Abstract
Introduction
Very low birth weight (VLBW) and premature infants are at risk of developing postnatal cytomegalovirus (CMV) disease, including CMV-related sepsis-like syndrome (CMV-SLS). Estimates of breast milk-acquired CMV infection and disease among these infants in the United States are lacking.
Methods
We performed a systematic review and meta-analysis to estimate the pooled proportions (and 95% confidence intervals) of VLBW and premature infants born to CMV-seropositive women with breast milk-acquired CMV infection and CMV-SLS. We combined these proportions with population-based rates of CMV seropositivity, breast milk feeding, VLBW and prematurity to estimate annual rates of breast milk-acquired CMV infection and CMV-SLS in the United States.
Results
In our meta-analysis, among 299 infants fed untreated breast milk, we estimated 19% (11%–32%) acquired CMV infection and 4% (2%–7%) developed CMV-SLS. Assuming these proportions, we estimated a rate of breast milk-acquired CMV infection among VLBW and premature infants in the United States of 6.5% (3.7%–10.9%) and 1.4% (0.7%–2.4%) of CMV-SLS, corresponding to 600 infants with CMV-SLS in 2008. Among 212 infants fed frozen breast milk, our meta-analysis proportions were 13% (7%–24%) for infection and 5% (2%–12%) for CMV-SLS, yielding slightly lower rates of breast milk-acquired CMV infection (4.4%; 2.4%–6.8%) but similar rates of CMV-SLS (1.7%; 0.7%–4.1%).
Conclusions
Breast milk-acquired CMV infection presenting with CMV-SLS is relatively rare. Prospective studies to better define the burden of disease are needed to refine guidelines for feeding breast milk from CMV-seropositive mothers to VLBW and premature infants.
Keywords: breast milk, cytomegalovirus, premature infant, very low birth weight infant, sepsis-like syndrome
INTRODUCTION
Cytomegalovirus (CMV) may be transmitted in utero as a result of primary maternal infection or recurrent infection resulting from re-infection with a new CMV strain or reactivation of latent virus.1 CMV may also be acquired perinatally via exposure to infected maternal genital secretions during delivery, or postnatally by blood transfusion or infected breast milk.2 CMV disease due to postnatally-acquired infection is uncommon in full-term infants, presumably because of protection from passive transfer of maternal antibodies that occur mostly in the third trimester, and the infant’s more mature immune system.3, 4 However, infants born <32 weeks gestational age or with a birth weight <1500g may be at higher risk of developing symptomatic postnatal CMV disease, characterized by hepatopathy, thrombocytopenia, neutropenia, petechiae, respiratory distress syndrome, and sepsis-like syndrome.4, 5
Breastfeeding is a common route for CMV transmission, particularly in populations with high CMV seroprevalence and high rates of breastfeeding.6 CMV is commonly excreted in breast milk from seropositive women, beginning during the first week postpartum, peaking at 4–8 weeks after delivery, and declining steadily thereafter. Infectious virus and CMV DNA and RNA have been isolated from cell-associated and whey fractions in the breast milk of 40%–97% of CMV-seropositive lactating women.7–17 In its 2012 Policy Statement on breastfeeding and use of human milk, the American Academy of Pediatrics stated: “The value of routinely feeding [fresh] human milk from [CMV] seropositive mothers to preterm infants outweighs the risks of clinical disease, especially because no long-term neurodevelopmental abnormalities have been reported”.18
In the United States, an estimated 58% of pregnancies occur among CMV-seropositive women.19 Because few data exist on the incidence of breast milk-acquired CMV infection and disease among preterm infants, we conducted a systematic review and meta-analysis of studies reporting on postnatal CMV infection and disease presumably acquired via consumption of breast milk among very low birth weight (VLBW) and premature infants born to CMV-seropositive women but uninfected at birth. We applied the results of our meta-analysis to US population-based data to estimate the annual rates in the United States of 3 outcomes: breast milk-acquired CMV infection, CMV-related symptoms, and CMV-related sepsis-like syndrome (CMV-SLS).
METHODS
Systematic Review
Studies published in English, French, Spanish, or Portuguese with no restriction on publication date were identified by searching Web of Science, PubMed, OVID/Medline and EMBASE databases, using the following keywords and variations: breast feeding or breast milk, premature or preterm, low birth weight or very low birth weight infants, cytomegalovirus or CMV, postnatal CMV infection, breast milk-acquired CMV infection. We included original studies providing data on postnatal CMV infection in VLBW and premature infants born to CMV-seropositive mothers, presumably acquired via consumption of untreated, frozen or pasteurized breast milk. We also included additional studies found in the references of studies identified during the literature search that were not among our search results. We excluded reviews, guidelines, expert opinions, multiple reports from the same authors reporting results from the same population, studies of non-VLBW infants, and case reports.
We reviewed each study for the following information: assessment of maternal CMV serological status; infant inclusion criteria (weight and gestational age at birth); infant exclusion criteria (methods for diagnosing congenital CMV infection); methods for diagnosing postnatal CMV infection among infants; breast milk handling process (pasteurization, freezing, no treatment); numbers of infants who acquired postnatal CMV infection and developed CMV-related symptoms or CMV-SLS born to and fed breast milk from CMV-seropositive mothers; infants’ birth weight and corrected gestational age at onset of CMV viruria; measures taken to prevent or identify CMV transmission from blood transfusion; and administration of prophylactic immunoglobulin. The risk of bias of individual studies was assessed by evaluating the study population (inclusion and exclusion criteria) and possible CMV transmission by means other than breast milk (e.g. blood transfusion).
Meta-Analysis
We included studies that reported the number of infants born to CMV-seropositive mothers, defined by assessment of maternal serological status, who were uninfected at birth (i.e. congenital CMV infection was excluded) and acquired CMV infection postnatally. Congenital CMV infection was defined as a positive viral culture, shell vial assay or CMV-DNA test in cord blood or urine within the first 3 weeks of life. Postnatal CMV infection was defined as a positive viral culture, shell vial assay or CMV-DNA test in urine not earlier than after 2 weeks of life when previous results were negative. We defined CMV-related symptoms as any of the following: neutropenia, thrombocytopenia, petechiae, hepatopathy, hyperbilirubinemia, elevated liver enzymes, jaundice or CMV pneumonia; and CMV-SLS as sepsis-like symptoms, such as bradycardia, apnea or respiratory deterioration, in the absence of bacterial infection and coincident with CMV viruria.
We grouped studies or subgroups within studies by infants who were fed: 1) untreated breast milk, 2) frozen breast milk, or 3) combinations of untreated, frozen or pasteurized breast milk, or not specified. We ran separate meta-analyses for each of these three groups and estimated the pooled proportions (and 95% confidence intervals; CI) of infants born to CMV-seropositive mothers who acquired CMV infection, developed CMV-related symptoms, or CMV-SLS. We used random effects (DerSimonian-Laird) models for the meta-analyses, which accounts for heterogeneity across studies by minimizing two sources of variance in measuring the true prevalence: within-study errors and variation across studies.20 We calculated the I2 statistic to assess the heterogeneity across the studies.21 To assess the robustness of the meta-analysis results, we performed a sensitivity analysis that included only the studies that attempted to rule out CMV infection acquired through transfused blood products and studies that attempted to prevent such transmission by using CMV seronegative or leukocyte-reduced blood products. All meta-analyses were done using Comprehensive Meta Analysis Version 2.2.064 (Biostat, Englewood, NJ, USA).
Estimated Rates of Breast Milk-Acquired CMV Infection and Disease in the United States
To estimate annual rates of breast milk-acquired CMV infection, CMV-related symptoms, and CMV-SLS in the United States, we used the pooled estimated proportions of these three outcomes from our meta-analyses with population-based data which accounted for differences in CMV seropositivity, breast milk feeding, VLBW and prematurity rates by maternal age and race/ethnicity in the United States. We used the following formula: Zi = (1-α)βμPi, weighted by the proportion of VLBW and premature infants by maternal age and race/ethnicity, where α is the birth prevalence of congenital CMV infection by maternal age and race/ethnicity, β is the age- and race/ethnicity-specific CMV seropositivity proportion among women, μ is the breastfeeding rates, and Pi, the meta-analysis pooled estimated proportions (and 95% CI) for each of the three outcomes i stated above, for infants fed: 1) untreated breast milk, 2) frozen breast milk, or 3) combinations of untreated, frozen or pasteurized breast milk or non-specified. For US estimates of congenital CMV birth prevalence22 and CMV seropositivity among women we used published data based on the 3rd US National Health and Nutrition Examination Survey.19 For breast milk feeding rates, we used published data on breast milk feeding from the California Perinatal Quality Care Collaborative which includes data on over 90% of NICUs in California.23 Because rates of breast milk feeding stratified by maternal age and race/ethnicity together were not available, we had to develop two separate models. Model 1 accounted for breast milk feeding rates by maternal race/ethnicity and Model 2, by maternal age. We then applied each of our estimated rates to the number of US infants born annually with birth weight <1500g and gestational age <32 weeks, based on 2008 national vital statistics data.24
RESULTS
Systematic Review
Of a total of 67 unique citations from 1980 to 2011, 50 (78%) were excluded. Excluded citations included 13 case reports, 12 reviews, guidelines or expert opinions, 10 studies assessing prevalence of perinatal infection or CMV transmission in non-VLBW infants, 6 reports from overlapping populations, and 9 studies unrelated to the scope of this review, including animal models and laboratory studies. The 17 studies included in this review were published during 2001–2011 (Table 1).8–17, 25–31 Nine studies were conducted in Europe (Germany, Italy, France, UK and Sweden), 4 in Asia (Japan and Taiwan), 2 in North America (Canada and United States), 1 in South America (Brazil), and 1 in the Middle-East (Israel).
Table 1.
Summary of 17 Studies from Systematic Review and Meta-Analysis
| Infants fed | Study, by year of publication, country |
Methods: Inclusion Criteria |
Infants fed breast milk from CMV- seropositive mothers |
Infants with breast milk-acquired CMV infection |
Infants with CMV- related symptomsa |
Infants with CMV-SLSb |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| n | n (%) | Onset of CMV viruria | n (%) | n (%) | Onset of CMV viruria |
||||||
|
|
|
|
|||||||||
| Birth weight, grams |
Gestational age, weeks |
Chronological age, days Median (range) |
Corrected age for gestation, weeks Median (range) |
Corrected age for gestation, weeks Median (range) |
|||||||
| Untreated breast milk | Hamprecht, 2001 Germany8 | <1500 | or | <32 | 90 | 33 (37) | 53.5 (27–120) | 34.5 (28.3–47.1) | 16 (18) | 4 (4) | 29.8 (28.3–32.1) |
| Mussi-Pinhata, 2004 Brazil (Subgroup)25 | <1500 | or | <34 | 49 | 13 (27) | 75 (32–140)± | NA | 0 (0) | 0 (0) | - | |
| Meier, 2005 Germany9 | ≤2,010* | <33 | 21 | 5 (24) | NA | NA | 2 (10) | 1 (5) | NA | ||
| Miron, 2005 Israel26 | <1500 | or | <32 | 70 | 4 (6) | 35 (28–49) | 34 (31–35) | 3 (4) | 1 (1) | 31 | |
| Croly-Labourdette, 2006 France10 | ≤2240* | <33 | 7 | 1(14) | 53 | 36.8 | 0 (0) | 0 (0) | - | ||
| Capretti, 2009 Italy 11 | <1500 | and | <32 | 62 | 9 (15) | 51 (36–80) | 36.1 (31–41.4) | 8 (13) | 3 (5) | 33.4 (31–34) | |
|
| |||||||||||
| Overall (n) | 299 | 65 | 29 | 9 | |||||||
| Pooled proportion from meta-analysis, % (95%CI) | 19 (11–32) | 10 (5–17) | 4 (2–7) | ||||||||
| I2 | 77 | 50 | 0 | ||||||||
|
| |||||||||||
| Frozen breast milk | Yasuda, 2003 Japan12 | <1200 | or | <34 | 34 | 3 (9) | 54 (42–84) | 33.3 (33.1–39.7) | 0 (0) | 0 (0) | - |
| Jim, 2004 Taiwan13 | <1500 | and | <35 | 40 | 6 (15) | 77§ (21–168) | NA | 6 (15) | 5 (13) | NA | |
| Lee, 2007 USA27 | <1500 | or | <32 | 23 | 2 (9) | 40.5 (37–44) | 33.1 (29.3–33.1) | 1 (4) | 0 (0) | - | |
| Jim, 2009 Taiwan14 | <1500 | and | <35 | 23 | 8 (35) | 63§ (SD=2.6) | NA | 3 (13) | 2 (9) | NA | |
| Buxmann, 2009 Germany28 | <1710* | <31 | 35 | 5 (14) | 73 (43–89) | 35.7 (32.7–39.6) | 2 (6) | 0 (0) | - | ||
| Chiavarini, 2011 Italy15 | <2000 | or | <32 | 57 | 1 (2) | 47 | 35.1 | 1 (2) | 1 (2) | 35.1 | |
|
| |||||||||||
| Overall (n) | 212 | 26 | 13 | 8 | |||||||
| Pooled proportion from meta-analysis, % (95%CI) | 13 (7–24) | 7 (3–14) | 5 (2–12) | ||||||||
| I2 | 62 | 37 | 33 | ||||||||
|
| |||||||||||
| Combinations of untreated, frozen, or pasteurized breast milk or non-specified | Mosca, 2001 Italy16 | ≤1380* | <34 | 30 | 5 (17) | 34 (NA) | NA | 0 (0) | 0 (0) | - | |
| Sharland, 2002 UK29 | NA | <32 | 18 | 1(6) | 62 | 32.9 | 0 (0) | 0 (0) | - | ||
| Mussi-Pinhata, 2004 Brazil (subgroup)25 | <1500 | or | <34 | 46 | 8 (17) | 75 (32–140)± | NA | 1 (2) | 0 (0) | - | |
| Doctor, 2005 Canada30 | <1000 | <28* | 61 | 4 (7) | 48–72 | NA | 1 (2) | 1 (2) | NA | ||
| Omarsdottir, 2007 Sweden17 | ≤1166* | <28* | 7 | 2 (29) | 34–46 | 31.3 (29.1–33.4) | 1 (14) | 1 (14) | 31.1 | ||
| Hayashi, 2011 Japan31 | <1000 | or | <28 | 22 | 1 (5) | 70 | 36.7 | 1 (5) | 0 (0) | - | |
|
| |||||||||||
| Overall (n) | 184 | 21 | 4 | 2 | |||||||
| Pooled proportion from meta-analysis, % (95%CI) | 13 (7–20) | 3 (1–8) | 3 (1–7) | ||||||||
| I2 | 26 | 0 | 0 | ||||||||
CMV-related symptoms defined as any of the following: neutropenia, thrombocytopenia, petechiae, hepatopathy, hyperbilirubinemia, elevated liver enzymes, jaundice or CMV pneumonia
CMV-SLS defined as sepsis-like symptoms, such as bradycardia, apnea or respiratory deterioration, in the absence of bacterial infection and coincident with CMV viruria.
The observed upper end of the birth weight and/or gestational age shown for studies that used only one criterion or none.
Median and range reported for all study infants, not just those shown in this category of breast milk
Mean; SD: Standard deviation
NA: Not available
All studies assessed maternal serological status by detection of CMV antibodies at delivery or up to 1 week postpartum. Mothers were tested for IgG and IgM antibodies in 9 studies8, 9, 11, 13–15, 26, 28, 31 and for IgG antibodies in 4 studies10, 12, 25, 30; 4 studies did not report which serological tests were performed16, 17, 27, 29. Studies varied regarding infant inclusion criteria, more commonly specifying a birth weight cutoff of <1500g (7 studies) or gestational age <32 weeks (6 studies) (Table 1). Infants with congenital CMV infection were excluded in all studies based on positive viral culture or CMV-DNA test in cord blood or urine within the first 3 weeks of life. Among infants who were uninfected at birth, postnatal CMV infection was determined through collection of infants’ urine weekly, biweekly or monthly until 8 to 12 weeks of age, and analysis by PCR8, 9, 11, 12, 14, 16, 25, 26, 28, 31, viral culture8, 10, 11, 13, 27, 28 or shell vial assay10, 16, 27, 29, 30.
Of the 17 studies, 5 included infants fed with untreated breast milk8–11, 26, 6 included infants fed breast milk that was frozen at temperatures of −18ºC to −20ºC for >24h or 72h12–15, 27, 28, 5 included infants fed untreated breast milk in combination with frozen breast milk and/or pasteurized breast milk from donors17, 25, 29–31, and in one study it was unclear whether the infants were given untreated or treated milk.16
Overall 695 infants born to and fed breast milk from CMV-seropositive mothers were identified in the 17 studies, with a median of 38 infants per study (range: 7–90) (Table 1). The reported proportions of infants with breast milk-acquired CMV infection varied from 2% to 37%; those who developed CMV-related symptoms and CMV-SLS varied from 0% to 18% and 0% to 14%, respectively.
The median time to first detection or onset of CMV viruria was 50 days (range: 27–120) among the 42 infants in the 8 studies that reported individual data.8, 11, 12, 17, 26–28, 31 The study by Jim et al. had the widest range in the time to first detection of CMV viruria, 21–168 days.13 In 11 studies, corrected gestational age at onset of CMV viruria was reported or could be calculated: among 45 infants with breast milk-acquired CMV infection, 34 (75%) had onset of CMV viruria between 28 and ≤37 weeks corrected age and 11 (25%), at age >37 weeks8, 10–12, 15, 17, 26–29, 31; among 19 infants who developed CMV-SLS, 6 (32%) had onset of CMV viruria at <32 weeks corrected age, 4 (21%) between 33–36 weeks, and 9 at unknown corrected age.8, 11, 15, 17, 26, 27
Three of 17 studies attempted to determine CMV viral load in breast milk and its association with transmission.12, 14, 17 In the Yasuda study, the maximum viral load in breast milk from mothers of 12 uninfected infants was higher than that from mothers of 3 infected infants.12 The Jim study found that at 4 weeks postpartum, viral load in breast milk from mothers of 8 infected infants was significantly higher than that of mothers of 15 uninfected infants.14 The Omarsdottir study found only 2 infected infants among 7 included.17 The numbers were too small to assess a possible association between viral load in breast milk and transmission or disease.
Fourteen of the 17 studies included information on the use of blood products, but most did not present data on the number of infants who received blood products. We found that the number of transfusions can be high: Hayashi reported that 20 of 27 infants (22 born to CMV-seropositive mothers and 5 to CMV-seronegative mothers) required a median of 2 (range=1–8) red blood cell transfusions, and 1 infant required a platelet transfusion.31 One of the 17 studies excluded infants that had received transfusion of blood products.10 In 10 studies, investigators reported the measures used to prevent CMV transmission from transfused blood products in their institutions, which included transfusion of CMV-IgG seronegative blood products in 5 studies8, 9, 27, 28, 30, use of leukocyte-depleted blood products in 5 studies11, 17, 26, 31, and both in one study.29 In 3 studies, actual samples of blood products administered to infants were tested as part of the study for CMV-DNA and all were negative.12, 16, 31 In one study, gamma-irradiated blood of unknown CMV status was used25, although this method does not inactivate CMV and may even increase CMV replication in latently infected cells.32 Three studies did not mention any attempt to prevent or identify CMV transmission through transfused blood products.13–15 In 2 studies, infants were prophylactically treated with intravenous immunoglobulin.11, 16
Meta-Analysis
Among the 695 infants, 299 were fed untreated breast milk8–11, 25, 26; 212 infants were fed frozen breast milk12–15, 27, 28, and 184 were fed a combinations of untreated, frozen or pasteurized breast milk or non-specified16, 17, 25, 29–31 (Table 1). Among infants who were fed untreated breast milk, 19% (95% CI=11%–32%) acquired CMV infection, 10% (95% CI=5%–17%) developed CMV-related symptoms and 4% (95%CI=2%–7%) developed CMV-SLS. Among infants who were fed frozen breast milk, 13% (95% CI=7%–24%) acquired CMV infection, 7% (95% CI=3%–14%) developed CMV-related symptoms, and 5% (95% CI=2%–12%) developed CMV-SLS. In the one available US study included in the meta-analysis, a lower proportion of infants born to CMV-seropositive mothers who received frozen breast milk acquired infection (9%) and developed CMV-related symptoms (4%), estimates that are within our observed ranges; none had CMV-SLS. Among infants who were fed combinations of untreated and frozen or pasteurized breast milk or non-specified, 13% (95% CI=7%–20%) acquired CMV infection, 3% (95% CI=1%–8%) developed CMV-related symptoms and 3% (95%CI=1%–7%) developed CMV-SLS. The I2 values for each of the meta-analyses for CMV infection and CMV-related symptoms varied widely, indicating considerable heterogeneity of the studies, but was low for CMV-SLS (Table 1).
Results of the sensitivity analysis limited to the 13 studies that ruled out CMV infection through transfused blood products10, 12, 16, 31 or that attempted to prevent such by using CMV seronegative8, 9, 27–30 or leukocyte-reduced blood products11, 17, 26, 29, 31 yielded results with CI that overlapped with those from the meta-analysis that included all studies (online supplemental material).
Estimated Rates of Breast Milk-Acquired CMV Infection and Disease in the United States, 2008
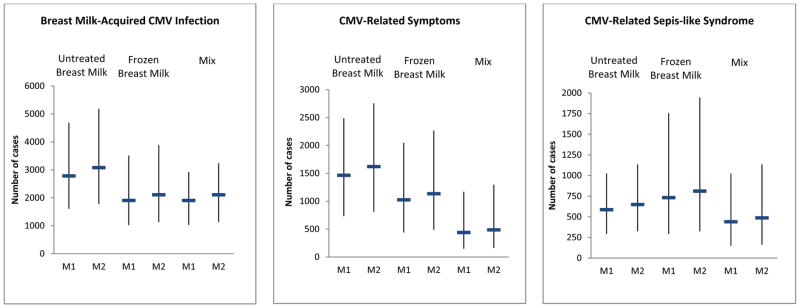
We combined the results of the meta-analysis with US population-based data (online supplemental material) to derive estimates of breast milk-acquired CMV infection and disease in the United States. Adjusting for breast milk feeding rates by maternal race/ethnicity, our estimated rates from model 1 assuming the meta-analysis results for the group of infants fed untreated milk were: 6.5% (95% CI=3.7%–10.9%) for breast milk-acquired CMV infection, 3.4% (95 CI=1.7%–5.8%) for CMV-related symptoms, and 1.4% (95% CI=0.7%–2.4%) for CMV-SLS (Table 2). These correspond to approximately 2800 infants with breast milk-acquired CMV infection, 1500 with CMV-related symptoms, and 600 with CMV-SLS in the United States in 2008, as a result of exposure to untreated breast milk (Figure 1). Point estimates for rates of breast milk-acquired CMV infection and CMV-related symptoms were lower assuming the meta-analysis results for the group of infants fed frozen milk, but the confidence limits overlapped the point estimates for the group fed untreated milk. Based on these estimates, 1900, 1000, and 700 infants acquired CMV infection, and developed CMV-related symptoms and CMV-SLS in the United States in 2008, as a result of exposure to frozen breast milk. Rates of breast milk-acquired CMV infection assuming the meta-analysis results for the group of infants fed combinations of untreated, frozen or pasteurized milk or non-specified were similar to that of the group fed frozen milk, but rates of CMV-related symptoms and CMV-SLS were lower. These corresponded to an estimated 1900 infants with breast milk-acquired CMV infection, 400 with CMV-related symptoms, and 400 with CMV-SLS in 2008. Point estimates for the 3 outcomes based on Model 2 adjusted for breast milk feeding rates by maternal age were slightly higher than those from Model 1.
Table 2.
Estimated Rates of Breast Milk-Acquired CMV Infection, CMV-Related Symptoms, and CMV-SLS among VLBW and Premature Infants Adjusting for Breast Milk Feeding Rates by Maternal Race/Ethnicity (Model 1) and Maternal Age (Model 2), United States – 2008
| Breast milk | Breast milk-acquired CMV infection | CMV-related symptomsa | CMV-SLSb | |
|---|---|---|---|---|
|
| ||||
| % (95% CI) | ||||
| Model 1 | Untreated | 6.5 (3.7–10.9) | 3.4 (1.7–5.8) | 1.4 (0.7–2.4) |
| Frozen | 4.4 (2.4–8.2) | 2.4 (1.0–4.8) | 1.7 (0.7–4.1) | |
| Mix | 4.4 (2.4–6.8) | 1.0 (0.3–2.7) | 1.0 (0.3–2.4) | |
|
| ||||
| Model 2 | Untreated | 7.2 (4.1–12.0) | 3.8 (1.9–6.4) | 1.5 (0.8–2.6) |
| Frozen | 4.9 (2.6–9.0) | 2.6 (1.1–5.3) | 1.9 (0.8–4.5) | |
| Mix | 4.9 (2.6–7.5) | 1.1 (0.4–3.0) | 1.1 (0.4–2.6) | |
CMV-related symptoms defined as any of the following: neutropenia, thrombocytopenia, petechiae, hepatopathy, hyperbilirubinemia, elevated liver enzymes, jaundice or CMV pneumonia
CMV-SLS defined sepsis-like symptoms, such as bradycardia, apnea or respiratory deterioration, in the absence of bacterial infection and coincident with CMV viruria.
Figure 1.
Estimated Number of VLBW and Premature Infants with Breast Milk-Acquired CMV Infection, CMV-Related Symptoms and CMV-Related Sepsis-like Syndrome, United States – 2008
Notes: Dash represents point estimates and whiskers represent 95% confidence interval; M1: Model 1, adjusting for breastfeeding rates by maternal race/ethnicity, M2: Model 2, adjusting for breastfeeding rates by maternal age. CMV-related symptoms defined as any of the following: neutropenia, thrombocytopenia, petechiae, hepatopathy, hyperbilirubinemia, elevated liver enzymes, jaundice or CMV pneumonia. CMV-related sepsis-like syndrome defined as sepsis-like symptoms, such as bradycardia, apnea or respiratory deterioration, in the absence of bacterial infection and coincident with CMV viruria.
DISCUSSION
According to our estimates, 0.3%–4.5% of VLBW and premature infants in the United States may develop CMV-SLS from breast-milk acquired CMV infection, resulting in up to approximately 2000 affected VLBW and premature infants in 2008. If all VLBW and premature infants were fed fresh breast milk, as recently recommended by the American Academy of Pediatrics 18, we estimate the rate of CMV-SLS from breast milk-acquired CMV infection would be approximately 2.5% (95% CI=1.3%–4.4%). Although breast milk provides many benefits to VLBW and preterm infants, those fed breast milk from CMV-seropositive mothers are at risk of postnatal CMV infection, in a minority of whom CMV-SLS may result, which have been associated with longer hospitalizations during infancy.9, 13, 28, 33
Our understanding of the degree to which freezing breast milk is effective for reducing CMV infection and disease VLBW and premature infants is incomplete. Although freezing breast milk is known to decrease viral titers, it has not been shown to reliably eliminate CMV completely.34, 35 Our meta-analysis suggests that the risk of breast milk-acquired CMV infection is lower if infants are fed frozen breast milk compared to untreated breast milk but the risk of developing CMV-SLS appears to be similar in both groups, a finding which persisted after exclusion of studies that failed to fully rule out CMV transmission from blood transfusion.13–15, 25 Nonetheless, some caveats need to be considered. Many of the studies we identified in the literature review had small numbers of participants or lacked control groups (i.e. did not directly compare feeding of untreated vs. treated breast milk). Most of the studies were conducted in other countries, where clinical practice, infection control measures, and transmission patterns might be different from those in the United States. Although some studies did not fully control for other sources of postnatal CMV infection, such as transfusion of blood products, based on our sensitivity analysis, this did not affect the direction of our findings. Also, the effectiveness of freezing to inactivate CMV may vary by storage temperature and length of time frozen; there were differences in freezing practices across studies included in our meta-analysis. Accurate categorization of exposure to untreated breast milk can be challenging; it is possible that some infants categorized as having received frozen milk had some exposures to untreated milk. These and other factors contributed to the lack of precision in our estimates for CMV infection and disease among infants who received untreated versus frozen breast milk.
Our review highlights the need for more robust studies of breast milk-acquired CMV infection and disease, particularly in the United States. The one US study included in the meta-analyses was conducted in a hospital in California where maternal milk is frozen at 20°C for at least 24 hours before feeding and fresh breast milk is not allowed until the infant is able to feed at the breast. Among 23 VLBW and premature infants who had CMV-seropositive mothers, 2 (9%) acquired CMV infection and 1 (4%) developed CMV-related symptoms coincident with the first positive test, but none had CMV-SLS.27 In contrast, in a US study excluded from our review because it did not report data on the number of infants fed breast milk from CMV-seropositive mothers, investigators found a 15% (5/33) rate of CMV-SLS among infants with birth weight <1100g and gestational age < 28 weeks36, which was much higher than our final estimates (1.0%–2.6%). In that study, all infected infants were fed untreated breast milk and had onset of CMV viremia or viruria between 35–60 days of life, corresponding to 30.4–33.7 weeks corrected age for gestation. It is possible that the population in the study we excluded, comprised of extremely premature infants with early postnatal virus transmission, was at higher risk of developing CMV-SLS.37 These two studies also suggest that current practices regarding use of breast milk in NICUs in the United States may vary substantially across NICUs.
To estimate annual rates of breast milk-acquired CMV infection and disease in the United States, we combined the results of the meta-analysis with US population-based data. The meta-analysis included data from studies conducted in different countries, but restricted to infants born to CMV-seropositive mothers. Rates of CMV excretion in breast milk may not differ substantially among CMV-seropositive women across populations, however detection rates could vary depending upon laboratory methods used across studies to detect virolactia (17%–58% with viral culture)11, 13, 14 or DNAlactia (67%–97% for PCR)8, 9, 12–14, 16, 17, timing of sample collection and storage. Nonetheless, rates of breast-milk acquired CMV infection and disease may vary widely across populations depending on population-specific rates of CMV seropositivity, breast milk feeding, VLBW and prematurity. Among the data we used to estimate these rates for the United States, the breastfeeding rates likely contribute the most uncertainty since they vary depending upon the medical condition of the infant (i.e. infants with serious medical conditions are less likely to be fed breast milk)23, 38, 39 and sociodemographic factors such as maternal race/ethnicity, age, education level and family income.40 In addition, little is known about breast milk handling and feeding practices for VLBW and premature infants or infection control policies in place to prevent CMV transmission in US NICUs. Monitoring these practices nationally is critical for understanding the burden of postnatal CMV disease among VLBW and premature infants.
Although an assessment of risk factors for CMV infection and disease was not a goal of our systematic review, we found some valuable data. The majority of infants who developed CMV-SLS from breast-milk acquired CMV infection had onset of CMV viruria before 32 weeks corrected gestational age. The study by Maschmann, which comprised the same population as the Hamprecht study included in our review, found that lower birth weight and early CMV transmission were risk factors for symptomatic infection.37 Considering risk factors for transmission, Mussi-Pinhata et al. found that infected infants were more likely to have either been fed untreated breast milk in the first month of life or fed breast milk for more than 1 month.25 In other studies, risk factors for transmission were early onset of CMV DNAlactia and virolactia8, prolonged viral excretion in breast milk14, and higher milk whey viral loads.14, 41 These may suggest that treating breast milk from CMV-seropositive mothers would only be necessary until the infant reaches a certain age or birth weight, after which the risk of symptomatic disease decreases. A better understanding of risk factors for CMV-SLS in VLBW and premature infants may help refining guidelines for feeding breast milk from CMV-seropositive mothers to these infants.
Breast milk is the optimal food for infants, including preterm infants, providing substantial nutritional and immunological benefits.42 Among preterm infants, breast milk feeding is associated with improved neurodevelopmental outcomes and a lower risk of retinopathy of prematurity, infections, and necrotizing enterocolitis.18, 42 These benefits appear to outweigh the risks of severe disease from breast milk-acquired CMV infection in the neonatal period, which has not been definitively associated with the delayed development or sensorineural hearing loss seen with congenital CMV infection.2, 11, 13, 26, 43–45 However, long term follow-up data on the effects of postnatal CMV infection are limited, with only a small number of infants studied into childhood. One recent study study found that the cognitive and motor function scores of VLBW infants with breast milk-acquired CMV infection were within normal ranges when examined at school-age but not as high as those of the controls (VLBW infants without CMV infection).46 More studies are needed to better describe risk factors for severe postnatal CMV disease and long-term neurodevelopmental outcomes. Data from these kinds of studies would be important to support or further refine future guidelines on breast milk feeding of VLBW and premature infants born to CMV-seropositive mothers, and will help health care providers and parents make decisions weighting the risk of CMV transmission and the benefits of breast milk.
Supplementary Material
Acknowledgments
The authors thank Laurence Grummer-Strawn, Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, CDC for valuable comments and Mary McCauley, Office of the Associate Director for Science, National Center for Immunization and Respiratory Diseases/CDC, for insightful editorial comments.
Abbreviations
- CMV
cytomegalovirus
- VLBW
very low birth weight
- SLS
sepsis-like syndrome
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor’s Statement:
Tatiana M. Lanzieri conceptualized and designed the study, reviewed the literature and carried out the analyses and interpretation of data, drafted the initial manuscript, and approved the final manuscript as submitted.
Sheila C. Dollard assisted with conceptualizing the systematic review and interpreting the findings of the systematic review and meta-analysis, critically revised the manuscript, and approved the final manuscript as submitted.
Cassandra D. Josephson assisted with interpreting the findings of the systematic review and meta-analysis, critically revised the manuscript, and approved the final manuscript as submitted.
Scott Schmid assisted with conceptualizing the systematic review, interpreting the findings of the systematic review and meta-analysis, critically revised the manuscript, and approved the final manuscript as submitted
Stephanie R. Bialek conceptualized and designed the study, interpreted the findings of the systematic review and meta-analysis, critically revised the manuscript, and approved the final manuscript as submitted.
Funding Source: No external funding was used for this study.
References
- 1.Ahlfors K, Ivarsson SA, Johnsson T, Svanberg L. Primary and secondary maternal cytomegalovirus infections and their relation to congenital infection. Analysis of maternal sera. Acta paediatrica Scandinavica. 1982;71(1):109–113. doi: 10.1111/j.1651-2227.1982.tb09380.x. [DOI] [PubMed] [Google Scholar]
- 2.Stagno S, Reynolds DW, Pass RF, Alford CA. Breast milk and the risk of cytomegalovirus infection. N Engl J Med. 1980;302(19):1073–1076. doi: 10.1056/NEJM198005083021908. [DOI] [PubMed] [Google Scholar]
- 3.Mussi-Pinhata MM, Pinto PC, Yamamoto AY, Berencsi K, de Souza CB, Andrea M, et al. Placental transfer of naturally acquired, maternal cytomegalovirus antibodies in term and preterm neonates. Journal of medical virology. 2003;69(2):232–239. doi: 10.1002/jmv.10271. [DOI] [PubMed] [Google Scholar]
- 4.Dworsky M, Yow M, Stagno S, Pass RF, Alford C. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics. 1983;72(3):295–299. [PubMed] [Google Scholar]
- 5.Vochem M, Hamprecht K, Jahn G, Speer CP. Transmission of cytomegalovirus to preterm infants through breast milk. Pediatr Infect Dis J. 1998;17(1):53–58. doi: 10.1097/00006454-199801000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Britt W. Infectious Diseases of the Fetus and Newborn (Seventh Edition) Philadelphia: W.B. Saunders; 2011. CHAPTER 23 - Cytomegalovirus; pp. 706–755. [Google Scholar]
- 7.Hamprecht K, Vochem M, Baumeister A, Boniek M, Speer CP, Jahn G. Detection of cytomegaloviral DNA in human milk cells and cell free milk whey by nested PCR. Journal of virological methods. 1998;70(2):167–176. doi: 10.1016/s0166-0934(97)00179-1. [DOI] [PubMed] [Google Scholar]
- 8.Hamprecht K, Maschmann J, Vochem M, Dietz K, Speer CP, Jahn G. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet. 2001;357(9255):513–518. doi: 10.1016/S0140-6736(00)04043-5. [DOI] [PubMed] [Google Scholar]
- 9.Meier J, Lienicke U, Tschirch E, Kruger DH, Wauer RR, Prosch S. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J Clin Microbiol. 2005;43(3):1318–1324. doi: 10.1128/JCM.43.3.1318-1324.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croly-Labourdette S, Vallet S, Gagneur A, Gremmo-Feger G, Legrand-Quillien MC, Ansquer H, et al. Pilot epidemiologic study about transmission of cytomegalovirus from mother to preterm infant by breastfeeding. [French] Archives De Pediatrie. 2006;13(7):1015–1021. doi: 10.1016/j.arcped.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Capretti MG, Lanari M, Lazzarotto T, Gabrielli L, Pignatelli S, Corvaglia L, et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother’s milk: a prospective study. J Pediatr. 2009;154(6):842–848. doi: 10.1016/j.jpeds.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda A, Kimura H, Hayakawa M, Ohshiro M, Kato Y, Matsuura O, et al. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics. 2003;111(6 Pt 1):1333–1336. doi: 10.1542/peds.111.6.1333. [DOI] [PubMed] [Google Scholar]
- 13.Jim WT, Shu CH, Chiu NC, Kao HA, Hung HY, Chang JH, et al. Transmission of cytomegalovirus from mothers to preterm infants by breast milk. Pediatr Infect Dis J. 2004;23(9):848–851. doi: 10.1097/01.inf.0000137571.35541.55. [DOI] [PubMed] [Google Scholar]
- 14.Jim WT, Shu CH, Chiu NC, Chang JH, Hung HY, Peng CC, et al. High cytomegalovirus load and prolonged virus excretion in breast milk increase risk for viral acquisition by very low birth weight infants. Pediatr Infect Dis J. 2009;28(10):891–894. doi: 10.1097/INF.0b013e3181a55c52. [DOI] [PubMed] [Google Scholar]
- 15.Chiavarini M, Bragetti P, Sensini A, Cenci E, Castronari R, Rossi MJ, et al. Breastfeeding and transmission of cytomegalovirus to preterm infants. Case report and kinetic of CMV-DNA in breast milk. Ital J Pediatr. 2011;37:6. doi: 10.1186/1824-7288-37-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosca F, Pugni L, Barbi M, Binda S. Transmission of cytomegalovirus. Lancet. 2001;357(9270):1800. doi: 10.1016/S0140-6736(00)04914-X. [DOI] [PubMed] [Google Scholar]
- 17.Omarsdottir S, Casper C, Zweygberg Wirgart B, Grillner L, Vanpee M. Transmission of cytomegalovirus to extremely preterm infants through breast milk. Acta Paediatr. 2007;96(4):492–494. doi: 10.1111/j.1651-2227.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827–841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 19.Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC infectious diseases. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Random-Effects Model. COMPLETE, editor. Introduction to Meta-Analysis. 2009 [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C, Zhang X, Bialek S, Cannon MJ. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;52(2):e11–13. doi: 10.1093/cid/ciq085. [DOI] [PubMed] [Google Scholar]
- 23.Lee HC, Gould JB. Factors influencing breast milk versus formula feeding at discharge for very low birth weight infants in California. J Pediatr. 2009;155(5):657–662. e651–652. doi: 10.1016/j.jpeds.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 24.CDC. National Center for Health Statistics. Number of live births <32 weeks gestational age and birth weight <1500g by maternal age and race/ethnicity. United States: 2008. [Google Scholar]
- 25.Mussi-Pinhata MM, Yamamoto AY, do Carmo Rego MA, Pinto PC, da Motta MS, Calixto C. Perinatal or early-postnatal cytomegalovirus infection in preterm infants under 34 weeks gestation born to CMV-seropositive mothers within a high-seroprevalence population. J Pediatr. 2004;145(5):685–688. doi: 10.1016/j.jpeds.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Miron D, Brosilow S, Felszer K, Reich D, Halle D, Wachtel D, et al. Incidence and clinical manifestations of breast milk-acquired Cytomegalovirus infection in low birth weight infants. J Perinatol. 2005;25(5):299–303. doi: 10.1038/sj.jp.7211255. [DOI] [PubMed] [Google Scholar]
- 27.Lee HC, Enright A, Benitz WE, Madan A. Postnatal cytomegalovirus infection from frozen breast milk in preterm, low birth weight infants. Pediatr Infect Dis J. 2007;26(3):276. doi: 10.1097/01.inf.0000254412.66944.3e. [DOI] [PubMed] [Google Scholar]
- 28.Buxmann H, Miljak A, Fischer D, Rabenau HF, Doerr HW, Schloesser RL. Incidence and clinical outcome of cytomegalovirus transmission via breast milk in preterm infants </=31 weeks. Acta Paediatr. 2009;98(2):270–276. doi: 10.1111/j.1651-2227.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- 29.Sharland M, Khare M, Bedford-Russell A. Prevention of postnatal cytomegalovirus infection in preterm infants. Archives of disease in childhood Fetal and neonatal edition. 2002;86(2):F140. doi: 10.1136/fn.86.2.F140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doctor S, Friedman S, Dunn MS, Asztalos EV, Wylie L, Mazzulli T, et al. Cytomegalovirus transmission to extremely low-birthweight infants through breast milk. Acta Paediatr. 2005;94(1):53–58. doi: 10.1111/j.1651-2227.2005.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi S, Kimura H, Oshiro M, Kato Y, Yasuda A, Suzuki C, et al. Transmission of cytomegalovirus via breast milk in extremely premature infants. J Perinatol. 2011;31(6):440–445. doi: 10.1038/jp.2010.150. [DOI] [PubMed] [Google Scholar]
- 32.Ohagen A, Gibaja V, Horrigan J, Lunderville D, Jayarama V, Marcello J, et al. Induction of latent human cytomegalovirus by conventional gamma irradiation and prevention by treatment with INACTINE PEN110. Vox sanguinis. 2004;87(1):1–9. doi: 10.1111/j.1423-0410.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- 33.Neuberger P, Hamprecht K, Vochem M, Maschmann J, Speer CP, Jahn G, et al. Case-control study of symptoms and neonatal outcome of human milk-transmitted cytomegalovirus infection in premature infants. J Pediatr. 2006;148(3):326–331. doi: 10.1016/j.jpeds.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 34.Dworsky M, Stagno S, Pass RF, Cassady G, Alford C. Persistence of cytomegalovirus in human milk after storage. J Pediatr. 1982;101(3):440–443. doi: 10.1016/s0022-3476(82)80081-4. [DOI] [PubMed] [Google Scholar]
- 35.Friis H, Andersen HK. Rate of inactivation of cytomegalovirus in raw banked milk during storage at −20 degrees C and pasteurisation. British medical journal. 1982;285(6355):1604–1605. doi: 10.1136/bmj.285.6355.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamele M, Flanagan R, Loomis CA, Stevens T, Fairchok MP. Severe morbidity and mortality with breast milk associated cytomegalovirus infection. Pediatr Infect Dis J. 2010;29(1):84–86. doi: 10.1097/INF.0b013e3181b6dbb5. [DOI] [PubMed] [Google Scholar]
- 37.Maschmann J, Hamprecht K, Dietz K, Jahn G, Speer CP. Cytomegalovirus infection of extremely low-birth weight infants via breast milk. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2001;33(12):1998–2003. doi: 10.1086/324345. [DOI] [PubMed] [Google Scholar]
- 38.Espy KA, Senn TE. Incidence and correlates of breast milk feeding in hospitalized preterm infants. Social science & medicine. 2003;57(8):1421–1428. doi: 10.1016/s0277-9536(02)00542-7. [DOI] [PubMed] [Google Scholar]
- 39.Pineda RG. Predictors of breastfeeding and breastmilk feeding among very low birth weight infants. Breastfeeding medicine : the official journal of the Academy of Breastfeeding Medicine. 2011;6(1):15–19. doi: 10.1089/bfm.2010.0010. [DOI] [PubMed] [Google Scholar]
- 40.Ryan AS. The resurgence of breastfeeding in the United States. Pediatrics. 1997;99(4):E12. doi: 10.1542/peds.99.4.e12. [DOI] [PubMed] [Google Scholar]
- 41.van der Strate BW, Harmsen MC, Schafer P, Swart PJ, The TH, Jahn G, et al. Viral load in breast milk correlates with transmission of human cytomegalovirus to preterm neonates, but lactoferrin concentrations do not. Clin Diagn Lab Immunol. 2001;8(4):818–821. doi: 10.1128/CDLI.8.4.818-821.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrence RM, Lawrence RA. Breastfeeding: more than just good nutrition. Pediatrics in review/American Academy of Pediatrics. 2011;32(7):267–280. doi: 10.1542/pir.32-7-267. [DOI] [PubMed] [Google Scholar]
- 43.Vollmer B, Seibold-Weiger K, Schmitz-Salue C, Hamprecht K, Goelz R, Krageloh-Mann I, et al. Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis J. 2004;23(4):322–327. doi: 10.1097/00006454-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Kumar ML, Nankervis GA, Jacobs IB, Ernhart CB, Glasson CE, McMillan PM, et al. Congenital and postnatally acquired cytomegalovirus infections: long-term follow-up. J Pediatr. 1984;104(5):674–679. doi: 10.1016/s0022-3476(84)80942-7. [DOI] [PubMed] [Google Scholar]
- 45.Paryani SG, Yeager AS, Hosford-Dunn H, Johnson SJ, Malachowski N, Ariagno RL, et al. Sequelae of acquired cytomegalovirus infection in premature and sick term infants. J Pediatr. 1985;107(3):451–456. doi: 10.1016/s0022-3476(85)80533-3. [DOI] [PubMed] [Google Scholar]
- 46.Bevot A, Hamprecht K, Krageloh-Mann I, Brosch S, Goelz R, Vollmer B. Long-term outcome in preterm children with human cytomegalovirus infection transmitted via breast milk. Acta Paediatr. 2012;101(4):e167–172. doi: 10.1111/j.1651-2227.2011.02538.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.