To the Editor
Although antibodies against the programmed death 1 (PD-1) receptor and one of its ligands (PD-L1) have produced tumor regressions in multiple cancer types, these therapies are untested in patients treated with long-term immunosuppressive medications.1 Here, we report a case in which PD-1 antibody was administered to a solid-organ transplant recipient with metastatic cutaneous squamous-cell carcinoma. The patient had a robust antitumor response along with allograft rejection. This case suggests that anti–PD-1 can be highly effective against cancers arising in the context of chronic immunosuppression. It also suggests that the PD-1 pathway may be critical in maintaining the partial tolerance that prevents T-cell–mediated allograft rejection.
A 57-year-old woman underwent kidney transplantation from a deceased donor in 1989. She received standard long-term immunosuppression, including cyclosporine and prednisone. She presented with metastatic cutaneous squamous-cell carcinoma in March 2014. Kidney function remained stable after reduction of immunosuppression to 5 mg of prednisone daily. Her cutaneous squamous-cell carcinoma progressed, first during cetuximab therapy and then during trametinib therapy (her tumor contained a loss-of-function mutation in NF1).
Administration of anti–PD-1 was then considered on the basis of her poor candidacy for cytotoxic chemotherapy (Eastern Cooperative Oncology Group [ECOG] performance status of 2 [on a 5-point scale, with higher scores indicating greater disability]), a 2-year overall survival rate of 40% among patients with metastatic cutaneous squamous-cell carcinoma,2 and ex vivo analyses of cutaneous squamous-cell carcinoma in solid-organ transplant recipients showing expression of PD-1 and its ligands, a finding that in other tumor histologic types is associated with an increased likelihood of response to PD-1 block-ade.1,3 The risk of immune-related toxic effects associated with anti–PD-1, including kidney-allograft rejection, were explicitly conveyed to the patient.4 With the endorsement of a virtual tumor board convened to evaluate relative risk and benefit, she began pembrolizumab (anti– PD-1) in September 2014.
Two months after initiation of anti–PD-1, the patient had acute allograft rejection. Despite administration of high-dose glucocorticoids, the patient's transplanted kidney did not recover. Histologic and immunohistochemical evaluation of the explanted kidney revealed severe acute and chronic cell-mediated rejection (Fig. 1).
Figure 1. Explanted Renal Allograft Showing Evidence of Rejection and Expression of PD-1 Pathway Molecules after Administration of PD-1 Antibody.
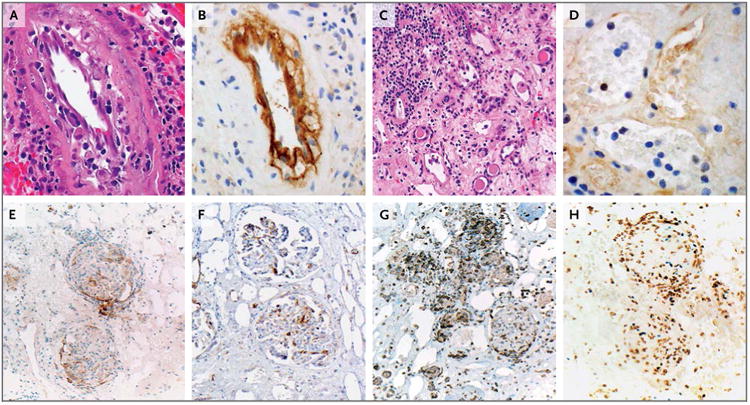
In Panel A (hematoxylin and eosin), arteries show intimal arteritis and focal intimal foam cells, findings consistent with chronic vasculopathy in the allograft. In Panel B, strong immunostaining for C4d (a product of the classical complement pathway) is present in the artery endothelium. Panel C (hematoxylin and eosin) shows glomerulitis, severe tubular loss, tubulitis, interstitial edema, and interstitial inflammation. In Panel D, peritubular capillaries show capillaritis but negative C4d immunostaining. In Panels E and F, immunostaining for programmed death 1 ligand 1 (PD-L1) and programmed death 1 ligand 2 (PD-L2), respectively, show that these molecules are present on endothelial cells and infiltrating immune cells associated with glomeruli. In Panel G, infiltrating T cells expressing programmed death 1 (PD-1) are associated with cells expressing PD-L1 and PD-L2. In Panel H, the infiltrating immune cells are predominantly CD8-positive and are coexpressed with Ki-67, findings consistent with an activated cytotoxic T-cell phenotype; shown are the results of double immunostaining for CD8 (brown chromagen) and Ki-67 (blue chromagen).
Evaluation of the patient's serum for antibodies to HLA class I and II antigens and angiotensin II type 1–receptor antibody was negative (see the Supplementary Appendix, available with the full text of this letter at NEJM.org). The excised kidney contained many infiltrating PD-1–positive T cells and PD-L1–positive and PD-L2–positive endothelial cells and immune cells associated with glomeruli, findings consistent with cell-mediated rejection induced by blockade of the PD-1 pathway.
The patient's cutaneous squamous-cell carcinoma expressed PD-L1 on tumor cells and infiltrating immune cells. Expression was spatially associated with an activated CD8-positive cytotoxic T-cell response, including lymphocytes expressing PD-L2 and PD-1 (Fig. S1 in the Supplementary Appendix).
Computed tomography performed 8 months after the initiation of pembrolizumab revealed an 85% reduction in tumor burden (according to Response Evaluation Criteria in Solid Tumors, version 1.1) (Fig. S2 in the Supplementary Appendix). The patient has not had autoimmune toxic effects. She is undergoing hemodialysis without unacceptable adverse events, and her ECOG performance status has returned to 0.
This case shows the critical role of the PD-1 pathway both in immune resistance by cancers arising in the context of long-term immunosuppression and in the maintenance of partial adaptive tolerance to transplanted organs. The latter finding contrasts with those of our earlier report, in which two organ-transplant recipients with melanoma who were treated with anti– cytotoxic T-lymphocyte antigen 4 (CTLA-4) did not have rejection of the transplanted kidneys,5 suggesting that the CTLA-4 pathway may play a lesser role than the PD-1 pathway in transplant tolerance. We speculate that PD-1 pathway agonists could be useful in the prevention of allograft rejection.
Supplementary Material
Acknowledgments
Supported by the Commonwealth Foundation for Cancer Research, Stand Up to Cancer, and grants (P30 CA006973 and R01CA142779) from the National Cancer Institute.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Evan J. Lipson, Email: evanlipson@jhmi.edu, Johns Hopkins University School of Medicine Baltimore, MD.
Serena M. Bagnasco, Johns Hopkins University School of Medicine Baltimore, MD.
Jack Moore, Jr, MedStar Georgetown Transplant Institute Washington, DC.
Sekwon Jang, Inova Dwight and Martha Schar Cancer Institute Fairfax, VA.
Manisha J. Patel, Johns Hopkins University School of Medicine Baltimore, MD.
Andrea A. Zachary, Johns Hopkins University School of Medicine Baltimore, MD.
Drew M. Pardoll, Johns Hopkins University School of Medicine Baltimore, MD.
Janis M. Taube, Johns Hopkins University School of Medicine Baltimore, MD.
Charles G. Drake, Johns Hopkins University School of Medicine Baltimore, MD.
References
- 1.Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL. Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol. 2015;42:587–600. doi: 10.1053/j.seminoncol.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunner M, Veness MJ, Ch'ng S, Elliott M, Clark JR. Distant metastases from cutaneous squamous cell carcinoma — analysis of AJCC stage IV. Head Neck. 2013;35:72–5. doi: 10.1002/hed.22913. [DOI] [PubMed] [Google Scholar]
- 3.Wang CQ, Yanofsky VR, Mitsui H, Krueger JG, Felsen D, Carucci JA. The PD-1 pathway is a potential drug target for squamous cell carcinoma in solid organ transplant recipients. J Invest Dermatol. 2013;133(Suppl 1):S10. abstract. [Google Scholar]
- 4.Teply BA, Lipson EJ. Identification and management of toxicities from immune checkpoint-blocking drugs. Oncology (Williston Park) 2014;28(Suppl 3):30–8. [PubMed] [Google Scholar]
- 5.Lipson EJ, Bodell MA, Kraus ES, Sharfman WH. Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol. 2014;32(19):e69–71. doi: 10.1200/JCO.2013.49.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


