Abstract
Objective
Opioid prescriptions have seen an increase across the United States (US), Canada, Europe and the United Kingdom (UK). In the US they have quadrupled from 1999 to 2010. Opioid use among patients with rheumatoid arthritis (RA) over time is not well described. This study examined trends of opioid use in patients with RA.
Methods
Retrospective prescription data was examined from 2005–2014 in a population-based incidence cohort of patients with RA by 1987 ACR criteria and comparable non-RA subjects. Differences in opioid use were examined with Poisson models.
Results
A total of 501 patients with RA (71% female) and 532 non-RA subjects (70% female) were included in the study. Total and chronic opioid use in 2014 was substantial in both cohorts 40% RA vs 24% non-RA and 12% RA vs. 4% non-RA, respectively. Opioid use increased by 19% per year in both cohorts during the study period (95% confidence interval [CI]: 1.15, 1.25). Relative risk (RR) of chronic opiate use for RA patients compared to non-RA subjects was highest in adults aged 50–64 years (RR: 2.82; 95%CI: 1.43–6.23). RA disease characteristics, biologic use at index, treated depression/fibromyalgia, education, smoking status were not significantly associated with chronic opiate use.
Conclusions
Over a third of patients with RA use opioids in some form, and in more than a tenth use is chronic. Use has increased in recent years. Patients aged 50–64 with RA use substantially more opioids than their non-RA counterparts.
Keywords: Rheumatoid arthritis, Opioid trends, Chronic pain management
The use of opioid analgesics is on the rise in the western world with increasing rates reported through most of Europe, Canada, United Kingdom (UK), and the United States (US) [1, 2]. The rise of the opioid analgesic use has been especially dramatic in the US as individual prescription sales quadrupled from 1999 to 2010 [3]. Along with the boom in sales, age-adjusted opioid drug poisoning death rates (81% of which are unintentional) have more than doubled from 2000 to 2013, from 6.2 to 13.8 per 100,000 [4, 5]. There are now more opioid-based analgesic users in the general patient population than ever before, but to what extent specific subpopulations are affected by this rise is in need of clarification. Traditionally, opioid therapy has been divided into two pain groups, cancer related and non-cancer related pain, as well as into two therapy duration groups, acute and chronic, of which non-cancer chronic opioid use is the fastest growing [3].
Although the efficacy and relative safety of short-term opioid use has been established, there is limited evidence regarding long-term opioid analgesic use for pain management [6]. Rates of diagnosis of chronic non-cancer pain have increased in recent years in the US population. Prevalence studies by random sampling in general adult communities have revealed rates as high as 64.4% [7], while others have estimated that as many as 25.3 million adults are suffering from chronic pain [8].
Patients with non-cancer related pain may be at a particularly high risk for opioid use. Rheumatoid arthritis (RA) is a chronic disease associated with chronic pain. Patients who suffer from RA repeatedly report that pain is the primary driver in seeking medical consultation [9]. Patients suffer from multiple inflammatory and noninflammatory chronic pain syndromes throughout their disease course [10].
From a public health perspective, RA is also a particularly important disease to be studied for opioid use since the Centers for Disease Control and Prevention (CDC) estimate that RA affected 1.5 million Americans in 2007 and that by the year 2030, 67 million persons in the US will have been diagnosed with some form of arthritis [11]. The aim of this study was to identify trends in opioid use among patients with RA compared to similar subjects without RA and to describe the characteristics of patients with RA at greatest risk for chronic opioid use.
Methods
Study Population
This historic cohort study was approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. Study subjects were identified using resources from the Rochester Epidemiology Project (REP), a special record-linkage system that records all inpatient and outpatient encounters among the residents of Olmsted County, Minnesota [12] [13], including prescription data. All Olmsted County, Minnesota residents aged 18 years or older who first fulfilled 1987 American College of Rheumatology criteria for RA in 1980–2004 were previously identified [14]. A comparison cohort of patients without RA with similar age, sex, and calendar year was randomly selected from the same population. The index date was defined to be January 1st 2005 and included all patients from the RA and non-RA cohorts who were alive and followed on that date. All subjects in both cohorts were followed longitudinally until death, migration, or December 31, 2014.
Data collection
All outpatient opioid prescriptions were identified by generic and trade names for 2004 to 2014. For each prescription, the frequency, dose, and refills were obtained and manually converted to days of use. Data on RA therapies and disease severity indicators was previously collected by manual record review. Biologic use at any time prior to index and current glucocorticoid therapy at index date was recorded for all patients with RA. Data on disease severity indicators included rheumatoid factor and/or anti-citrullinated protein antibodies (RF/ACPA) positivity, rheumatoid nodules, erosions/destructive changes on radiographs and erythrocyte sedimentation rate (ESR) values. The time from first joint swelling to fulfillment of ACR criteria was abstracted to assess delay in diagnosis of RA.
Medical records of all subjects with an electronically coded diagnosis of depression and fibromyalgia were identified then manually reviewed and data on the use of medical therapy for these diagnoses at index were collected. Subsequently only those individuals receiving current medical therapy for either depression and/or fibromyalgia after the index date were labeled as such. Diagnoses of comorbidities prior to index were obtained electronically for both cohorts and classified using the Deyo adaptation of the Charlson Comorbidity Index [15]. These included myocardial infarction, peripheral vascular disease, cerebrovascular disease, cancer, peptic ulcer and diabetes mellitus. Smoking status and educational achievement were obtained from self-answered questionnaires routinely administered at clinic visits.
Opioid use definitions
Any opioid use was defined as one or more opioid prescriptions in the study period. Chronic use was defined as a prescription(s) for 60 or more days at usual dose and usual schedule (table 1) within a 6 month period or those subjects using fentanyl, methadone and controlled/extended release oxycodone. Opioid presentation, formulation, and strength considered for the study are listed in table 1.
Table 1.
Usual dose and Usual Schedule Definitions for Opioid Use
| Opioid Receptor Strength | Drug | Usual Dose | Usual Schedule | Milligrams per day | Morphine Equivalent Dosing (MED) |
|---|---|---|---|---|---|
| Mild to Moderate Agonist | Codeine | 15mg Tablet 30mg/5mL Sol. |
2 Tab q6hrs 5 mL q6hr |
120mg/24hr 120mg/24hr |
18mg 18mg |
| Hydrocodone/Acetaminophen | 5mg/500mg Tablet 7.5mg/500mg/15mL Sol. |
1 Tab q4hr 15mL q6hr |
30mg/24hr 30mg/24hr |
30mg 30mg |
|
| Oxycodone | 5mg Tablet 5mg/5mL Sol. 10mg CR Tablet |
1 Tab q6hr 5mL q6hr 1 Tab q12hr |
20mg/24hr 20mg/24hr 20mg/24hr |
30mg 30mg 30mg |
|
| Oxycodone/Acetaminophen | 5/325mg Tablet | 1 Tab q6hr | 20mg/24hr | 30mg | |
| Hydrocodone/Acetaminophen | 5mg/500mg Tablet 7.5mg/500mg/15mL Sol. |
1 Tab q4hr 15mL q6hr |
30mg/24hr 30mg/24hr |
30mg 30mg |
|
| Propoxyphene | 100mg Tablet (Napsylate) | 1 Tab q6hr | 400mg/24hr | N/A (~30–60) | |
| Mixed Receptor Agonist | Pentazocine/naloxone | 50mg/0.5mg | 1 Tab q4hr | 300mg/24hr | N/A (~16.7) |
| Strong Agonist | Morphine | 15mg Tablet 30mg SR Tab 10mg/5mL Sol. 10mg Supp. 10mg/mL Inj. Sol. |
1 Tab q4hr 1 Tab q12hr 5ml q4hr 1 Supp. Q4hr SC/IM/IV 5mg q6hr |
90mg/24hr 60mg/24hr 60mg/24hr 60mg/24hr 20mg/24hr |
90mg 60mg 60mg 60mg 20mg |
| Atypical | Tramadol | 50mg Tablet 100mg ER Tab |
1 Tab q6hr 1 Tab q24hr |
200mg/24hr 100mg/24hr |
40mg 20mg |
| Tramadol/Acetaminophen | 37.5/325mg Tablet | 2 Tab q6hr | 300mg/24hr | 60mg |
Abbreviations: Solution (Sol.), Milligram (mg), Milliliter (mL), Sustained Release (SR), Suppository (Supp.), Injectable Solution (Inj. Sol.), Every 4 hours (q4hrs), Every 6 hours (q6hrs), Every 12 hours (q12hrs), Not Available (N/A)
Statistical analysis
Descriptive statistics (means, percentages, etc.) were used to summarize the characteristics of each cohort. Characteristics were compared between cohorts using Chi-square and rank sum tests. Person-year methods were used to compute the overall rate of first chronic opioid use in the time period of interest as the number of patients meeting the definition of chronic opioid use divided by the number of person-years of follow-up. Patients who had any opioid prescription in 2004 were excluded from analyses of first chronic use of opioids in an attempt to estimate the rate of incident chronic use of opioids. Poisson regression models were used to estimate the relative risk of first chronic opioid use among those with RA compared to those without RA.
Yearly estimates of percentage of subjects with any opioid use were computed as the number of patients with any opioid prescription in a particular calendar year divided by the person-years of follow up in that calendar year. Yearly estimates of the percentage of subjects with chronic opioid use were computed similarly. To avoid overestimation of the rate of chronic use of opioids, after having met our definition of chronic use, subjects were returned to non-chronic use when there was a year without an opioid prescription.
Cox models adjusted for age, sex, and other characteristics were used to examine difference in time to first opioid use during the time frame of interest between the cohorts. Cox models were also used to examine characteristics associated with first chronic opioid use among the patients with RA. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 501 patients with RA (71% female) and 532 non-RA subjects (70% female) were included in the study. The patients with RA had a mean (±SD) age at index date of 61.3 ± 14.5 years, with a mean follow-up from index of 8.5 ± 2.7 years. The non-RA subjects had a mean age at index date of 62.6 ± 14.7 years, with a mean follow-up from index of 8.7 ± 2.4 years. Any opioid prescription in the year prior to the index date was significantly higher in the RA cohort, 31% RA vs 20% non-RA (p<0.001). Further characteristics found to be significantly different between the RA and non-RA subjects at index were peripheral vascular disease 19% RA vs. 13% non-RA (p=0.006), and a prior peptic ulcer 19% RA vs 13% non-RA (p=0.005). There were no apparent differences at index data between subjects with and without RA regarding a previous diagnosis of diabetes mellitus, cancer, myocardial infarction, congestion heart failure or cerebrovascular disease (table 2). The opioids identified by prescription frequency were oxycodone (39%), hydrocodone (18%), tramadol (22%), fentanyl (6%) morphine (3%), propoxyphene (4%) and codeine (5%).
Table 2.
Baseline Characteristics of Rheumatoid Arthritis (RA) cases versus non-RA comparator subjects
| Characteristic | Non-RA N=532 |
RA N=501 |
P-value |
|---|---|---|---|
|
| |||
| Sex | 0.69 | ||
| Female | 372 (69.9%) | 356 (71.1%) | |
| Male | 160 (30.1%) | 145 (28.9%) | |
|
| |||
| Age at Index | 0.13 | ||
| Mean (SD) | 62.6 (14.7) | 61.3 (14.5) | |
| Median | 63.1 | 61.4 | |
|
| |||
| Ethnicity | 0.27 | ||
| Unknown | 3 | 2 | |
| American Indian | 1 (0.2%) | 3 (0.6%) | |
| Asian | 11 (2.1%) | 14 (2.8%) | |
| African American | 7 (1.3%) | 3 (0.6%) | |
| Native Hawaiian | 0 (0.0%) | 2 (0.4%) | |
| White | 507 (95.8%) | 471 (94.4%) | |
| Mora than one race | 3 (0.6%) | 6 (1.2%) | |
|
| |||
| Education Level | <.001 | ||
| Missing | 6 | 16 | |
| <High School | 43 (8.2%) | 36 (7.4%) | |
| High School | 156 (29.7%) | 173 (35.7%) | |
| Technical School/College | 244 (46.4%) | 243 (50.1%) | |
| Graduate School | 83 (15.8%) | 33 (6.8%) | |
|
| |||
| Length of Follow-up, years | |||
| Mean (SD) | 8.7 (2.4) | 8.5 (2.7) | |
|
| |||
| Comorbidities Prior to Index | |||
| Myocardial Infarct | 48 (9.0%) | 44 (8.8%) | 0.89 |
| Congestive Heart Failure | 53 (10.0%) | 53 (10.6%) | 0.74 |
| Peripheral Vascular Disease | 67 (12.6%) | 94 (18.8%) | 0.006 |
| Ulcer Disease | 67 (12.6%) | 95 (19.0%) | 0.005 |
| Cerebrovascular Disease | 78 (14.7%) | 67 (13.4%) | 0.55 |
| Diabetes mellitus | 76 (14.3%) | 70 (14.0%) | 0.88 |
| Cancer | 168 (31.6%) | 150 (29.9%) | 0.57 |
|
| |||
| Smoking Status at RA Incidence | 0.015 | ||
| Never | 287 (53.9%) | 226 (45.1%) | |
| Former | 158 (29.7%) | 171 (34.1%) | |
| Current | 87 (16.4%) | 104 (20.8%) | |
|
| |||
| Treatment for Depression at Index | 71 (13.3%) | 72 (14.4%) | 0.63 |
|
| |||
| Treatment for Fibromyalgia at Index | 10 (1.9%) | 20 (4.0%) | 0.043 |
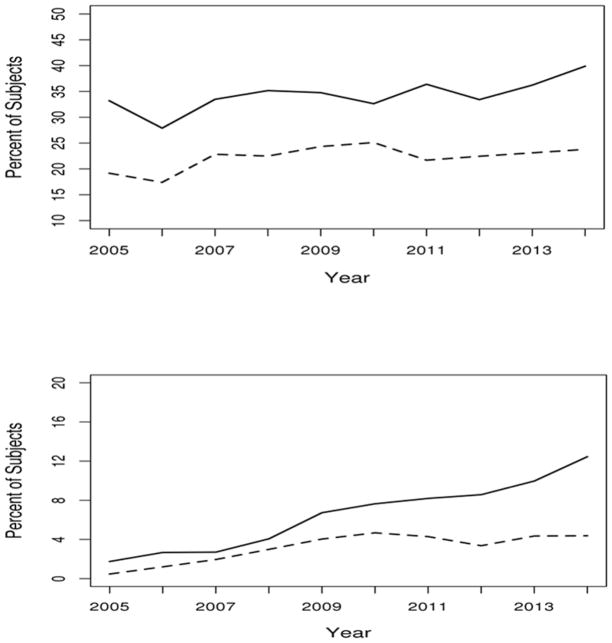
Over the observed ten-year period any opioid use was found to be common in both cohorts with 40% of patients with RA and 24% non-RA subjects having used an opioid in 2014 (figure 1, upper panel). Patients with RA were 1.5 times more likely to have experienced any opioid use than non-RA subjects (age and sex adjusted hazard ratio [HR]: 1.58; 95% confidence interval [CI]: 1.37, 1.82).
Figure 1.
Percentage of subjects with any (upper panel) and chronic (lower panel) opioid prescription by year and cohort. Rheumatoid arthritis (RA) shown with a solid line and non-RA shown with a dashed line).
To estimate incidence of chronic opioid use, the 156 patients with RA and 105 non-RA subjects who had an opioid prescription in 2004 were excluded from analysis of chronic opioid use. Chronic opioid use was also substantial in both cohorts, 12% RA vs 4% non-RA in 2014, and this rate increased over time (relative risk [RR]: 1.19 per year; 95% CI: 1.15, 1.25; figure 1, lower panel). Patients with RA were nearly twice as likely to require chronic opioid therapy as comparator subjects (age and sex adjusted HR 1.90; 95% CI: 1.32, 2.72). The higher rate of chronic use in RA subjects remained unchanged after adjusting for known general population risk factors for opioid use including smoking status, educational level, treatment for depression or fibromyalgia, and Charlson comorbidity index (HR 1.92; 95% CI: 1.33, 2.78).
Additional analysis of the opioid use trend by sex and age group showed that chronic opioid use was considerably higher in patients with RA aged 50–64 years, 2.3% vs. 0.8% in non-RA adults (relative risk [RR]: 2.82; 95% CI: 1.43, 6.23; table 3). There was no identified difference in risk of chronic opioid use in younger adults age 18 to 49 or in adults 65 years or older. Adult women with RA at any age were more likely to use chronic opioid therapy than comparator non-RA women (RR 1.80; 95% CI: 1.19, 2.75). The greatest (threefold) increased risk for chronic opioid use was noted in females with RA age 50–64 compared to non-RA (RR 3.35; 95% CI: 1.47, 9.37).
Table 3.
First Chronic Opioid Use Rates in Patients with Rheumatoid Arthritis (RA) Compared to Subjects without RA
| Sex | Age Group, years | RA Rate (per 100 py) | Non-RA Rate (per 100 py) | Relative Risk (95% CI) |
|---|---|---|---|---|
| Female | 18–49 | 0.54 | 0.48 | 1.14 (0.16, 8.15) |
| 50–64 | 2.45 | 0.69 | 3.35 (1.47, 9.37) | |
| 65+ | 3.38 | 2.20 | 1.54 (0.94, 2.54) | |
| Total | 2.56 | 1.42 | 1.80 (1.19, 2.75) | |
| Male | 18–49 | 1.28 | 0.81 | 1.58 (0.10, 24.36) |
| 50–64 | 1.91 | 0.97 | 1.88 (0.60, 7.10) | |
| 65+ | 2.04 | 1.65 | 1.25 (0.50, 3.05) | |
| Total | 1.92 | 1.31 | 1.46 (0.73, 2.95) | |
| Total | 18–49 | 0.67 | 0.55 | 1.22 (0.24, 6.08) |
| 50–64 | 2.27 | 0.78 | 2.82 (1.43, 6.23) | |
| 65+ | 2.98 | 2.02 | 1.47 (0.95, 2.27) | |
| Total | 2.37 | 1.39 | 1.71 (1.20, 2.45) |
Abbreviations: Person Years (py), Confidence Interval (CI)
Among patients with RA, those on systemic glucocorticoid therapy at index were at markedly higher risk for chronic opioid use (HR 2.48; 95% CI: 1.51, 4.06). Disease severity factors for RA were not significantly associated with an increased risk of chronic opioid use, including RF/ACPA positivity, rheumatoid nodules, and ESR at diagnosis (table 4). Delay in diagnosis, as measured by time from first joint swelling to fulfillment of criteria for RA, was not associated with chronic opioid use (p=0.98). The presence of erosions/destructive changes approached significance (HR: 1.50; 95% CI: 0.93, 2.41). Biologic therapy use before index was also not associated with either a decreased or increased risk (HR 1.18; 95% CI: 0.55, 2.50).
Table 4.
Characteristics associated with first chronic opioid in patients with rheumatoid arthritis (RA).
| Characteristic | Hazard Ratio* (95% CI) | P-value |
|---|---|---|
|
| ||
| Smoking Status (vs. Never smokers) | 0.19 | |
| Former | 1.23 (0.70, 2.15) | |
| Current | 1.75 (0.96, 3.22) | |
|
| ||
| Highest Education (vs. High School) | 0.24 | |
| Graduate School | 1.77 (0.80, 3.94) | |
| Technical School/College | 0.81 (0.47, 1.40) | |
| <High School | 1.33 (0.54, 3.27) | |
|
| ||
| Treatment for Depression at Index | 0.78 (0.34, 1.83) | 0.57 |
|
| ||
| Treatment for Fibromyalgia at Index | 1.84 (0.58, 5.88) | 0.30 |
|
| ||
| Biologic Use Prior to or at Index | 1.18 (0.55, 2.50) | 0.67 |
|
| ||
| Glucocorticoid Use at Index | 2.48 (1.51, 4.06) | <0.001 |
|
| ||
| RF/ACPA positivity | 1.27 (0.75, 2.16) | 0.37 |
|
| ||
| Rheumatoid nodules | 0.82 (0.49, 1.38) | 0.46 |
|
| ||
| Erosions/destructive changes at index | 1.50 (0.93, 2.41) | 0.10 |
|
| ||
| ≥3 ESR measures of ≥60 mm/1 hour** | 0.72 (0.26, 2.02) | 0.54 |
|
| ||
| Years from first joint swelling to fulfillment of criteria for RA | 1.00 (0.90, 1.11) | 0.98 |
Abbreviations: CI=confidence interval, RF=rheumatoid factor,
ACPA=anti-citrullinated protein antibodies, ESR=erythrocyte sedimentation rate
Adjusted for age, sex and duration of rheumatoid arthritis at index date
Prior to index with a minimum interval of 30 days between 2 measurements
Discussion
In this study, opioid use was common and has increased in recent years in patients with RA and non-RA populations. Over one third of patients with RA are prescribed an opioid and disturbingly, one in ten use opioids chronically during their disease course. This is a significantly greater opioid usage than that seen in an already opioid-burdened general population, and the data suggest this will increase in the coming years [4][5]. While the current study could not specifically interrogate the indications for opioid use in individual patients, these high use rates are particularly concerning as available evidence does not support the efficacy of chronic opioid use for chronic non-cancer pain management [6] [16] [17].
The decision to use a sixty day or two-month definition for chronic opioid use is largely derived from data gathered and released by the CDC on the safety of opioid use. Persons at highest risk to suffer serious harm from opioids are all those aged 65 years or less who are prescribed therapy for 6 weeks (or longer) and all persons, regardless of age, using extended use formulations such as fentanyl patches, OxyContin®, and methadone [16]. In this context, there are currently no reported randomized placebo-controlled trials on the effectiveness of opioid therapy in RA. As well, there is no evidence that the analgesic effectiveness of opioids can be maintained past eight weeks of continuous use in any form of chronic non-cancer pain [6]. Therefore, the suggested definition for chronic opioid use is both purposeful and representative of known data and it does not necessarily reflect previously suggested definitions, such as a three-month period of use. This time frame of 90 days has been broadly borrowed from other studies of chronic pain, that is, pain persisting past acute tissue injury [18]. A 60 day definition encompasses both the longest period of demonstrated effectiveness for opioid based analgesics and those who at two months are already at high risk to suffer harm from continuing therapy with opioids.
Pain prevalence studies reflect a growing need for effective pain management with pain rates as high as 64.4% in the general population. Through random sampling of 6,000 adults age over 30 years of age by survey conducted in 2004, over a fourth of those affected were dissatisfied with their healthcare providers’ analgesic solutions [7]. For patients who suffer from RA, pain is what most often motivates the patient visit, but does not necessarily correlate with disease activity [9] [19], underlining an added need for pain management in addition to treatment of active inflammatory disease to reduce progressive joint damage. It is entirely plausible that because opioid analgesics are a potent and effective tool in the management of moderate to severe pain in the acute phase, particularly when pain is resistant to traditional non-opioid based therapies, they have been resorted to for longer term management. However, transferring the proven short-term effectiveness of opioids to a continuous long-term effective pain management strategy for non-cancer related pain has been largely unsuccessful [4, 16, 17, 20, 3, 6].
The lack of association of chronic opioid use with traditional RA disease severity markers such as RF/ACPA positivity and previous biologic therapy may indirectly be suggestive of the importance of another pain pathway closely related to the chronic inflammatory disease state of RA. Although not assessed in this study, the elicitation of pain as part of the vital signs recorded in every clinical encounter may offer a way to raise awareness about patients’ pain experience and the need for more directed therapy, especially in those who suffer from RA.
In recent years most patients with RA have decreased progression of joint damage, significantly greater decreases in inflammatory markers, increased likelihood of achieving remission, and an overall lower morbidity/mortality when compared to pre-biologic therapy cohorts [19] [21] [22]. Yet, opioid use is higher in recent years. Reasons for this are unclear, but may represent response to patient expectation of better pain management and secular trends in acceptability of opioid prescribing and use among patients and health care providers.
The reason for the observed relationship between opioid use and systemic glucocorticoid (GC) use is unclear. It may relate to a pain rebounding effect after discontinuation of GCs, which have intrinsic analgesics effects [23]. A recent observation from this same cohort of patients with RA revealed that in recent years more patients are prescribed GCs earlier in their RA disease course when compared to previous years and this increase was most evident in the first four years after diagnosis, while still showing a larger proportion of patients discontinuing GCs [24]. It is possible that the loss of analgesia produced by GCs may account for a part of this increase use of opioids in recent years.
Comorbid depression and fibromyalgia are more common in RA patients than non-RA comparators [25]. Depression can increase patient reported pain and/or tenderness as captured on both physician and patient assessment scales and is correlated with disease severity measures [10][26] [27]. In the present study, the higher rate of opioid use in RA was not associated with treatment for depression and fibromyalgia. Unlike other studies from the general population, smoking and educational achievement were not found to be significantly associated with opioid use rates in the RA population [18]. These findings suggest that other factors possibly related to the RA disease process overshadow these risk factors for chronic opioid use.
Another factor related to chronic pain in patients with RA is fatigue. While not addressed in the current study, a report from the British Society for Rheumatology Biologics Register for RA (BSRBR-RA) in over 2,500 patients with RA failed to identify a relationship between changes in inflammation or disease activity and changes in fatigue, but did find that changes in patient reported pain accounted for most of the differences in fatigue [28]. Other studies have reported that pain persists past remission and is dissociated from active disease processes in RA [9] [19] [22]. It is possible, but not well studied, that persistent pain even in remission is addressed with chronic opioid therapy.
This is the first study of opioid use trends in a well described and modern RA cohort over the most recent decade in which prescription data was analyzed from a robust data collection system. Limitations include the lack of data on why opioids were prescribed, and on the amount of opioid patients actually used. Detailed prescription data including pill counts were obtained, however actual use by the patients is uncertain, as there is wide variability in actual consumption of pain medication. Opioid prescriptions given by providers outside of Olmsted County, MN, were not included and additional prescriptions sought elsewhere were not captured. However, it is not likely that this differed for patients with RA and comparators. The population of Olmsted County is stable, with few migrations making it more likely that the prescription data are accurate and complete. In addition, both cohorts are primarily made up of Caucasians and differences in opioid use by ethnicity were not examined. This was a study to describe opioid use and not opioid adverse effects or burden. While deaths related to opioid use are of public health concern, they could not be ascertained or inferred in this study.
The findings in the present study demonstrate that chronic opioid use in patients with RA is common and has increased in recent years, even as disease control and damage from disease has generally declined. Concern about future trends in use must take into account both the potential benefit, but increasingly also, the harm inherent with chronic opioid therapy including significant morbidity and mortality from dependency [29]. Research into pain mechanisms and alternative pain management therapies for chronic non-cancer related pain is needed to improve the lives of patients with RA and reduce the burden of chronic opioid therapy.
Acknowledgments
Grants/Financial Supports: This study was funded by a grant from the National Institutes of Health, NIAMS (R01 AR46849) and made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676 and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors disclose that they have no relevant conflicts of interest to disclose.
References
- 1.Weisberg DF, Becker WC, Fiellin DA, Stannard C. Prescription opioid misuse in the United States and the United Kingdom: cautionary lessons. Int J Drug Policy. 2014;25(6):1124–30. doi: 10.1016/j.drugpo.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 2.van Amsterdam J, van den Brink W. The Misuse of Prescription Opioids: A Threat for Europe? Curr Drug Abuse Rev. 2015;8(1):3–14. doi: 10.2174/187447370801150611184218. [DOI] [PubMed] [Google Scholar]
- 3.Nelson LS, Perrone J. Curbing the opioid epidemic in the United States: the risk evaluation and mitigation strategy (REMS) JAMA. 2012;308(5):457–8. doi: 10.1001/jama.2012.8165. [DOI] [PubMed] [Google Scholar]
- 4.CDC. NCHS Data on Drug Poisoning Deaths. 2015 http://www.cdc.gov/nchs/data/factsheets/factsheet_drug_poisoning.pdf.
- 5.CDC. Injury Prevention & Control: Prescription Drug Overdose. 2010. [Google Scholar]
- 6.Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain. 2004;112(3):372–80. doi: 10.1016/j.pain.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 7.Watkins EA, Wollan PC, Melton LJ, 3rd, Yawn BP. A population in pain: report from the Olmsted County health study. Pain medicine. 2008;9(2):166–74. doi: 10.1111/j.1526-4637.2007.00280.x. [DOI] [PubMed] [Google Scholar]
- 8.Nahin RL. Estimates of Pain Prevalence and Severity in Adults: United States, 2012. The journal of pain : official journal of the American Pain Society. 2015;16(8):769–80. doi: 10.1016/j.jpain.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiberg T, Finset A, Uhlig T, Kvien TK. Seven year changes in health status and priorities for improvement of health in patients with rheumatoid arthritis. Annals of the rheumatic diseases. 2005;64(2):191–5. doi: 10.1136/ard.2004.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee YC. Effect and treatment of chronic pain in inflammatory arthritis. Current rheumatology reports. 2013;15(1):300. doi: 10.1007/s11926-012-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. Arthritis-Related Statistics. 2015 http://www.cdc.gov/arthritis/data_statistics/arthritis_related_stats.htm.
- 12.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic proceedings. 1996;71(3):266–74. doi: 10.1016/S0025-6196(11)63966-9. [DOI] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–24. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and rheumatism. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.CDC. Prescription Opioid Analgesic Use Among Adults: United States, 1999–2012. 2015. [PubMed] [Google Scholar]
- 17.Whittle SL, Richards BL, van der Heijde DM, Buchbinder R. The efficacy and safety of opioids in inflammatory arthritis: a Cochrane systematic review. The Journal of rheumatology Supplement. 2012;90:40–6. doi: 10.3899/jrheum.120341. [DOI] [PubMed] [Google Scholar]
- 18.Hooten WM, St Sauver JL, McGree ME, Jacobson DJ, Warner DO. Incidence and Risk Factors for Progression From Short-term to Episodic or Long-term Opioid Prescribing: A Population-Based Study. Mayo Clinic proceedings. 2015;90(7):850–6. doi: 10.1016/j.mayocp.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YC, Cui J, Lu B, Frits ML, Iannaccone CK, Shadick NA, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis research & therapy. 2011;13(3):R83. doi: 10.1186/ar3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657–9. doi: 10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- 21.Dale J. Advances in the management of rheumatoid arthritis. Scottish medical journal. 2015 doi: 10.1177/0036933015592761. [DOI] [PubMed] [Google Scholar]
- 22.Andersson ML, Forslind K, Hafstrom I. Comparing Five Year Out-Come in Two Cohorts of Patients with Early Rheumatoid Arthritis - A BARFOT Study. The open rheumatology journal. 2015;9:8–15. doi: 10.2174/1874312901409010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mensah-Nyagan AG, Meyer L, Schaeffer V, Kibaly C, Patte-Mensah C. Evidence for a key role of steroids in the modulation of pain. Psychoneuroendocrinology. 2009;34(Suppl 1):S169–77. doi: 10.1016/j.psyneuen.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Makol A, Davis JM, 3rd, Crowson CS, Therneau TM, Gabriel SE, Matteson EL. Time trends in glucocorticoid use in rheumatoid arthritis: results from a population-based inception cohort, 1980–1994 versus 1995–2007. Arthritis care & research. 2014;66(10):1482–8. doi: 10.1002/acr.22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin MC, Guo HR, Lu MC, Livneh H, Lai NS, Tsai TY. Increased risk of depression in patients with rheumatoid arthritis: a seven-year population-based cohort study. Clinics (Sao Paulo) 2015;70(2):91–6. doi: 10.6061/clinics/2015(02)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imran MY, Saira Khan EA, Ahmad NM, Farman Raja S, Saeed MA, Ijaz Haider I. Depression in Rheumatoid Arthritis and its relation to disease activity. Pakistan journal of medical sciences. 2015;31(2):393–7. doi: 10.12669/pjms.312.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz J, Rosenbloom BN, Fashler S. Chronic Pain, Psychopathology, and DSM-5 Somatic Symptom Disorder. Canadian journal of psychiatry Revue canadienne de psychiatrie. 2015;60(4):160–7. doi: 10.1177/070674371506000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Druce KL, Jones GT, Macfarlane GJ, Basu MN. Determining pathways to improvements in Rheumatoid Arthritis fatigue: Results from the BSRBR-RA. Arthritis & rheumatology. 2015 doi: 10.1002/art.39238. [DOI] [PubMed] [Google Scholar]
- 29.Cazacu I, Mogosan C, Loghin F. Safety issues of current analgesics: an update. Clujul Med. 2015;88(2):128–36. doi: 10.15386/cjmed-413. [DOI] [PMC free article] [PubMed] [Google Scholar]