Abstract
INTRODUCTION
Few studies have examined the effects of amyloid and APOE genotype on cognition among middle-aged individuals.
METHODS
We included 464 cognitively normal, test-naïve, participants with PiB-PET amyloid imaging, mean age of 62.7 (range 51–71 years), enrolled in the Mayo Clinic Study of Aging. Participants completed multiple cognitive assessments, including a standard neuropsychological battery and the CogState computerized battery, over 30 months of follow-up. Linear mixed models were used to examine the effects of amyloid and APOE genotype on baseline cognition and cognitive decline.
RESULTS
Elevated amyloid was not associated with tests of episodic memory, but did predict declines on tests of executive function. APOE genotype was not associated with cognition. Among APOE ε4 non-carriers, higher amyloid was predictive of decline on tests of executive function and on one episodic memory test.
DISCUSSION
Elevated amyloidosis and APOE genotype does not appear to exert a dramatic influence on cognition in middle-age.
Keywords: Amyloid, APOE, Alzheimer’s disease, Cognitive aging, Cohort studies, PET
1. Introduction
The pathophysiological brain changes associated with Alzheimer’s disease (AD) are thought to begin decades before clinical symptoms based on autopsy and clinical studies [1–3]. Therefore, the earliest identification of at-risk individuals will provide the greatest window of opportunity to prevent or delay disease symptoms. AD secondary prevention trials of at risk cognitively normal individuals, defined as significant amyloid- beta accumulation or the presence of at least one apolipoprotein E (APOE) ε4 allele, are in the planning stages or ongoing. Most studies examining the effects of amyloid and APOE genotype on cognition have utilized cohorts aged 65 and older, with mean ages of the cohorts typically in the mid- to late 70’s [4–12]. It is important to also determine the effects of amyloid and APOE genotype on cognition in younger cohorts. This information may help better discern at what age population screening for amyloid and APOE genotype is most predictive of cognitive decline if potential secondary preventive treatments are found to be effective.
A challenge in assessing the impact of an anti-Alzheimer therapy in middle-age is that, in general, there is little cognitive change but notable practice effects [13]. As a result, it is difficult to quantify the effects of risk and biological factors, such as amyloid and APOE genotype, on cognitive decline and to estimate potential treatment effects. Several studies are now focused on identifying neuropsychological tests that are more sensitive to subtle cognitive changes among cognitively normal individuals. One area of interest is computerized testing.
A computerized battery may have advantage over standard neuropsychological tests or other cognitive screening measures by being more sensitive and efficient, removing ceiling and floor effects, providing real-time data entry and precise recording of accuracy and speed, and minimizing practice effects [14,15]. Few studies have examined the performance of computerized cognitive batteries in a large middle-aged cohort. Characterization of the longitudinal trajectories of cognitively normal individuals in middle-age on standard neuropsychological and computerized cognitive tests for practice effects and sensitivity to change is critical. We recently described the feasibility of the CogState computerized battery, and factors that affect performance among 1,660 non-demented individuals enrolled in the Mayo Clinic Study of Aging [16]. In the present study, we first assessed cognitive changes over 30 months across standard neuropsychological measures and the CogState computerized battery in a cohort of 464 cognitively normal individuals, mean [SD] age of 62.7 [5.4] years, enrolled in the Mayo Clinic Study of Aging. We then examined the influence of amyloid and APOE genotype on baseline cognition and cognitive trajectories.
2. Methods
2.1. Participants
The Mayo Clinic Study of Aging (MCSA) is a population-based study of cognitive aging among Olmsted County, MN residents that initially began in October 2004, enrolling individuals aged 70 to 89 years, as previously described [17]. Given the importance of understanding risk factors and the development and progression of AD pathophysiology in late middle-age, we expanded the study to also enroll a population-based sample of individuals aged 50–69 using the same stratified random sampling methodology as in the original cohort. The Olmsted County population, aged 50–69 (n = 31,502), was sampled by 5-year age groups and sex beginning on November 1, 2011. The present study included 464 individuals, aged 50–69, who were cognitively normal and test naïve at baseline, and had amyloid imaging and APOE genotype.
2.2. Standard protocol approvals, registrations, and patient consents
The study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participants provided written informed consent.
2.3. Participant assessment
MCSA full study visits occurred every 15 months and included a physician examination, an interview by a study coordinator, and neuropsychological testing [17]. The physician examination included a medical history review, complete neurological examination, and administration of the Short Test of Mental Status [18]. The study coordinator interview included demographic information, medical history and questions about memory to both the participant and an informant using the Clinical Dementia Rating scale [19]. During the time with the study coordinator, the participants also completed the CogState computerized battery.
The neuropsychological battery was administered by a psychometrist and included nine tests covering four domains: 1) memory (Auditory Verbal Learning Test Delayed Recall Trial [20]; Wechsler Memory Scale-Revised Logical Memory II & Visual Reproduction II) [21]; 2) language (Boston Naming Test [22] and Category Fluency [23]; 3) executive function (Trail Making Test B [24] and WAIS-R Digit Symbol subtest [25]; and 4) visuospatial skills (WAIS-R Picture Completion and Block Design subtests) [25]. Trail Making Test A [24] was also administered. Blood was collected and APOE genotype was determined.
2.4. Diagnostic determination
For each participant, performance in a cognitive domain was compared with the age-adjusted scores of cognitively normal individuals previously obtained using Mayo’s Older American Normative Studies [26]. This approach relies on prior normative work and extensive experience with the measurement of cognitive abilities in an independent sample of subjects from the same population. Subjects with scores of ≥1.5 SD below the age-specific mean in the general population were considered for possible cognitive impairment. A final decision to diagnose mild cognitive impairment (MCI) was based on a consensus agreement between the study coordinator, examining physician, and neuropsychologist, after taking into account education, prior occupation, visual or hearing deficits, and reviewing all other participant information [17,27]. Individuals who performed in the normal range and did not meet criteria for MCI or dementia were deemed cognitively normal. Performance on the CogState computerized cognitive battery was not used to determine a diagnosis.
2.5. CogState computerized battery administration
Participants completed the CogState battery every 7.5 months (at the full in-clinic study visit every 15 months, and also at a CogState-only visit at the midpoint between the full study visits). Administration of the CogState battery included four card tasks and the Groton Maze Learning Test (GMLT), as previously described [16,28]. Given the previous literature showing an initial practice/learning effect between the first and second administration [29], at the baseline visit we administered a short practice battery, followed by a two-minute rest period, then the complete battery. Subsequent visits included a brief introduction to the test (i.e., the participant completed a few practice attempts), followed immediately by the complete battery. The computerized battery included measures of psychomotor function (Detection; DET), attention (Identification; IDN), visual episodic memory (One Card Learning; OCL), and visual working memory (One Back; ONB). The GMLT measures spatial working memory, learning efficiency, and error monitoring (outcomes included moves per second and total number of errors).
The CogState battery provides a large number of equivalent alternative forms for serial assessments. This is achieved by having a large stimulus set from which exemplars are randomly chosen, resulting in a different set of exemplars that are used each time an individual takes the test. The paradigm remains constant but the items are randomly chosen. Furthermore, the correct response (either yes or no) is randomly chosen for each trial of the task, and the inter-stimulus interval has a random interval that varies for each trial of the task. For the GMLT there are 20 possible hidden pathways, matched for number of tiles and turns, that are presented in a random, non-recurring order.
2.6. Amyloid PET methods
PET images were acquired using a PET/CT scanner (DRX or DRXT, GE Healthcare). A CT image was obtained for attenuation correction. The 11C PiB-PET scan, consisting of four 5-minute dynamic frames, was acquired from 40–60 minutes after injection. Image analysis was done using our in-house fully automated image processing pipeline [30]. An amyloid PET standardized uptake value ratio (SUVR) was determined by calculating the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus regions of interest (ROIs) for each subject and dividing this meta ROI by the median uptake over voxels in the cerebellar gray matter ROI of the atlas. The data were partial volume corrected using a 2-compartment approach (brain and non-brain). The median value of PET voxels corresponding to grey matter was calculated for each ROI. We primarily analyzed amyloid as a continuous variable. We also utilized cut-offs for elevated amyloid of SUVR ≥1.4 [30].
2.7 Statistical methods
Raw scores of all individual computerized and neuropsychological tests were converted to z-scores using baseline results. Select z-scores were inverted so that higher z-scores meant better performance for all cognitive tests. The trajectories of each computerized test, individual neuropsychological test and domain-specific z-scores over the 30-month follow-up were examined using linear mixed models with maximum likelihood estimation and an unstructured covariance matrix. This approach permitted assessment of the average rate of change per year in all cognitive tests while accounting for the dependence of within-subject repeated measures over time. Multivariable models were adjusted for age, sex, and education.
We also used linear mixed models to separately examine the effect of baseline amyloid (as a continuous variable) and the presence of an APOE ε4 allele on baseline cognition and longitudinal average rate of change per year for all cognitive tests. Models included terms for baseline amyloid or presence of an APOE ε4 allele (indicating the relationship between amyloid or APOE ε4 and baseline cognitive z-score), time (indicating annual change in the cognitive z-score over the follow-up), and the interaction between either amyloid or presence of an APOE ε4 allele and time (indicating whether baseline amyloid or APOE ε4 predicted change in cognition). To examine the interaction between amyloid and presence of an APOE ε4 allele on baseline and longitudinal cognition, we utilized the linear mixed models for amyloid (as described above) and stratified by the presence of an APOE ε4 allele. We did examine interactions between amyloid and sex in predicting cognitive change, including stratifying by the presence of APOE ε4 allele. However, none of the interactions were significant so these results are not presented.
Permutation testing was performed for the mixed models to address the large number of statistical tests. Between-subject effects were permuted as to maintain within-subject correlations. A random subset of 10,000 combinations from all possible permutations were selected and used for analysis. After permutation testing, P < .05 was considered statistically significant. All statistical analyses were performed using SAS (SAS Institute, Cary, NC).
3. Results
The baseline characteristics of the 464 cognitively normal participants are shown in Table 1. The mean [SD] age of the sample was 62.7 [5.4] years and 52% were men. The mean PiB-PET SUVR [SD] was 1.3 [1.2] and 81 (17.5%) had elevated brain amyloid based on the cutpoint of SUVR ≥1.4. The age of the youngest individual with elevated amyloid was 59 years. Compared to individuals with amyloid SUVR<1.4, those with SUVR ≥1.4 were significantly older, had less education, more frequently had an APOE ε4 allele and performed worse on some CogState and neuropsychological tests in unadjusted analyses (Table 1). Individuals with an APOE ε4 allele, versus those without, had higher amyloid levels, and performed worse on some standard neuropsychological tests in the executive and language domains in unadjusted analyses.
Table 1.
Participant characteristics (N = 464)
| Characteristics | ALL | Aβ≥1.4 (N=81) | Aβ<1.4 (N=383) | APOE E4+ (N=130) | APOE E4− (N=334) |
|---|---|---|---|---|---|
| Mean (SD)/N(%) | Mean (SD)/N(%) | Mean (SD)/N(%) | Mean (SD)/N(%) | Mean (SD)/N(%) | |
| Age | 62.7 (5.4) | 66.6 (2.8) | 61.9 (5.5)*** | 63.2 (5.2) | 62.5 (5.5) |
| Men | 240 (51.7%) | 35 (43.2%) | 205 (53.5%) | 66 (50.8%) | 174 (52.1%) |
| Education | 15.3 (2.3) | 14.7 (2.5) | 15.4 (2.3)** | 15.0 (2.3) | 15.4 (2.3) |
| Amyloid SUVR | 1.3 (0.2) | 1.6 (0.3) | 1.3 (0.1) | 1.4 (0.3) | 1.3(0.1)** |
| Aβ≥1.4 SUVR | 81 (17.5%) | 37 (28.5%) | 44 (13.2%)*** | ||
| Any APOE E4 allele | 130 (28.0%) | 37 (45.7%) | 93 (24.3%)*** | ||
| CogState Tests | |||||
| Detection (DET) | 2.6 (0.1) | 2.6 (0.1) | 2.6 (0.1) | 2.6 (0.1) | 2.6 (0.1) |
| Identification (IDN) | 2.7 (0.1) | 2.8 (0.1) | 2.7 (0.1)** | 2.7 (0.1) | 2.7 (0.1) |
| One Card Learning (OCL) | 1.0 (0.1) | 1.0 (0.1) | 1.0 (0.1)* | 1.0 (0.1) | 1.0 (0.1) |
| One Back (ONB) | 2.9 (0.1) | 2.9 (0.1) | 2.9 (0.1)** | 2.9 (0.1) | 2.9 (0.1) |
| GMLT Total Errors | 50.7 (18.1) | 56.1 (17.6) | 49.4 (18.0)** | 50.4 (17.9) | 50.8 (18.2) |
| GMLT Speed | 0.6 (0.1) | 0.5 (0.1) | 0.6 (0.1)*** | 0.6 (0.1) | 0.6 (0.1) |
| Neuropsychological Tests (Raw Scores) | |||||
| Logical Memory delayed recall | 21.9 (7.5) | 21.5 (7.3) | 21.9 (7.6) | 21.4 (8.3) | 22.0 (7.2) |
| AVLT delayed recall | 9.3 (3.3) | 8.8 (3.1) | 9.5 (3.3) | 9.1 (3.2) | 9.4 (3.3) |
| Visual Reproduction delayed recall | 27.4 (6.9) | 25.3 (6.6) | 27.8 (6.9)*** | 27.2 (7.1) | 27.4 (6.9) |
| WAIS-R Digit Symbol | 53.1 (10.7) | 49.5 (9.5) | 53.9 (10.8)** | 51.3 (10.9) | 53.8 (10.6)* |
| TMT-A | 149.0 (9.2) | 146.1 (10.1) | 149.7 (8.9)** | 147.8 (10.3) | 149.5 (8.7) |
| TMT-B | 230.0 (24.4) | 222.9 (24.2) | 231.5 (24.2)*** | 225.9 (25.4) | 231.6 (23.9)* |
| WAIS-R Picture Completion | 14.8 (2.4) | 14.2 (2.6) | 14.9 (2.4)* | 14.6 (2.5) | 14.9 (2.4) |
| WAIS-R Block Design | 29.8 (8.9) | 26.2 (8.0) | 30.5 (8.9)*** | 29.0 (8.5) | 30.0 (9.0) |
| Boston Naming Test | 56.5 (3.3) | 56.4 (3.3) | 56.6 (3.3) | 55.8 (3.2) | 56.8 (3.3)*** |
| Category Fluency | 49.7 (10.1) | 49.1 (9.9) | 49.8 (10.1) | 48.4 (10.0) | 50.2 (10.1) |
| Global and Domain-specific Scores | |||||
| Global z-score | 0.0 (0.6) | −0.2 (0.6) | 0.1 (0.6)*** | −0.1 (0.7) | 0.1 (0.6)* |
| Memory z-score | 0.0 (0.8) | −0.1 (0.8) | 0.1 (0.8)* | 0.0 (0.9) | 0.1 (0.8) |
| Language z-score | 0.0 (0.8) | 0.0 (0.8) | 0.0 (0.8) | −0.2 (0.8) | 0.1 (0.8)** |
| Executive function z-score | 0.0 (0.9) | −0.3 (0.8) | 0.1 (0.9)*** | −0.1 (0.9) | 0.1 (0.8)* |
| Visual spatial z-score | 0.1 (0.8) | −0.3 (0.8) | 0.1 (0.8)*** | 0.0 (0.8) | 0.1 (0.8) |
| Incident MCI | 5 (1.1%) | 1 (1.2%) | 4 (1.0%) | 2 (1.5%) | 3 (0.9%) |
| Number of CogState exams | 3.5 (1.0) | 3.5 (1.0) | 3.5 (1.0) | 3.5 (1.0) | 3.5 (1.0) |
| Number of Neuropsychological exams | 2.1 (0.6) | 2.2 (0.6) | 2.1 (0.6) | 2.1 (0.6) | 2.1 (0.6) |
Abbreviations: APOE, apolipoprotein E; SUVR, Standard Uptake Value Ratio; GMLT, Groton Maze Learning Test; AVLT, Auditory Verbal Learning Test; WAIS-R, Wechsler Memory Scale-Revised Logical Memory II; TMT-A, Trail Making Test, Part A; TMT-B, Trail Making Test, Part B. MCI, Mild Cognitive Impairment.
P < .001,
P < .01,
P < .05.
3.1. Cognitive trajectories
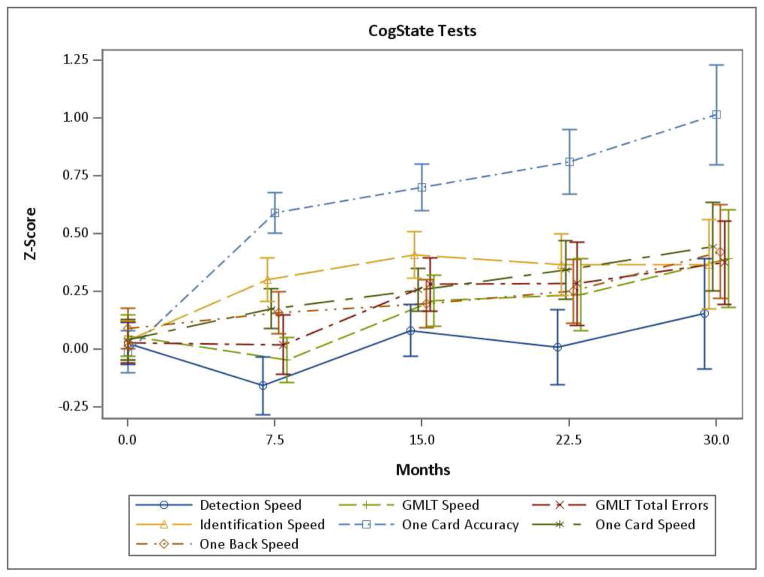
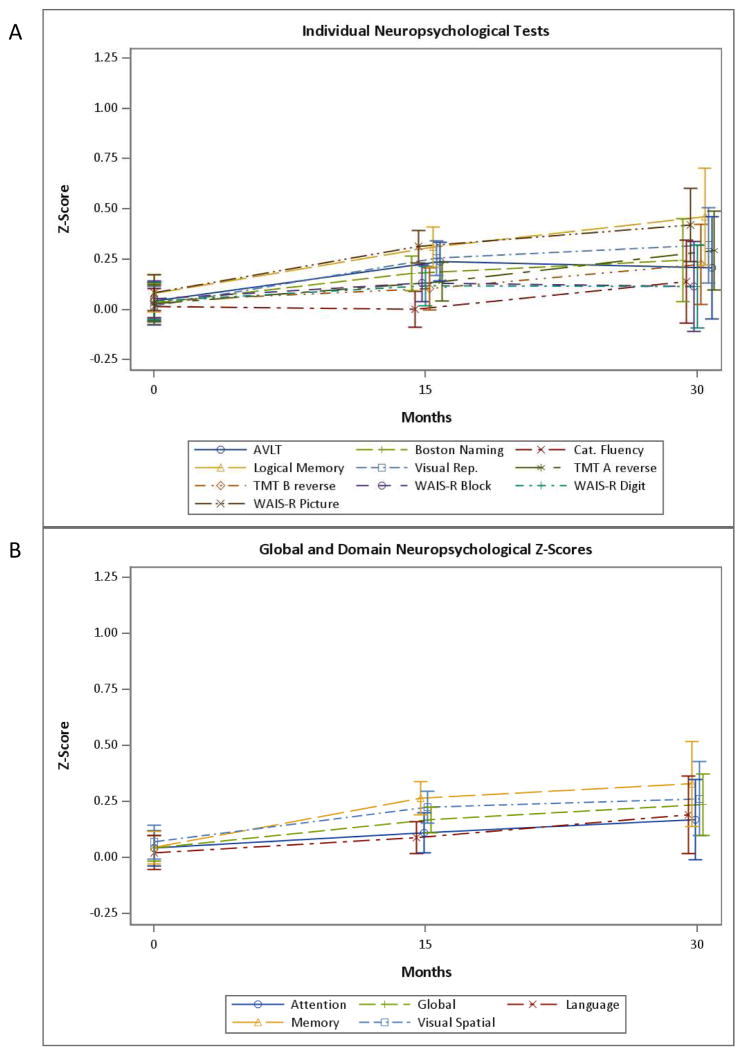
The longitudinal performance on the CogState tests, over 5 visits (30 months), are shown in Fig. 1 and Supplementary Table 1. Essentially all CogState tests showed better performance over time in linear mixed models adjusting for age, sex, and education. Most of the computerized tests primarily showed a practice effect at the second and third visits. However, mean levels of OCL accuracy (Fig. 1, One Card Accuracy) showed continued practice effects for each subsequent visit up to 5 visits. The longitudinal performance on the standard neuropsychological tests and domain-specific z-scores are shown in Figs. 2A and 2B and Supplementary Table 2. All domain-specific and many individual test z-scores also demonstrated better performance over time, adjusting for age, sex, and education, with the exception of Category Fluency, WAIS-R Block Design, and Language z-score. The longitudinal trajectories of most domain-specific and individual neuropsychological test z-scores were similar to most computerized tests, with an initial practice effect followed by a leveling off or slight increase (see Fig. 2).
Fig. 1. The longitudinal performance on all CogState computerized tests over 5 visits (30 months).
GMLT, Groton Maze Learning Test.
Fig. 2. The longitudinal performance of: A) the individual standard neuropsychological tests, and B) domain-specific z-scores over 3 visits (30 months).
AVLT, Auditory Verbal Learning Test; TMT-A, Trail Making Test, Part A; TMT-B, Trail Making Test, Part B; WAIS-R, Wechsler Memory Scale-Revised Logical Memory II.
3.2. Effects of amyloid, APOE ε4 allele, and their interaction for predicting cognitive decline
Overall, there were few associations between baseline amyloid and either baseline cognition or cognitive change, including tests of episodic memory. At baseline, increasing amyloid SUVR was associated with poorer performance on computerized tests of psychomotor speed (DET; b = −0.92, P = .002), visual working memory (ONB; b = −0.62, P = .014), and spatial working memory (GMLT Total Errors; b = −0.71, P = .013), but did not predict cognitive decline on these tests (Table 2). Higher baseline amyloid was predictive of cognitive decline on the WAIS-R Digit Symbol (b = −0.24, P = .011) and the Executive domain (b = −0.22, P = .011). APOE genotype alone was not cross-sectionally associated with any cognitive test and also did not longitudinally predict change on any test.
Table 2.
Multivariable linear mixed models examining the effect of amyloid on cognitive decline*
| Baseline Amyloid | Time | Baseline Amyloid * time | ||||
|---|---|---|---|---|---|---|
| Cognitive z-scores | b(se) | P value** | b(se) | P value** | b(se) | P value** |
| CogState Tests | ||||||
| Detection (DET) | −0.92 (0.28) | .002 | −0.17 (0.21) | .399 | 0.19 (0.15) | .208 |
| Identification (IDN) | −0.43 (0.24) | .074 | 0.03 (0.14) | .814 | 0.13 (0.11) | .218 |
| One Card Learning (OCL) | −0.13 (0.24) | .569 | 0.38 (0.18) | .033 | 0.05 (0.13) | .719 |
| One Back (ONB) | −0.62 (0.24) | .014 | 0.10 (0.14) | .446 | 0.02 (0.10) | .817 |
| GMLT Total Errors | −0.71 (0.27) | .013 | 0.13 (0.23) | .543 | 0.04 (0.17) | .786 |
| GMLT Speed | −0.27 (0.24) | .272 | 0.32 (0.15) | .032 | −0.10 (0.11) | .326 |
| Individual Standard Neuropsychological Tests | ||||||
| Logical Memory delayed recall | −0.10 (0.24) | .822 | −0.15 (0.18) | .052 | 0.21 (0.13) | .330 |
| AVLT delayed recall | 0.04 (0.25) | .880 | 0.18 (0.16) | .246 | −0.04 (0.12) | .704 |
| Visual Reproduction delayed recall | −0.10 (0.24) | .690 | −0.15 (0.18) | .407 | 0.21 (0.13) | .120 |
| WAIS-R Digit Symbol | −0.33 (0.24) | .173 | 0.39 (0.12) | .004 | −0.24 (0.09) | .011 |
| TMT-A | −0.18 (0.25) | .456 | 0.12 (0.19) | .528 | −0.02 (0.14) | .905 |
| TMT-B | −0.09 (0.25) | .710 | 0.31 (0.17) | .068 | −0.19 (0.12) | .114 |
| WAIS-R Picture Completion | 0.13 (0.22) | .581 | 0.04 (0.18) | .799 | 0.07 (0.13) | .608 |
| WAIS-R Block Design | −0.17 (0.24) | .465 | 0.05 (0.14) | .730 | −0.005 (0.10) | .966 |
| Boston Naming Test | 0.16 (0.24) | .512 | −0.03 (0.13) | .837 | 0.07 (0.09) | .484 |
| Category Fluency | −0.05 (0.22) | .833 | 0.13 (0.15) | .382 | −0.10 (0.11) | .372 |
| Global and Domain-specific Scores | ||||||
| Global z-score | −0.09 (0.14) | .527 | 0.11 (0.06) | .057 | −0.03 (0.04) | .510 |
| Memory z-score | −0.08 (0.67) | .679 | 0.09 (0.10) | .364 | 0.03 (0.07) | .704 |
| Language z-score | 0.06 (0.19) | .770 | 0.05 (0.10) | .605 | −0.02 (0.07) | .823 |
| Executive function z-score | −0.21 (0.21) | .330 | 0.35 (0.11) | .004 | −0.22 (0.08) | .011 |
| Visual spatial z-score | −0.03 (0.19) | .862 | 0.03 (0.12) | .763 | 0.04 (0.09) | .656 |
Abbreviations: GMLT, Groton Maze Learning Test; AVLT, Auditory Verbal Learning Test; WAIS-R, Wechsler Memory Scale-Revised Logical Memory II; TMT-A, Trail Making Test, Part A; TMT-B, Trail Making Test, Part B.
Models adjust for baseline age, sex, and education.
Adjusted P value obtained through permutation testing.
Notes: Baseline amyloid refers to the cross-sectional association between baseline amyloid (as a continuous variable) and baseline cognitive performance. Time refers to the annual change in z-score for the cognitive dependent variable. The baseline amyloid*time variable refers to annual rate of change in the dependent cognitive variable for each unit increase in baseline amyloid SUVR.
The relationship between baseline amyloid and baseline and longitudinal performance on some of the cognitive tests differed by the presence of an APOE ε4 allele (Table 3). Among APOE ε4 carriers, higher baseline amyloid was associated with poorer performance on baseline psychomotor speed (DET; b = −1.26, P = .002) and spatial working memory (GMLT Total Errors; b = −1.01, P = .017). There was also a trend for poorer baseline performance on the Wechsler Memory Scale-Revised Logical Memory II delayed recall (b = −0.069, P = .053). However, among APOE ε4 carriers, amyloid did not predict change on any cognitive test.
Table 3.
Multivariate linear mixed models examining the effect of amyloid and APOE E4 genotype on baseline cognition and cognitive decline
| Cognitive z-scores | No APOE E4 allele (N=337) | ≥1 APOE E4 allele (N=131) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Amyloid | Time | Baseline Amyloid * time | Baseline Amyloid | Time | Baseline Amyloid * time | |||||||
| b(se) | P value** | b(se) | P value** | b(se) | P value** | b(se) | P value** | b(se) | P value** | b(se) | P value** | |
| CogState Tests | ||||||||||||
| Detection (DET) | 0.20 (0.48) | .683 | 0.02 (0.30) | .939 | 0.04 (0.23) | .861 | −1.26 (0.40) | .002 | −0.29 (0.34) | .379 | 0.27 (0.24) | .236 |
| Identification (IDN) | 0.50 (0.43) | .254 | 0.05 (0.22) | .831 | 0.12 (0.17) | .446 | −0.28 (0.34) | .410 | −0.01 (0.22) | .967 | 0.15 (0.15) | .314 |
| One Card Learning (OCL) | −0.27 (0.45) | .553 | −0.11 (0.22) | .605 | 0.19 (0.16) | .228 | −0.51 (0.31) | .099 | 0.23 (0.19) | .219 | −0.09 (0.13) | .519 |
| One Back (ONB) | 0.01 (0.43) | .981 | 0.52 (0.28) | .068 | −0.04 (0.21) | .839 | −0.36 (0.31) | .250 | 0.20 (0.25) | .417 | 0.15 (0.17) | .387 |
| GMLT Total Errors | −0.16 (0.74) | .720 | 0.06 (0.35) | .847 | 0.08 (0.26) | .732 | −1.01 (0.37) | .017 | 0.26 (0.36) | .414 | −0.04 (0.25) | .859 |
| GMLT Speed | 0.36 (0.43) | .410 | 0.33 (0.22) | .135 | −0.13 (0.17) | .429 | −0.53 (0.34) | .124 | 0.46 (0.22) | .040 | −0.16 (0.15) | .287 |
| Individual Standard Neuropsychological Tests | ||||||||||||
| Logical Memory delayed recall | 1.30 (0.46) | .005 | 0.66 (0.25) | .010 | −0.38 (0.19) | .047 | −0.69 (0.35) | .053 | 0.10 (0.23) | .650 | 0.03 (0.16) | .840 |
| AVLT delayed recall recall | 0.28 (0.47) | .553 | 0.37 (0.25) | .136 | −0.19 (0.19) | .294 | −0.03 (0.30) | .930 | 0.06 (0.21) | .780 | 0.05 (0.15) | .756 |
| Visual Reproduction delayed recall | 0.52 (0.45) | .241 | −0.02 (0.29) | .954 | 0.11 (0.22) | .608 | −0.48 (0.30) | .109 | −0.27 (0.25) | .266 | 0.28 (0.17) | .107 |
| WAIS-R Digit Symbol | −0.01 (0.46) | .994 | 0.69 (0.20) | .003 | −0.48 (0.15) | .004 | −0.40 (0.30) | .181 | 0.28 (0.14) | .069 | −0.14 (0.10) | .186 |
| TMT-A | 0.55 (0.43) | .198 | 0.42 (0.29) | .141 | −0.23 (0.22) | .278 | −0.45 (0.37) | .216 | −0.21 (0.30) | .467 | 0.17 (0.21) | .381 |
| TMT-B | 0.29 (0.45) | .497 | 0.41 (0.23) | .077 | −0.26 (0.17) | .120 | −0.05 (0.36) | .876 | 0.25 (0.32) | .393 | −0.15 (0.22) | .454 |
| WAIS-R Picture Completion | 0.55 (0.42) | .196 | 0.21 (0.27) | .436 | −0.07 (0.20) | .724 | −0.04 (0.29) | .881 | 0.07 (0.26) | .791 | 0.09 (0.18) | .633 |
| WAIS-R Block Design | 0.35 (0.45) | .447 | −0.01 (0.21) | .960 | 0.02 (0.16) | .879 | 0.44 (0.29) | .123 | 0.22 (0.21) | .291 | −0.09 (0.15) | .531 |
| Boston Naming Test | 0.68 (0.15) | .147 | −0.06 (0.20) | .762 | 0.07 (0.15) | .614 | 0.05 (0.29) | .862 | 0.16 (0.19) | .389 | −0.02 (0.13) | .859 |
| Category Fluency | 0.43 (0.41) | .296 | 0.10 (0.24) | .662 | −0.08 (0.18) | .675 | −0.19 (0.29) | .545 | 0.14 (0.20) | .472 | −0.11 (0.14) | .408 |
| Global and Domain-specific Scores | ||||||||||||
| Global | 0.45 (0.25) | .077 | 0.23 (0.09) | .010 | −0.13 (0.07) | .063 | −0.28 (0.19) | .145 | 0.08 (0.08) | .345 | 0.01 (0.06) | .918 |
| Memory | 0.69 (0.36) | .052 | 0.32 (0.16) | .049 | −0.14 (0.13) | .246 | −0.46 (0.25) | .071 | −0.07 (0.14) | .632 | 0.14 (0.10) | .155 |
| Language | 0.55 (0.35) | .123 | 0.01 (0.16) | .929 | 0.01 (0.12) | .974 | −0.07 (0.25) | .786 | 0.15 (0.14) | .283 | −0.07 (0.10) | .482 |
| Executive function | 0.14 (0.39) | .706 | 0.54 (0.16) | .002 | −0.37 (0.12) | .004 | −0.23 (0.27) | .401 | 0.25 (0.18) | .150 | −0.14 (0.12) | .251 |
| Visual spatial | 0.44 (0.25) | .224 | 0.10 (0.18) | .581 | −0.03 (0.13) | .847 | −0.26 (0.25) | .287 | 0.13 (0.18) | .456 | 0.01 (0.12) | .938 |
Abbreviations: APOE, apolipoprotein E; GMLT, Groton Maze Learning Test; AVLT, Auditory Verbal Learning Test; WAIS-R, Wechsler Memory Scale-Revised Logical Memory II; TMT-A, Trail Making Test, Part A; TMT-B, Trail Making Test, Part B.
Models adjust for baseline age, sex, and education.
Adjusted P value obtained through permutation testing
Notes: Baseline amyloid refers to the cross-sectional association between baseline amyloid (as a continuous variable) and baseline cognitive performance. Time refers to the annual change in z-score for the cognitive dependent variable. The baseline amyloid*time variable refers to annual rate of change in the dependent cognitive variable for each unit increase in baseline amyloid SUVR.
Interestingly, and in contrast to the above results, among APOE ε4 non-carriers higher baseline amyloid was associated with better baseline performance on the Wechsler Memory Scale-Revised Logical Memory II delayed recall (b = 1.30, P = .005; Table 3). Further, higher baseline amyloid predicted cognitive decline on the Wechsler Memory Scale-Revised Logical Memory II delayed recall (b = −0.038, P = .047), WAIS-R Digit Symbol (b = −0.24, P = .011) and the Executive domain (b = −0.22, P = .011).
3.3. Sensitivity analyses
In additional analyses we utilized an amyloid PiB-PET cutoff of SUVR ≥1.4. Participants with SUVR ≥1.4 did not perform worse on any cognitive test at baseline or longitudinally. In other analyses, we restricted the sample to the 121 individuals with data at all visits but the results did not differ. We also reran the analyses excluding the 5 individuals with incident MCI but the results remained. Lastly, we determined the effect of the APOE genotype and amyloid level on censoring, but neither was predictive of missing data or loss-to-follow-up.
4. Discussion
In this study of late middle-aged cognitively normal individuals enrolled in the population-based Mayo Clinic Study of Aging, we found few associations between brain amyloid levels, as measured by PiB PET, and cross-sectional and longitudinal measures of cognition over 30 months, including tests of episodic memory. APOE genotype alone was not cross-sectionally or longitudinally associated with cognition. However, some of the associations between amyloid and cognition varied by APOE genotype.
Observational studies examining the effect of amyloid and APOE genotype on cognition in cognitively normal individuals, who are substantially older than in the current study, have been inconsistent [4–12,31–34]. A meta-analysis focused on cognitively normal older individuals reported that amyloid was associated with worse episodic memory and there were trends for associations with executive function and global cognition [35]. However, while the associations were statistically significant, the effect sizes were small. Notably, most studies examining the effect of amyloid and APOE ε4 genotype on cognition in older individuals are of individuals aged 70 and older. The age of the study population is important because the appearance of abnormal amyloidosis increases with age beginning in the late 50s [36]. Declines in memory become more rapid after the seventh decade, corresponding to the period in which the symptoms of sporadic AD begin to increase in prevalence [37,38].
Recent studies of younger individuals have not found a relationship between amyloid and episodic memory. A cross-sectional study of 137 individuals, mean age of 60 (range 30–89 years), found a significant dose-response effect of mean cortical amyloid on processing speed and fluid reasoning, but not on working memory, episodic memory, or crystallized intelligence [12]. Another study of 201 individuals with a mean age of 64 (range 46–73 years) also did not find an association between amyloid burden and cognition [39]. Our observations are unique in the literature due to the larger sample size of 464 late middle-aged participants with a mean age of 62.6 (range 51–71 years). Overall, we found few associations between amyloid and cognition. Further, amyloid was more strongly associated with measures of attention and executive function than with measures of episodic memory. Among individuals with an APOE ε4 allele, we did observe cross-sectional associations between amyloid and visual working memory, and a trend for an association between higher amyloid and delayed episodic memory on the Logical Memory test. However, amyloid was not predictive of cognitive decline in APOE ε4 carriers. Only among APOE ε4 non-carriers did we find that higher amyloid was predictive of decline on one episodic memory test and on tests of executive function. It is possible that, when stratifying by APOE ε4 carrier status, our sample size among APOE ε4 carriers did not allow for enough power to detect an association. However, there were no trends and our sample was still twice the size of previous studies of middle-aged individuals. We conducted several sensitivity analyses to assure there was not a methodological reason for this observation, but we did not identify one. There are multiple possible explanations for these findings. First, some studies suggest that changes in executive function may precede declines in memory among individuals who later develop AD [40–42]. Our results would support these deductions. Second, it is possible that APOE ε4 carriers had cognitive decline prior to enrolling in the study, and therefore did not have substantial additional cognitive decline over the next 30 months. Thus, we only saw an association between amyloid and cognition among non APOE ε4 carriers. Lastly, a large number of statistical tests were performed. While we did utilize permutation testing, it is still possible that some of the observed associations may be due to type 1 error. Longitudinal studies with longer follow-up in young- and middle-aged adults with serial imaging and cognition are needed to further elucidate our results.
Much research has focused on identifying the best compilation of neuropsychological test items aimed at detecting the smallest amount of cognitive change in the shortest period of time in cognitively normal individuals e.g., [43,44]. A computerized battery may have an advantage over standard neuropsychological tests or other cognitive screening measures by being more sensitive and efficient, removing ceiling and floor effects, providing real-time data entry, suitability for off-site or long-distance use and longitudinally screening for and assessing cognition at the population-level [14–16]. However, few studies have examined the longitudinal performance of computerized cognitive batteries in middle-aged individuals. In the present study, we assessed cognition with both the CogState computerized battery and standard neuropsychological tests. With the exception of the CogState OCL accuracy measure, practice effects on the CogState computerized tests and standard neuropsychological tests were similar in this group of middle-aged individuals. Because computerized testing may be more suitable to longitudinal screening, and screening large populations, these results suggest that computerized tests may offer a means for longitudinally assessing cognition in this age group. Indeed, we have previously demonstrated the acceptability and feasibility of computerized testing, in the clinic and at home, among non-demented individuals aged 50 and older enrolled in the Mayo Clinic Study on Aging [28]. We did not, however, expect the sustained practice effects observed for the OCL accuracy measure in this age group. Previous studies have not observed such large practice effects on the OCL, including when administered 4 times in one day [29]. Reasons for our observation are not fully understood but are important for discerning the use of this test as a cognitive outcome measure in both clinical trials and longitudinal epidemiological studies. Implicit memory may play a role in this phenomenon wherein the participant derives some benefit from having completed the procedure previously despite unpredictability in the interstimulus intervals and the response required for successful completion of each item.
Strengths of our study include the large number of late middle-aged individuals, the population-based prospective cohort design, and the comprehensive evaluation of participants. One limitation is that we only assessed the effect of amyloid on cognition in this analysis. Neurodegenerative markers are altered closer in time to the development of cognitive symptoms [1–3,45]. Future analyses will need to assess the effects of neurodegeneration, including cortical thickness and tau pathology in this age group. Further longitudinal exploration of computerized tests in this age group is also warranted to better understand the observed practice effects and how they might affect estimates of treatment effects in clinical trials.
Supplementary Material
Research in context.
1. Systematic review
We reviewed the literature in PubMed that focused on detecting early cognitive changes and the effects of amyloid and APOE genotype on cognition. Several studies are now focused on identifying neuropsychological tests that are more sensitive to subtle cognitive changes. However, few studies have examined the performance of computerized cognitive batteries in a large population-based middle-aged cohort. Studies examining the effects of amyloid and APOE genotype on cognition have primarily utilized cohorts aged 65 an older.
2. Interpretation
With the exception of the CogState One Card Learning (OCL) accuracy measure, practice effects on the CogState computerized tests and standard neuropsychological tests were similar in this group of middle-aged individuals aged 51–71. However, a sustained practice effect was observed for the OCL accuracy measure in this age group. Elevated amyloidosis and APOE genotype did not appear to exert a dramatic influence on cognition in middle-age. If anything, amyloid was more strongly associated with measures of attention and executive function than with measures of episodic memory.
3. Future Directions
Neurodegenerative markers may be more predictive of cognitive symptoms. Future analyses will need to assess the effects of neurodegeneration, including cortical thickness and tau pathology, on cognition in middle-aged individuals. In addition, longitudinal exploration of computerized tests in this age group is critical to better understand the observed practice effects and how they might affect estimates of treatment effects in clinical trials.
Acknowledgments
This study was supported by funding from the National Institutes of Health/National Institute on Aging grants U01 AG006786, RO1 AG011378, and R01 AG041851; the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program, the Walter S. and Lucienne Driskill Foundation, the Elsie and Marvin Dekelboum Family Foundation, and was made possible by the Rochester Epidemiology Project (R01 AG034676).
Abbreviations
- AD
Alzheimer’s disease
- APOE
apolipoprotein E
- AVLT
Auditory Verbal Learning Task
- DET
detection
- GMLT
Groton Maze Learning Test
- IDN
identification
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- OCL
One Card Learning
- ONB
One Back
- PiB PET
Pittsburgh compound B positron emission tomography
- ROIs
regions of interest
- SUVR
standardized uptake value ratio
- TMT-A
Trail Making Test, Part A
- TMT-B
Trail Making Test, Part B
- WAIS-R
Wechsler Memory Scale-Revised Logical Memory II
Footnotes
Statistical analyses were conducted by: C. E. Hagen, MS and T. J. Christianson, BSc, Division of Biomedical Statistics and Informatics, Mayo Clinic.
Supplementary data related to this article can be found online.
Disclosure Statement: Dr. Mielke served as a consultant to AbbVie and Lysosomal Therapeutics, Inc., and receives research support from the NIH/NIA and the Michael J. Fox Foundation. Dr. Machulda, Mr. Hagen, and Dr. Roberts receive research support from the National Institutes of Health. Dr. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRx Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH. Dr. Lowe serves on scientific advisory boards for Piramal Imaging and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI). Dr. Kremers reports no disclosures. Dr. Jack has provided consulting services for Eli Lilly. He receives research funding from the National Institutes of Health, and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Petersen serves on scientific advisory boards for Pfizer, Inc., Janssen Alzheimer Immunotherapy, Roche, Inc., Merck, Inc., and Genentech, Inc.; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH/NIA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle M. Mielke, Email: Machulda.Mary@mayo.edu.
Clinton E. Hagen, Email: Hagen.Clinton@mayo.edu.
Teresa J. Christianson, Email: Christianson.Teresa@mayo.edu.
Rosebud O. Roberts, Email: roberts.rosebud@mayo.edu.
David S. Knopman, Email: Knopman@mayo.edu.
Prashanthi Vemuri, Email: Vemuri.Prashanthi@mayo.edu.
Val J. Lowe, Email: vlowe@mayo.edu.
Walter K. Kremers, Email: kremers.walter@mayo.edu.
Clifford R. Jack, Jr, Email: jack.clifford@mayo.edu.
Ronald C. Petersen, Email: peter8@mayo.edu.
References
- 1.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6:295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 3.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnick SM, Sojkova J, Zhou Y, An Y, Ye W, Holt DP, et al. Longitudinal cognitive decline is associated with fibrillar amyloid-beta measured by [11C]PiB. Neurology. 2010;74:807–15. doi: 10.1212/WNL.0b013e3181d3e3e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78:1576–82. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–81. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, et al. Abeta and cognitive change: examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement. 2014;10:743–51. e1. doi: 10.1016/j.jalz.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 8.Kawas CH, Greenia DE, Bullain SS, Clark CM, Pontecorvo MJ, Joshi AD, et al. Amyloid imaging and cognitive decline in nondemented oldest-old: the 90+ Study. Alzheimers Dement. 2013;9:199–203. doi: 10.1016/j.jalz.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doraiswamy PM, Sperling RA, Johnson K, Reiman EM, Wong TZ, Sabbagh MN, et al. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Mol Psychiatry. 2014;19:1044–51. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, et al. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–92. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mormino EC, Betensky RA, Hedden T, Schultz AP, Ward A, Huijbers W, et al. Amyloid and APOE epsilon4 interact to influence short-term decline in preclinical Alzheimer disease. Neurology. 2014;82:1760–7. doi: 10.1212/WNL.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, et al. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78:387–95. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machulda MM, Pankratz VS, Christianson TJ, Ivnik RJ, Mielke MM, Roberts RO, et al. Practice effects and longitudinal cognitive change in normal aging vs. incident mild cognitive impairment and dementia in the Mayo Clinic Study of Aging. Clin Neuropsychol. 2013;27:1247–64. doi: 10.1080/13854046.2013.836567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder PJ, Jackson CE, Petersen RC, Khachaturian AS, Kaye J, Albert MS, et al. Assessment of cognition in mild cognitive impairment: a comparative study. Alzheimers Dement. 2011;7:338–55. doi: 10.1016/j.jalz.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild K, Howieson D, Webbe F, Seelye A, Kaye J. Status of computerized cognitive testing in aging: a systematic review. Alzheimers Dement. 2008;4:428–37. doi: 10.1016/j.jalz.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mielke MM, Machulda MM, Hagen CE, Edwards KK, Roberts RO, Pankratz VS, et al. Performance of the CogState computerized battery in the Mayo Clinic Study on Aging. Alzheimers Dement. doi: 10.1016/j.jalz.2015.01.008. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–8. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 19.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 20.Rey A. L’examen Clinique en Psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 21.Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 22.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 23.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 2006. [Google Scholar]
- 24.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 25.Wechsler D. Wechsler Adult Intelligence Scale-Revised [Manual] San Antonio, TX: Psychological Corporation; 1981. [Google Scholar]
- 26.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo’s Older Americans Normative Studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol. 1992;6:1–30. [Google Scholar]
- 27.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology. 2010;75:889–97. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mielke MM, Weigand SD, Wiste HJ, Vemuri P, Machulda MM, Knopman DS, et al. Independent comparison of CogState computerized testing and a standard cognitive battery with neuroimaging. Alzheimers Dement. 2014;10:779–89. doi: 10.1016/j.jalz.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc. 2003;9:419–28. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- 30.Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Lowe V, Vemuri P, et al. Amyloid-first and neurodegeneration-first profiles characterize incident amyloid PET positivity. Neurology. 2013;81:1732–40. doi: 10.1212/01.wnl.0000435556.21319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, et al. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361:255–63. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiepers OJ, Harris SE, Gow AJ, Pattie A, Brett CE, Starr JM, et al. APOE E4 status predicts age-related cognitive decline in the ninth decade: longitudinal follow-up of the Lothian Birth Cohort 1921. Mol Psychiatry. 2012;17:315–24. doi: 10.1038/mp.2010.137. [DOI] [PubMed] [Google Scholar]
- 33.Kozauer NA, Mielke MM, Chan GK, Rebok GW, Lyketsos CG. Apolipoprotein E genotype and lifetime cognitive decline. Int Psychogeriatr. 2008;20:109–23. doi: 10.1017/S104161020700587X. [DOI] [PubMed] [Google Scholar]
- 34.Bunce D, Fratiglioni L, Small BJ, Winblad B, Backman L. APOE and cognitive decline in preclinical Alzheimer disease and non-demented aging. Neurology. 2004;63:816–21. doi: 10.1212/01.wnl.0000137041.86153.42. [DOI] [PubMed] [Google Scholar]
- 35.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–8. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, sex, and APOE epsilon4 effects on memory, brain structure, and beta- amyloid across the adult life span. JAMA Neurol. 2015;72:511–9. doi: 10.1001/jamaneurol.2014.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocca WA, Petersen RC, Knopman DS, Hebert LE, Evans DA, Hall KS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Dement. 2011;7:80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51:728–33. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- 39.Johnson SC, Christian BT, Okonkwo OC, Oh JM, Harding S, Xu G, et al. Amyloid burden and neural function in people at risk for Alzheimer’s Disease. Neurobiol Aging. 2014;35:576–84. doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrington MG, Chiang J, Pogoda JM, Gomez M, Thomas K, Marion SD, et al. Executive function changes before memory in preclinical Alzheimer’s pathology: a prospective, cross-sectional, case control study. PLoS One. 2013;8:e79378. doi: 10.1371/journal.pone.0079378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson MC, Xue QL, Zhou J, Fried LP. Executive decline and dysfunction precedes declines in memory: the Women’s Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2009;64:110–7. doi: 10.1093/gerona/gln008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baddeley AD, Bressi S, Della Sala S, Logie R, Spinnler H. The decline of working memory in Alzheimer’s disease. A longitudinal study Brain. 1991;114( Pt 6):2521–42. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- 43.Ayutyanont N, Langbaum JB, Hendrix SB, Chen K, Fleisher AS, Friesenhahn M, et al. The Alzheimer’s prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer’s disease treatments in presenilin 1 E280A mutation carriers. J Clin Psychiatry. 2014;75:652–60. doi: 10.4088/JCP.13m08927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langbaum JB, Hendrix SB, Ayutyanont N, Chen K, Fleisher AS, Shah RC, et al. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014;10:666–74. doi: 10.1016/j.jalz.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jack CR, Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.