 6-phosphogluconate dehydrogenase, decarboxylating | PGD | 53139 | 6.8 | 5226 | P52209 | |||
| 1327 | −1.23 | 0.014 | Protein disulfide-isomerase A4 | PDIA4 | 72932 | 4.96 | 9601 | P13667 |
| Prolyl endopeptidase | PREP | 80699 | 5.53 | 5550 | P48147 | |||
| 2621 | −1.23 | 0.049 | Actin, cytoplasmic 2 | ACTG1 | 41792 | 5.31 | 71 | P63261 |
| COP9 signalosome complex subunit 4 | COPS4 | 46268 | 5.57 | 51138 | Q9BT78 | |||
| 1786 | −1.22 | 0.0028 | 60 kDa heat shock protein, mitochondrial | HSPD1 (HSP60) | 61054 | 5.70 | 3329 | P10809 |
| 1377 | −1.21 | 0.049 | 78 kDa glucose-regulated protein | HSPA5 (Grp78) | 72332 | 5.07 | 3309 | P11021 |
| Heat shock cognate 71 kDa protein | HSPA8 | 70898 | 5.37 | 3312 | P11142 |
aSpot numbering to indicate the positions of spots in preparative gel.
bAverage abundance ratio (Obese/Non-obese hA-MSCs) as calculated by DeCyder Analysis.
cP-value at Student’s t test.
dGene symbol from NCBI.
eTheoretical molecular weight (Da).
fTheoretical pI.
gGene ID from NCBI.
hProtein code from Swiss Prot.
Figure 2. Pathways predicted by the DAVID and KEGG databases and list of proteins involved in pathways predicted by both tools.
(a) Functional annotation clustering obtained with DAVID: cluster 1 (response to unfolded protein; stress response; response to protein stimulus; heat shock; stress-induced protein; response to organic substance); cluster 2 (pigment granule/melanosome; vesicle); cluster 3 (molecular chaperone; stress response; heat shock protein 70; unfolded protein binding; chaperone; protein folding); cluster 4 (molecular chaperone; ATP; ATPase activity); cluster 5 (ATP-binding; nucleotide-binding); cluster 6 (cytoskeleton; intracellular non-membrane-bounded organelle); and cluster 7 (acetylated amino end; duplication; annexin; calcium binding; phospholipase/lipase inhibitor activity; enzyme inhibitor activity; phospholipid binding; calcium). (b) Statistically significant pathways predicted by KEGG: protein processing in the endoplasmic reticulum; glycolysis/gluconeogenesis; metabolic pathways; focal adhesion; the pentose phosphate pathway; fructose and mannose metabolism; arrhythmogenic right ventricular cardiomyopathy; RNA degradation; regulation of actin cytoskeleton. Grey bar represent the protein counts, that is the number of proteins included in each pathway. (c) List of proteins involved in statistical significant pathways predicted by both tools. *Proteins tested by Western Blot.
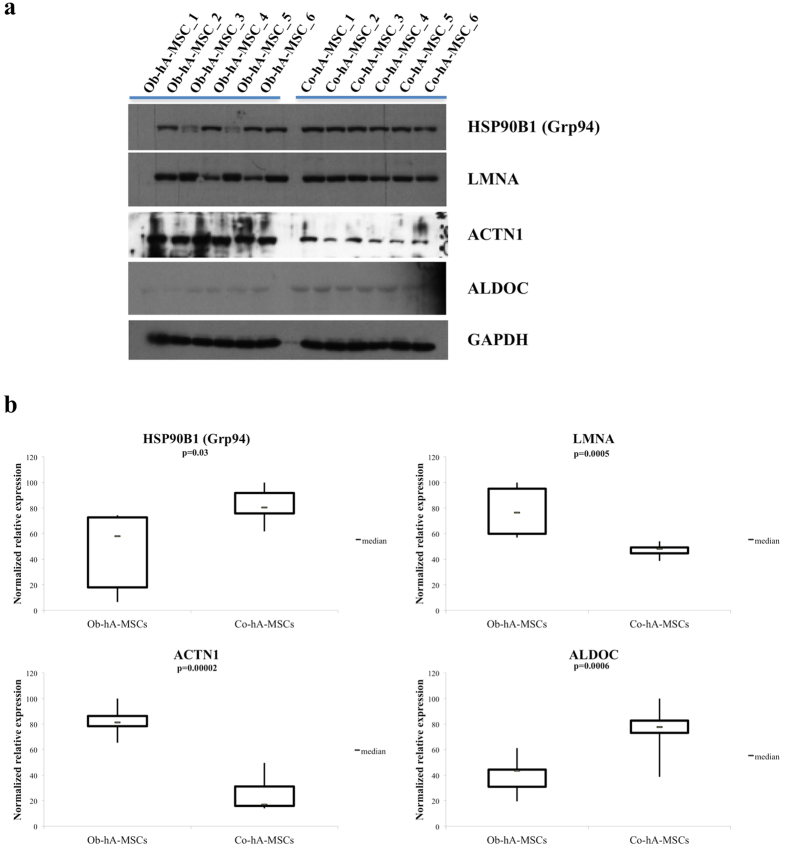
To confirm the protein levels obtained at 2D-DIGE, we measured the expression of nine proteins [4/9 up-expressed: pyruvate kinase (PKM), alpha-actinin 1 (ACTN1), prelamin A/C (LMNA), annexin A2 (ANXA2)], and 5/9 down-expressed: endoplasmin (HSP90B1/Grp94), aldolase C (ALDOC), heat-shock protein beta-1 (HSPB1/HSP27), 79 kDa glucose-regulated protein (HSPA5/Grp78), enolase 1 (ENO1)] in Ob- versus Co-hA-MSCs], by western blot (WB). The proteins were selected by arbitrary selection criteria: 7/9 proteins predicted by both DAVID and KEGG tools, 1/9 of those predicted only by DAVID and 1/9 of those predicted only by KEGG. For all nine tested proteins, the expression levels obtained at WB analysis confirmed the trend obtained at 2D-DIGE, and the differences in the WB expression levels (mean/SEM) between Ob- and Co-hA-MSCs were statistically significant (P < 0.05) for 4/9 tested proteins: HSP90B1 (0.23/0.06 and 0.41/0.03), LMNA (0.86/0.23 and 0.52/0.05), ACTN1 (1.41/0.08 and 0.43/0.10) and ALDOC (0.62/0.09 and 1.16/0.13) (Fig. 3a,b; Supplementary Figure S1). Full-length blots of each tested protein are reported in Supplementary Figure S2.
Figure 3. Western blot evaluation of selected proteins.
(a) Western blot showing statistically significant different levels (P < 0.05) of four proteins (HSP90B1, LMNA, ACTN1 and ALDOC) in Ob-hA-MSCs vs Co-hA-MSCs. In the figure are reported the cropped gels/blots obtained by each protein evaluation. All gels were run in the same experimental conditions (see material and methods for details). (Full-length blots of each tested protein are reported in Supplementary Figure S2). (b) Quantification of the expression of the tested four proteins. The optical density of each sample was measured and normalized using a GAPDH run on the same gel. The data are expressed as percent relative expression, i.e. for each protein, the sample with the highest expression of that protein was set at 100%. The bottom and top of each box represent the 25th and 75th percentile, respectively; the thick band within each box shows the 50th percentile (the median). The ends of the whiskers represent the minimum and maximum values of each group of data.
Discussion
In this study, we show that the proteome of hA-MSCs isolated from obese women at delivery differs significantly from that of control women. This finding, together with our previous report of enhanced adipogenesis in Ob-hA-MSCs and of an altered miRNA expression profile in amnion of obese women at delivery5,8, helps to shed light on the alterations that occur in the intrauterine environment in human obesity.
Globally, we identified, at 2D-DIGE followed by mass spectrometry, 62 proteins that were differently expressed in Ob- vs Co-hA-MSCs, and that were predicted by bioinformatics to alter many relevant cellular pathways. However, given the large number of proteins/pathways predicted to be deregulated, we focused our efforts on three groups of proteins that are involved in the stress response, the cytoskeleton and metabolism, respectively, that, based on findings obtained in pregnant obese animal models and their offspring10,11, and in tissues from adult obese subjects6, could be of significance in predisposing newborn of obese women to obesity or to obesity-related diseases.
Regarding the stress response, we found that a group of HSP proteins involved in this pathway (HSPA8, HSPD1, HSPB1, HSP90B1 and HSPA5) were down-expressed in Ob- vs Co-hA-MSCs. Heat shock proteins have been linked to obesity, inflammation and insulin resistance12. Heat shock proteins, which are stress-induced and mainly involved in cellular protein processing, are a group of highly conserved and ubiquitously expressed proteins discovered 50 years ago when, the accidental application of heat shock to Drosophila induced their expression13. HSPA8, a member of the HSP 70 family, is mainly expressed in the cytosol and nucleus, and represents up to 1% of the total cellular protein content14. HSPA8 binds to nascent or unfolded proteins in an ADP/ATP-dependent manner and exerts its chaperone activity often cooperating with other co-chaperones14. It is required for clathrin-mediated endocytosis, protein folding and protein degradation in the ubiquitin-proteasome pathway, and it plays a decisive role in autophagy by targeting proteins for lysosomal degradation14,15. HSPA8 is also endowed with shuttling capacity, being able to import/export proteins from the cytoplasm to the nucleus and vice versa, but this property can be inhibited by stress16, a condition in which HSPA8 is confined to the nucleus. Lastly, HSPA8 is involved in the mitochondrial import of proteins via its interaction with HSP9017.
Also HSP90B1 was decreased in our Ob-hA-MSCs. HSPD1, a mitochondrial chaperone of the HSP family, exerts a fundamental role in mitochondrial homeostasis and insulin sensitivity. In fact, in a murine model of type II diabetes mellitus, downregulation of mitochondrial HSPD1 caused hypothalamic insulin resistance and mitochondrial dysfunction consequent to disruption of leptin signaling18. HSPB1 is an ATP-independent molecular chaperone that enables the cell to respond to and overcome different stress conditions19. HSPA5 is located in the endoplasmic reticulum where it is involved in the import and folding of newly synthesized proteins14. The HSP90B1, HSPA5 and HSPD1 proteins have been implicated in inflammatory responses and stress response20.
Globally, the above five HSP proteins are involved in protecting cell integrity and in defending cells against oxidative stress. Consequently, their decreased levels in hA-MSCs in cases of maternal obesity is compatible with a defective response to stress in these cells. Maternal obesity exacerbates perinatal stress. In fact, placentas obtained from obese women show macrophage accumulation and inflammation similar to adipose tissue from obese subjects21. In animal models, maternal obesity results in the development of obesity-related diseases in offspring10. In fact, pancreatic and liver oxidative stress and apoptosis markers were significantly higher both in fetal tissue and in adult offspring of 25-week-old obese dams than in offspring from control dams, irrespective of their diet, and the increase was maintained up to the age of 30 weeks10. Moreover, metabolic alterations were associated with mitochondrial dysfunction and reduced anti-oxidant ability in the circulation and in the liver of offspring from diet-induced obese dams11. In particular, offspring developed hyperinsulinemia and fatty liver at 8 weeks of age, with no difference in body weight or adiposity with respect to controls: this finding is suggestive of differential programming of metabolic tissues leading to diabetes and fatty liver, which are well known obesity-related disorders11.
Another group of deregulated proteins that we identified in Ob-hA-MSCs is involved in the cytoskeleton and/or in cellular vesicle trafficking. In detail, ACTN1, several ANXs (ANX1, ANX2, ANX5, ANX6), LMNA and VIM were up-regulated in Ob-hA-MSCs vs Co-hA-MSCs. ACTN1 is a cytoskeletal protein that belongs to the superfamily of filamentous actin crosslinking proteins. It is a membrane-associated protein present in non-muscle cells, where it is concentrated in actin stress fiber ends and in adherens junctions22. ACTN1 plays a relevant role in cell motility; notably, in the immune response it drives cells to sites of inflammation22. ANXs are a family of Ca+2-regulated proteins: they are membrane-binding proteins that regulate the stabilization of membrane domains, membrane-cytoskeleton linking, and exocytic and endocytic events23. ANXA2 is an actin-binding protein involved in such cellular activities as the organization of membrane lipids at the site of actin cytoskeleton attachment, and whose activity correlates with inflammation. In fact, ANXA2 activates and stimulates the production of cytokines IL-6 and TNF-α as well as ICAM-1 production in macrophages in vitro24. Furthermore, in adipocytes, ANXA2 is a positive mediator of actin-dependent transport of GLUT4 vesicles on the membrane surface. In fact, ANXA2 inhibition in 3T3-L1 adipocytes resulted in reduction of GLUT4 translocation, whereas glucose-uptake and GLUT4 translocation were significantly higher in adipocytes transfected with wild-type ANXA2 than in cells transfected with control vector25. LMNA is a component of the nuclear lamina, which is the layer between chromatin and the inner nuclear membrane26. Accumulation of LMNA in hMSCs determines a premature aging phenotype that ultimately reduces the functionality of these cells27. LMNA accumulation in hMSCs deranges lipid metabolism and leads to structural and functional alterations of mitochondria and of the endoplasmic reticulum, which are organelles important for lipid homeostasis28. These alterations have been reported in such lipid-related metabolic diseases as obesity and type 2 diabetes29. Finally, VIM expression was enhanced in Ob-hA-MSCs. VIM is involved in fat droplet reorganization and its disruption during adipose differentiation of 3T3-L1 cells inhibits lipid droplet accumulation30; conversely, its increase could promote the lipid droplet accumulation in Ob-hA-MSCs. In line with the latter hypothesis, we previously observed an increased adipogenic potential in Ob-hA-MSCs with respect to Co-hA-MSCs8. Our previous finding of increased levels of ANXs, LMNA, VIM in visceral adipose tissue in morbidly obese subjects31 supports a link between these proteins and obesity. Lastly, levels of LMNA mRNA were found to be higher in the subcutaneous adipose tissue of obese subjects than in controls32.
The third group of proteins we identified were predicted to deregulate metabolic pathways being down- (ALDOC and ENO1) or up-expressed (PKM, PFKP and HK1) in Ob- vs Co-hA-MSCs. Protein ALDOC, by promoting the association of F-actin with GLUT4, was found to play a role in intracellular GLUT4 sequestration in subcutaneous adipose tissue33. It was hypothesized that ALDOC reduction could promote translocation of the protein GLUT4 in the membrane, thereby increasing glucose uptake and triglyceride synthesis and storage33. However, given the lack of experimental data, the role, if any, of these proteins in obesity or in obesity-associated disorders requires further investigation.
In conclusion, exposure to an obesogenic environment in utero is associated with proteome alterations in hA-MSCs. The proteins deranged are involved in the stress response, cytoskeleton and metabolic pathways, which are often associated with obesity-related phenotypes10,11,25,28. The possible role of these protein alterations in enhancing susceptibility to obesity or to obesity-related disorders in newborns of obese mothers requires further investigations. Notably, studies conducted in animal models of obesity during pregnancy10,11 indicate that cellular stress could trigger in human placenta metabolic alterations that predispose offspring to obesity-related disorders such as insulin resistance and fatty liver in fetal life and later in life. Our Ob-hA-MSC proteome data suggest that a similar mechanism could occur also in humans.
Material and Methods
Patients
Twelve obese (mean age 34.0 years) and 6 non-obese pregnant women (mean age 34.3 years) with pre-pregnancy BMI (mean/SD) 42.7/7.7 and 21.3/3.3 kg/m2, respectively, were enrolled at the Dipartimento di Neuroscienze e Scienze Riproduttive ed Odontostomatologiche, University of Naples Federico II. The exclusion criteria were neoplasia, viral infections, diabetes and metabolic syndrome. The clinical, anamnestic and family history, and biochemical data of each woman were recorded immediately before delivery. The birth weight, length, head circumference, 1 and 5 min Apgar indexes of infants were measured at delivery. All patients and controls gave their informed consent to the study. The study was performed according to the Helsinki II Declaration and was approved by the Ethics Committee of School of Medicine, University of Naples Federico II (authorization n. 248/08, 23/02/2009; amendment n. 248/08/ES1, 1/10/2014).
Sample collection
Two maternal blood samples were collected from each enrolled woman immediately before delivery. One sample was used for DNA extraction, whereas the other was centrifuged and the serum was stored at −80 °C until required for the measurement of the main biochemical parameters and leptin/adiponectin concentrations by routine methods or by immunoassay (Bio-Rad, Hemel Hempstead, Herts, UK), respectively. The term placentas were collected at delivery by cesarean section. Placentas were processed immediately to isolate the hA-MSCs from the amniotic membranes, according to Parolini et al.34. The isolation, DNA typing (to confirm fetal origin) and immunophenotyping of hA-MSCs were performed as described elsewhere8.
Two-Dimensional Fluorescence Difference Gel Electrophoresis
We pooled the hA-MSCs from two obese women to obtain a biological replicate (Ob-hA-MSCS). We performed a comparative experiment using six biological replicates of hA-MSCs from obese women and six biological replicates of hA-MSCs from non-obese women. DIGE experiments were performed as previously described35. The hA-MSCs were collected and resuspended in 0.5 ml of lysis buffer, containing 7M urea, 2M thiourea, 4% w/v chaps (3-[(3-cholamidopropyl)-dimethylammonium]-1-propane sulfonate), 30 mM Tris-HCl pH 7.5, cocktail of protease inhibitors (GE Healthcare, Piscataway, NJ). Cell debris was removed by centrifugation at 14,000 rpm at 4° C for 45 min. The cell lysate supernatant was precipitated using a 2D Clean up kit according to manufacturer’s instructions (GE Healthcare) and resuspended in 100 μL of lysis buffer.
The protein extract concentrations were determined and equal amounts of the protein lysates were labeled in vitro using two fluorescent cyanine minimal dyes (Cy3 and Cy5) that differed in excitation and emission wavelengths. A third cyanine dye (Cy2) was used to label a mixture of all samples as internal standard. The three differently labeled protein mixtures were pooled and subjected to isoelectric focusing through a non-linear pH range of 3–10 over a strip length of 24 cm. Strips were rehydrated without protein samples, with 450 μL of rehydration buffer containing 450 μL of DeStreak rehydration solution and 2% IPG Buffer pH 3−10 NL (GE Healthcare, Buckinghamshire, UK) overnight at room temperature. After rehydration, the strips were transferred to the Ettan IPGphor system (GE Healthcare) for isoelectric focusing. The samples were loaded on the strips with an equal volume of sample buffer containing 7M urea, 2M thiourea, 4% CHAPS, 0.2% DTT and 2% IPGphor buffer. The IPG strips were focused for 18 h at 20 °C as follows: 500 V for 4 h, linear gradient to 1000 V in 4 h, linear gradient to 10000 V in 4 h, step at 10000 V in 3 h, 300 V for 10 h.
The reducing and alkylating steps were performed between the first and the second electrophoretic step. Acrylamide strips were then transferred to the top of a classical SDS PAGE gel for a second orthogonal electrophoresis analysis. The Cy2, Cy3 and Cy5 images were obtained by scanning each of the four DIGE gels at an excitation/emission wavelength of 480/530 nm for Cy2, 520/590 nm for Cy3 and 620/680 nm for Cy5 using a Typhoon 9410 TM scanner (GE Healthcare). The semi-preparative gel was prepared according to the previously described procedure35, using 450 μg of protein from all replicates enrolled in this study. The semi-preparative gel was stained using GelCode™ Blue Stain Reagent (Thermo Fisher, Scientific, MA, USA) overnight. The semipreparative gel was scanned at 480/633 nm wavelengths using the Typhoon 9400 scanner (GE Healthcare). The images were analyzed with DeCyder software version 5.2 (GE Healthcare) in Batch Processing Mode. The maximum number of estimated spots per gel was fixed at 8000.
Detection and quantification of protein spots were carried out using the differential in-gel analysis (DIA), whereas protein spot matching among different gels was obtained using the biological variation analysis (BVA). The DIA module was used to compare Cy3/Cy5 image pairs with Cy2 internal standard from each gel. The DIA model was used also to detect spot boundaries and to calculate spot volume, normalized versus the volume of the corresponding spot present in the pool standard of the same gel. This analysis enables automated first level matching (“within-gel”) with a low experimental variation36. Image pairs were then matched between gels using the BVA feature. The results of the intra-gel comparison (DIA module) were imported into the BVA module. The Cy2 image containing the highest number of spots was designated the “master image” and used as template. The protein spots of the remaining internal standard images were automatically matched with the “master image”. Each spot intensity was expressed as a mean value of the 6 gels in order to reduce inter-gel variation. Spot intensities were then compared between the hA-MSCs from obese and from non-obese pregnant women. The Student’s t-test was used to determine the statistical significance of differences in spot intensity. Differentially regulated spots were defined as having a variation higher than 1.2 (P < 0.05) as reported elsewhere37. The accuracy of spots matching was verified, manually. The spot map containing spots differentially expressed in hA-MSCs from obese vs non-obese pregnant women were excised from the semi-preparative gel using the Ettan Spot Picker robotic system (GE Healthcare).
Proteomic analysis
The spots of interest were excised, hydrolyzed and the peptide mixtures analyzed by mass spectrometry, MALDI-MS and LC-MS/MS using respectively 4800 Plus MALDI TOF/TOF™ Analyzer, Applied Biosystems 4800 Proteomics Analyzer (Applied Biosystems, Framingham, MA, USA) and a LC/MSD Trap XCT Ultra (Agilent Technologies, Palo Alto, CA, USA) equipped with a 1100 HPLC system and a chip cube (Agilent Technologies). MALDI spectra were acquired in the positive ion reflector mode using delayed extraction in the mass range between 800 and 4000 Da. LC-MSMS analysis was performed using data-dependent acquisition of one MS scan followed by MS/MS scans of the three most abundant ions in each MS scan. Raw data analyses were converted into a Mascot format text to identify proteins using Matrix Science software. The protein search considered the following parameters: non-redundant protein sequence database (NCBInr), specificity of the proteolytic enzyme used for the hydrolysis (trypsin), taxonomic category of the sample, no protein molecular weight was considered, up to one missed cleavage, cysteines as S-carbamidomethylcysteines, unmodified N- and C-terminal ends, methionines both unmodified and oxidized, putative pyro-Glu formation by Gln, precursor peptide maximum mass tolerance of 200 ppm, and a maximum fragment mass tolerance of 200 ppm.
Western Blot Analysis
Protein extracts (30 μg) from Ob- and Co-hA-MSCs were analyzed by Western blot. Proteins were separated by 10% SDS-PAGE, and electroblotted onto nitrocellulose membranes (GE Healthcare). Nitrocellulose membranes were blocked with 5% Milk in PBS buffer with 0.1% v/v Tween (PBS-T), 20 for 2 h at room temperature. Immunoblotting was performed with the goat polyclonal antibody, anti-HSP90B1, anti-HSPB1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal antibody anti-LMNA, anti-ENO1, anti-HSPA5 (Santa Cruz Biotechnology), anti-PKM (Adcam Discover More, Northampton, UK), mouse antibody anti-ANXA2, anti-ACTN1, anti-GAPDH (Santa Cruz Biotechnology). Human anti-ALDOC antibody (kindly donated by Prof. F. Salvatore, CEINGE-Biotecnologie Avanzate, Naples, Italy) was used as previously described38 to test ALDOC expression. Goat polyclonal antibodies were used at a 1:1000 dilution in milk/PBS-T 1% w/v, the rabbit polyclonal antibodies at a 1:1000 dilution, the mouse monoclonal antibodies at a 1:500 dilution, the anti-goat secondary antibody (Sigma Aldrich) at a 1:10000 dilution, and the anti-rabbit and anti-mouse secondary antibodies (GE Healthcare) at a 1:5000 dilution. Immunoblots were detected using HRP-conjugated secondary antibodies and enhanced chemiluminescence (GE Healthcare). The resulting Western blot images were scanned using Chemi Doc software (Biorad, Hercules, CA, USA) and analyzed using Quantity One software (Biorad). The optical density of each sample was measured and normalized using a GAPDH run on the same gel. The data are expressed as percent relative expression, namely, for each protein, the sample with the highest expression of that protein was set 100%.
Statistical Analysis
The investigated parameters were expressed as mean ± standard deviation or mean/SEM. Student’s t test was used to compare group means, and a p-level < 0.05 was considered statistically significant. Statistical analyses were carried out with the PASW package for Windows (Ver.18; SPSS Inc. Headquarters, Chicago, Ill). In all cases, not-paired two-tailed Student’s t-tests were used to determine significance of the 2D-DIGE results, and false discovery rates were applied to all comparisons. We used the KEGG and DAVID bioinformatics tools to identify pathways containing altered proteins.
Additional Information
How to cite this article: Capobianco, V. et al. Proteome analysis of human amniotic mesenchymal stem cells (hA-MSCs) reveals impaired antioxidant ability, cytoskeleton and metabolic functionality in maternal obesity. Sci. Rep. 6, 25270; doi: 10.1038/srep25270 (2016).
Supplementary Material
Acknowledgments
The authors thank Jean Ann Gilder (Scientific Communication srl., Naples) for editing the text, and Vittorio Lucignano, CEINGE–Biotecnologie Avanzate for technical assistance related to graphics. This work was supported by: Grant POR CAMPANIA FSER 2007-2013 Project DIAINTECH and Grant CAMPUS-Bioframe, from Regione Campania, Italy; Grant PON02_00677 (BIOGENE) Pot. lab.8 A/B - 2012 from the Italian Ministry of University and Research.
Footnotes
Author Contributions L.S. and P.P. conceived study design; L.D.V and L.P. devised and supervised part of the experimental work; A.S. and P.M. provide the patients’ samples. V.C., M.C., L.I. and C.N. performed the experiments and carried out the analyses, and all authors participated in data interpretation and manuscript writing. All authors approved the final version.
References
- Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family. Growth Matern Child Health J 13, 268–273 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. M. et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347, f4539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy C. E. et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119, 323–335 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop K. A. & Burdge G. C. Epigenetic changes in early life and future risk of obesity. Int J Obes 35, 72–83 (2011). [DOI] [PubMed] [Google Scholar]
- Nardelli C. et al. Characterization and predicted role of the microRNA expression profile in amnion from obese pregnant women. Int J Obes 38, 466–469 (2014). [DOI] [PubMed] [Google Scholar]
- Oliva K. et al. The effect of pre-existing maternal obesity on the placenta proteome: two-dimensional difference gel electrophoresis coupled with mass spectrometry. J Mol Endocrinol 48, 139–149 (2012). [DOI] [PubMed] [Google Scholar]
- Pappa K. I. & Anagnou N. P. Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med 4, 423–433 (2009). [DOI] [PubMed] [Google Scholar]
- Iaffaldano L. et al. High aminopeptidase N/CD13 levels characterize human amniotic mesenchymal stem cells and drive their increased adipogenic potential in obese women. Stem Cells Dev 22, 2287–2297 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen K. M., Catalano P. M. & Yaktine A. L. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 21, 521–526 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad M. I. et al. Maternal obesity and malnourishment exacerbate perinatal oxidative stress resulting in diabetogenic programming in F1 offspring. J Endocrinol Invest. 10.1007/s40618-015-0413-5 (2015). [DOI] [PubMed] [Google Scholar]
- Alfaradhi M. Z. et al. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am J Physiol Regul Integr Comp Physiol. 307, R26–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge D. C., Whitham M. & Febbraio M. A. Chaperoning to the metabolic party: The emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol Metab. 3, 781–93 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. A new puffing pattern induced by temperature shock and DNP in Drosophila. Experientia 18, 571e573 (1962). [Google Scholar]
- Stricher F., Macri C., Ruff M. & Muller S. HSPA8/HSC70 chaperone protein: structure, function, and chemical targeting. Autophagy. 9, 1937–54 (2013). [DOI] [PubMed] [Google Scholar]
- Ciechanover A. The ubiquitin-proteasome pathway: on protein death and cell life. EMBO J. 17, 7151–60 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodiha M., Chu A., Lazrak O. & Stochaj U. Stress inhibits nucleocytoplasmic shuttling of heat shock protein hsc70. Am J Physiol Cell Physiol. 289, C1034–41 (2005). [DOI] [PubMed] [Google Scholar]
- Young J. C., Hoogenraad N. J. & Hartl F. U. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 112, 41–50 (2003). [DOI] [PubMed] [Google Scholar]
- Kleinridders A. et al. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J Clin Invest 123, 4667–4680 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsogiannou M., Andrieu C. & Rocchi P. Heat shock protein 27 phosphorylation state is associated with cancer progression. Front Genet 5, 1–5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims-Robinson C., Zhao S., Hur J. & Feldman E. L. Central nervous system endoplasmic reticulum stress in a murine model of type 2 diabetes. Diabetologia 55, 2276–2284 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challier J. C. et al. Obesity in pregnancy stimulates macrophage accumulation and inflammation in the placenta. Placenta. 29, 274–81 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomou K. G., Zachou K. & Dalekos G. N. Alpha-actinin: a multidisciplinary protein with important role in B-cell driven autoimmunity. Autoimmun Rev 10, 389–396 (2011). [DOI] [PubMed] [Google Scholar]
- Gerke V., Creutz C. E. & Moss S. E. Annexins: linking Ca2+ signaling to membrane dynamics. Nat Rev Mol Cell Biol 6, 449–461 (2005). [DOI] [PubMed] [Google Scholar]
- Swisher J. F., Khatri U. & Feldman G. M. Annexin A2 is a soluble mediator of macrophage activation. J Leukoc Biol 82, 1174–1184 (2007). [DOI] [PubMed] [Google Scholar]
- Huang J., Hsia S. H., Imamura T., Usui I. & Olefsky J. M. Annexin II is a thiazolidinedione-responsive gene involved in insulin-induced glucose transporter isoform 4 translocation in 3T3-L1 adipocytes. Endocrinology 145, 1579–1586 (2004). [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Gruenbaum Y., Moir R. D., Shumaker D. K. & Spann T. P. Nuclear lamins: building blocks of nuclear architecture. Genes Dev 16, 533–547 (2002). [DOI] [PubMed] [Google Scholar]
- Infante A. et al. Prelamin A accumulation and stress conditions induce impaired Oct-1 activity and autophagy in prematurely aged human mesenchymal stem cell. Aging 6, 264–280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez P. et al. Age-Related Lipid Metabolic Signature in Human LMNA-Lipodystrophic Stem Cell-Derived Adipocytes. J Clin Endocrinol Metab 100, E964–E973 (2015). [DOI] [PubMed] [Google Scholar]
- Rieusset J. Mitochondria and endoplasmic reticulum: mitochondria-endoplasmic reticulum interplay in type 2 diabetes pathophysiology. Int J Biochem Cell Biol 43, 1257–1262 (2011). [DOI] [PubMed] [Google Scholar]
- Lieber J. G. & Evans R. M. Disruption of the vimentin intermediate filament system during adipose conversion of 3T3-L1 cells inhibits lipid droplet accumulation. J Cell Sci 109, 3047–3058 (1996). [DOI] [PubMed] [Google Scholar]
- Capobianco V. et al. miRNA and protein expression profile of visceral adipose tissue reveal miR-141/YWHAG and miR-520e/RAB11A as two potential miRNA/protein target pairs associated with severe obesity. J Proteome Res 11, 3358–3369 (2012). [DOI] [PubMed] [Google Scholar]
- Miranda M. et al. LMNA mRNA expression is altered in human obesity and type 2 diabetes. Obesity (Silver Spring). 16, 1742–8 (2008). [DOI] [PubMed] [Google Scholar]
- Kao A. W., Noda Y., Johnson J. H., Pessin J. E. & Saltiel A. R. Aldolase mediates the association of F-actin with the insulin-responsive glucose transporter GLUT4. J Biol Chem 274, 17742–17747 (1999). [DOI] [PubMed] [Google Scholar]
- Parolini O. et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 26, 300–311 (2008). [DOI] [PubMed] [Google Scholar]
- Caterino M. et al. Transcription factor TBX1 overexpression induces downregulation of proteins involved in retinoic acid metabolism: a comparative proteomic analysis. J Proteome Res 8, 1515–1526 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont M. et al. Integrated technology platform for fluorescence 2-D difference gel electrophoresis. Life Science News (Amersham Biosciences) 7, 1–3 (2001). [Google Scholar]
- Caterino M. et al. Differential proteomic analysis in human cells subjected to ribosomal stress. Proteomics 13, 1220–1227 (2013). [DOI] [PubMed] [Google Scholar]
- Langellotti S. et al. A novel anti-aldolase C antibody specifically interacts with residues 85–102 of the protein. MAbs 6, 708–717 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.