Abstract
Recently, there has been great attention given to the possibility of combating obesity by targeting brown fat activity or increasing differentiation of brown adipocytes in white fat depots through a process termed ‘browning'. Sympathetic innervation of brown and white adipose tissues provides adrenergic input that drives thermogenesis and regulates fatty acid metabolism, as well as stimulating adipogenesis of recruitable brown adipocyte tissue (rBAT, also known as beige or brite) in white fat. Other factors acting in an endocrine or autocrine/paracrine manner in adipose tissue may also stimulate browning. There have been significant recent advances in understanding the mechanisms of increasing adipose tissue energy expenditure, as well as how brown adipocytes appear in white fat depots, including via de novo adipogenesis from tissue precursor cells. In this article, we integrate this new knowledge with a historical perspective on the discovery of ‘browning'. We also provide an overview of constitutive BAT vs rBAT in mouse and human.
Obesity represents a major risk factor for the development of several of our most common medical conditions, including type 2 diabetes mellitus, dyslipidemia, non-alcoholic fatty liver, cardiovascular disease and even some cancers.1 Increased adiposity is the main characteristic of obesity; however, not all fat depots are created solely for energy storage.2 Adipocytes found in white adipose tissue (WAT) contain a single large lipid droplet and have well-characterized roles in fuel storage and immune-endocrine functions. By contrast, brown adipose tissue (BAT) is specialized for energy expenditure. Adipocytes in BAT contain many small, multilocular lipid droplets, and are tightly packed with mitochondria. In addition, BAT is highly vascularized and densely innervated by the sympathetic nervous system (SNS). BAT uniquely expresses uncoupling protein 1 (UCP1), which is localized to the inner mitochondrial membrane, and acts to uncouple oxidative phosphorylation from ATP production, resulting in the electron gradient being dissipated as heat in a process termed thermogenesis.3, 4, 5, 6 In response to cold, or other stimuli such as diet or activation of β3-adrenergic receptors (ADRB3), thermogenesis is activated as a result of increased sympathetic input to BAT.7, 8
In addition to thermogenesis, recent studies have demonstrated that BAT is involved in triglyceride clearance9 and glucose disposal,10 and is a source of adipokines (which we call ‘BATokines' for BAT adipokines8), including FGF21,11, 12 Irisin/FNDC5(refs. 13, 14) and interleukin-6(ref. 15); (reviewed in Villarroya et al.16). Following the rediscovery of functionally active BAT in adult humans,17, 18, 19, 20, 21, 22, 23 brown fat has become an exciting area for obesity research. Given BAT's immense capacity for energy expenditure and its newly recognized effects on fatty acid and glucose metabolism, there is great hope that BAT's energetic capacity may be tapped by various medical interventions as a means to increase whole-body energy expenditure and reduce adiposity.8, 24, 25 Undoubtedly, improved knowledge about the regulation of brown fat formation, activation and communication with the central nervous system (CNS) are required to make such therapeutic approaches conceivable.
Although interscapular BAT is the major brown fat depot in mice and is constitutively expressed (constitutive BAT, cBAT), multilocular and UCP1-positive brown fat cells can be found in different anatomical locations as well. These ‘inducible' or ‘recruitable' brown fat cells (recruitable brown adipose tissue, rBAT),26 also known as ‘beige'27 or ‘brite'28 adipocytes, are found to be highly enriched in WAT and skeletal muscle in obesity-resistant strains of mice.29, 30, 31 Physiological stimuli, such as cold exposure and sympathetic activation, are also known to induce brown adipogenesis in white fat depots.32 This phenomenon, known as ‘browning', has been described in WAT, especially in the subcutaneous depot and after cold exposure or CNS manipulations that increased sympathetic outflow to these white fat depots.33, 34 Similarly, when mice are given chronic daily injections of CL 316,243, an ADBR3 agonist, subcutaneous WAT also undergoes ‘browning' and body temperature rises.35 In addition to SNS input, cBAT sends sensory nerve afferents to the CNS, which also are involved in the regulation of thermogenesis.36
Browning and cBAT vs rBAT: historical perspectives and current findings
The process of browning of white fat was first described in the 1984 FEBS paper by Young et al.,37 who wrote ‘…during a preliminary study of the effects of cold acclimation on female BALB/c mice, we noticed some brown areas in the perigenital fat pad. Since this observation was contrary to the popular view of distinct white and brown fat regions, we decided to set up this study so that morphological and biochemical criteria could be used to differentiate between white and brown adipocytes…' These brown areas were close to blood vessels and had UCP1 (assessed by radioimmunoassay). The cells were centrally nucleated with multilocular lipid droplets and a high concentration of mitochondria (measured by electron microscopy), and the tissue displayed high activities of mitochondrial enzymes.37 This finding was confirmed in 1986 using a cat model,38 and was followed up by several other observations,39, 40, 41 including studies in animals after treatment with ADBR3 agonists.42, 43
More recently, browning has been described as being induced by numerous factors,27, 44 including cardiac-derived natriuretic peptides,45 action of central and peripheral SIRT1,46, 47 central brain-derived neurotrophic factor action, as well as brain-derived neurotrophic factor induced by animals being housed in an enriched environment,48 central and possibly peripheral orexin action,49 muscle-derived irisin,13 heart-derived natriuretic peptides,45 liver- and BAT-derived FGF2150, 51 and bone morphogenetic proteins (BMPs)\transforming growth factor beta (TGFβ),35, 52, 53, 54, 55 among others. Other triggers shown to induce browning are as follows: overexpression of perilipin in WAT,56 addition of a PGC1α adenovirus to human white preadipocytes,57 prolactin receptor knockout,58 exercise,59 knockdown of neuropeptide Y in the dorsomedial hypothalamus60 and vascular endothelial growth factor-A overexpression in WAT.61 New signaling pathways have been implicated in the process of brown adipogenesis in WAT, including retinaldehyde dehydrogenase,62 4E-BP1,63 Rb,64 RIP140,65 LiverXRa,66 FoxC2,67 TIF2,68 p107,69 TNFαR70 and others. MicroRNAs (miRs) have also been linked with the control of brown adipogenesis, such as mir-193b-365,71 mir-196a,72 mir-133,73 miR-106b-93(ref. 74) and mir-155.75 Likely, these miRs have a role in regulating gene expression of transcription factors involved in controlling the brown adipocyte fate during cellular differentiation (reviewed in Trajkovski and Lodish76), and could also represent an aspect of epigenetic regulation of brown adipogenesis.
Whether the browning observed in the studies outlined above is the result of de novo adipogenesis of precursor cells into brown adipocytes and whether mature white adipocytes may transdifferentiate directly into mature brown adipocytes are theories still up for debate. Transdifferentiation is characterized by the presence of paucilocular UCP1+ adipocytes appearing in white fat,77, 78, 79 and has been described as early as 1966 by Hull and Segall.80 They suggested that white and brown adipocytes are essentially two forms of the same tissue, potentially representing a metabolic flexibility to either produce heat or store lipids, depending on the needs of the body.80 In support of this notion, UCP1-positive brown adipocytes were found in inguinal WAT between 10 and 21 days of age in mice maintained at room temperature, but these cells disappeared by 60 days of age.81 Interestingly, these cells could re-emanate upon cold exposure or could be suppressed by undernutrition from birth to 21 days post natal, suggesting a high degree of plasticity. A recent lineage-tracing study purported to reveal that mature adipocytes are capable of making a bi-directional switch: from mature white to mature brown adipocytes during cold exposure, and back again during re-warming.82 However, the cells pictured retain some multilocular morphology and low leptin expression even after re-warming; thus, the cell type with apparent plasticity in response to changes in environmental temperature may represent a distinct ‘recruitable' cell that does not, in fact, return to a truly white, unilocular adipocyte state with low levels of UCP1 and few mitochondria. Furthermore, it is interesting that these transdifferentiating cells occur in pockets, which may represent their close proximity to both sympathetic innervation and vascular supply, in order to mediate their flexibility in gene expression and thermogenic potential. It is certainly likely that sympathetic neurites undergo their own plasticity in response to numerous bouts of cold exposure, and may innervate and activate a slightly different group of precursor cells with each bout.
On the other hand, numerous studies have demonstrated that the appearance of brown adipocytes in WAT depots is the result of de novo adipogenesis. Precursor cells expressing stem cell antigen-1 and platelet-derived growth factor receptor-α in the abdominal fat pad similarly exhibit the dual potential to differentiate into either rBAT or WAT.35, 83 Interestingly, the stem cell antigen-1-positive cells isolated from cBAT and WAT express distinct molecular signatures and respond differently to inductive cues.35 Of the PDGFRα cells in WAT, a CD24+ population was identified that loses expression of CD24, as it more fully commits to the adipocyte lineage.84 Subtypes of precursor cells that can differentiate into brown adipocytes from white fat depots have been identified, including those expressing CD137, Tmem26 and Tbx1,85 but these cells have not been directly monitored in vivo as they undergo adipogenesis. A recent publication utilizing the AdipoChaser mouse, a doxycycline-inducible, mature adipocyte-specific tracing system, developed to answer the question of whether rBAT arises from a lineage distinct from white adipocytes.86 The AdipoChaser mouse was pulse-chased to indelibly label mature adipocytes, enabling the discovery that most of the rBATs appear in subcutaneous WAT in response to cold or treatment with β3-adrenergic agonists as the result of de novo adipogenesis, rather than preexisting white adipocytes.
Brown adipogenesis also occurs in situations with a reduction in autophagy in Myf5+ precursor cells (with a deletion of Atg7, a key gene for autophagy), which then increases rBAT.87 This is intriguing, given that autophagy is a situation responsive to nutrient status and is involved in lipid metabolism (reviewed in Christian et al.88). Targeting Myf5+ adipocyte precursors is significant, given the findings in mice that the Myf5+ lineage contributes to the development of the interscapular, or cBAT, whereas Myf5− cells largely comprise white fat depots and the rBAT compartment.89 Deletion of the type 1 bone morphogenetic protein receptor BMPR1A from Myf5+ cells results in a severe paucity of cBAT with a resulting compensation of rBAT in WAT depots.90 As a result, the knockout mice could maintain proper body temperature as adults and are resistant to high-fat diet-induced obesity. These data highlight the existence of a physiological system for thermoregulation and energy homeostasis by modulating total BAT-mediated thermogenic capacity. Mechanistically, this is likely due to feedback from the impaired cBAT to brain, and a resulting increase of SNS input to WAT in order to drive brown adipogenesis and rBAT development. Alternatively, the cross talk between cBAT and rBAT could be mediated by secreted factors presumably produced by residual cBAT in the Myf5-BMPR1A knockout mice. The understanding of fat depot cross talk is in its infancy, although it is known that sympathetic denervation of one WAT depot will influence norepinephrine turnover in the intact depots of WAT and BAT, but will not affect depot weights.91 Likely, this occurs via CNS integration of sensory afferents from adipose depots.92 More research is needed to better understand how adipose depots communicate with each other via CNS and SNS intermediaries.
Interestingly, ablation of the PI3K signaling inhibitor phosphatase and tensin homolog in Myf5+ precursors leads to a remodeling of adipose tissues in the body with increased adiposity of WAT and BAT, especially in the neck and shoulder regions.93 As a result, the cBAT in the interscapular region was more lipid laden. This study also revealed that a subset of cells in white fat depots, such as interscapular WAT and retroperitoneal WAT, in fact comprises a mix of Myf5− and Myf5+ precursors.93 Given the difficulty in comparing WAT and BAT depots across different studies, owing to variations in nomenclature and anatomical dissections, as well as differences conferred by mouse strain (reviewed in Yadav and Rane94), it will likely be important for the field to develop a recognized characterization of mouse fat depots. Such an advance would accommodate our growing knowledge about the differences among white fat depots and their relative response to browning, including new insights stemming from lineage-tracing studies (reviewed in Sanchez-Gurmaches and Guertin95).
Several new concepts have arisen in the study of rBAT, including whether the brown adipocytes interspersed between muscle fibers also contribute to anti-obesity effects,31, 35 and whether preadipocytes directly sense cold temperature and thereby turn on a thermogenic program or differentiate into brown adipocytes. A recent study demonstrated that 3T3-F442A cells exposed to cold temperatures in vitro exhibit a mild increase in UCP1 gene expression.96 It is interesting to note that single-celled organisms such as the bacterium Escherichia coli can directly sense and respond to environmental temperature.97 Such behavior suggests the possibility that CNS–SNS-independent pathways may exist for cell autonomous temperature sensation.
Human vs mouse BAT
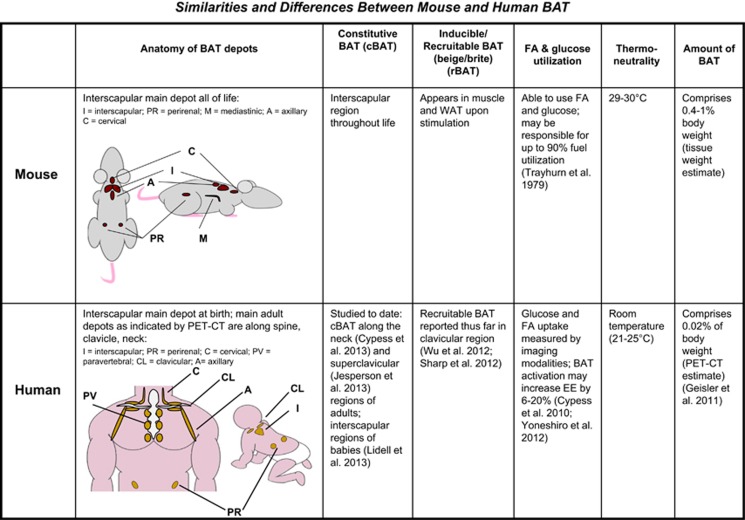
To date, there have been few studies in humans to identify non-cold-temperature means of activating BAT or increasing its mass, but a multitude of rodent studies have revealed many potential options for this purpose. Although the function of BAT appears similar between rodents and humans, making mice a useful model species, there are a few important distinctions. Rodents, such as laboratory mice, have a different distribution of BAT vs humans (Figure 1), which in mice includes a large, discrete interscapular pad that is similar to what is observed in human babies.98, 99 In adult humans, BAT is mainly clustered around the neck, clavicle and spinal cord.18, 19, 21 Mice contain other smaller depots of BAT, such as around the kidneys, cervical spine and heart,100 as well as the rBAT found in white fat and skeletal muscle.101 Humans also possess various small BAT depots including around organs such as kidney and heart, and in other subcutaneous depots such as under the arms.98, 102 Whether or not humans can boost their available BAT mass was previously unclear, but two recent studies have demonstrated that cold acclimation in humans, after repeated daily cold exposure, elevates BAT volume and activity, and increases energy expenditure.103, 104
Figure 1.
Mouse vs human BAT. Anatomical and other similarities, and differences between mouse and human BAT.
In additional, mice are often housed at a room temperature that is not thermoneutrality for them, forcing them to undergo thermogenesis at room temperature in order to maintain their body temperature. Such an adaptation renders their BAT chronically activated. Humans, on the other hand, live at thermoneutrality in addition to heating their homes and wearing clothing, which likely maintains BAT at a lower level of activation.
Another difference between rodent and human BAT has to do with the second population of brown adipocytes, the inducible or rBAT (brite or beige) as described above, which can be found in white fat depots or in the muscle of mice. These cells develop from a different lineage than the cBAT found in the interscapular region (reviewed in Townsend and Tseng8), and are recruitable in the sense that SNS stimulation (in the form of β-adrenergic agonists or cold exposure) or activation of signaling pathways (such as those reviewed in the sections above) can induce the presence of these brown adipocytes. It is believed that these cells can then heighten the energy expenditure of the whole animal. On the other hand, human BAT is now believed to be comprised of either cBAT (as is found in human babies' interscapular region99 and the neck,105 or supraclavicular region106 of adults), or human BAT comprises rBAT (as is found in the clavicular region85 and the retroperitoneal, intra-abdominal and other regions.107) The constitutive and recruitable types of brown adipocytes display a different gene expression signature,85, 100 but whether there is a functional difference in addition to a different lineage for these two types of adipocytes remains to be determined.
BAT mass in rodents is ~0.4–1% of body weight (Townsend and Tseng, unpublished dissection data, and Geisler108), whereas in humans it is estimated at ~0.02% of body weight,108 and thus humans have relatively less BAT than rodents. Small mammals may utilize BAT to oxidize up to 90% of daily fuel intake; BAT has been shown to account for the majority uptake of ingested glucose and the most cold-stimulated triglyceride clearance.109, 110 Lipogenesis in BAT may account for 40% of the total in cold-exposed rats.111 In humans, it has been estimated that a few grams of BAT may increase daily energy expenditure from 6 to 20%.112, 113 Regardless of the differences in the amount or anatomical locations of brown fat between rodents and humans, both human and rodent BAT possesses great capacity for thermogenesis, and serves as an important site for glucose and fatty acid metabolism (described above and Ouellet et al.114).
Although adipose tissues express numerous subtypes of the adrenergic receptor, ADBR3 has a more limited distribution in mice and is most highly expressed in BAT and WAT, thereby making it the most likely adrenergic receptor isoform to mediate sympathetic effects on energy expenditure in murine adipose tissues.115 On the other hand, humans have more widespread expression of ADBR3, including in adipose tissues, urinary bladder, smooth muscle and gut.116 In humans, the blockade of β-adrenergic receptors by propranolol before cold exposure does not inhibit cold-induced thermogenesis, suggesting that skeletal muscle uncoupling downstream of the β2-receptor may be the culprit.117 In mice, the triple β-receptor knockout is cold intolerant and obese.118, 119 It was recently demonstrated that the β1-receptor in mice mediates most of the cold- and diet-induced thermogenesis,120 further supporting the involvement of multiple adrenergic receptors in regulating adipose tissue energy expenditure in response to catecholamine release from sympathetic nerve terminals.
In rodents, ADRB3 agonists effectively activate BAT, leading to weight loss and improved insulin sensitivity.43, 121, 122 However, the effect of these compounds in humans appears negligible, which has been attributed to reduced ligand-binding ability or bioavailability to human ADRB3.123, 124 (After submission of the original manuscript, a new study demonstrated that mirabegron, a highly specific β3-adrenergic receptor agonist, can stimulate human brown fat thermogenesis (Cypess et al., Cell Metab 2015; 21: 33–38)) Furthermore, the cross-reactivity of these agonists to β1 or β2 adrenergic receptors may lead to unwanted cardiovascular side effects in humans.
Concluding remarks and future perspectives
New knowledge about the presence and activity of human BAT represents an exciting opportunity to exploit physiological pathways for increasing thermogenesis and energy expenditure as a potential therapeutic target for human obesity. Using mouse models, several novel pathways regulating BAT and WAT energy expenditure have been identified. The ability to utilize human subjects to obtain a better understanding of BAT function and activity is also improving. Considering the similarities and differences between mouse and human BAT, together these studies should provide important translational work to enable development of new therapeutics targeting BAT for the treatment of obesity and other metabolic diseases. The field is moving toward identification of cell types and molecular pathways mediating the development of rBAT in WAT depots. Such studies could determine whether these cells are identical in thermogenic function to cBAT,125 or whether different fat depots give rise to different subtypes of rBAT cells through mechanisms such as de novo adipogenesis or transdifferentiation. Finally, it remains to be resolved whether increasing rBAT will alone suffice to increase energy expenditure and restore metabolic health to obese humans, but given the data outlined here, the prospect is a promising one.
Acknowledgments
We thank E Caniano for administrative assistance and MD Lynes for assistance with the mouse and human drawings in Figure 1. This work was supported in part by National Institutes of Health (NIH) grants R01 DK077097 (to Y-HT) and P30 DK036836 (Diabetes Research Center to the Joslin Diabetes Center), a research grant from the American Diabetes Association (to Y-HT), and funding from the Harvard Stem Cell Institute (to Y-HT). KLT was funded by NIH grants T32 DK007260-33 and F32 DK091996 as well as a BADERC P&F, a BNORC P&F, and an ADA Junior Faculty Award 1-14-JF-55.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This article is published as part of a supplement sponsored by the Université Laval's Research Chair in Obesity, in an effort to inform the public on the causes, consequences, treatments and prevention of obesity.
Footnotes
Y-HT has received consulting fees from Ember Therapeutics, lecture fees from Pfizer Inc., and received grant support from Chugai Pharma Co., Ltd and MedImmune LLC. KLT declares no conflict of interest.
References
- Lazar MA. How obesity causes diabetes: not a tall tale. Science 2005; 307: 373–375. [DOI] [PubMed] [Google Scholar]
- Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell 2007; 131: 242–256. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 2000; 404: 652–660. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev 2009; 20: 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes Rev 2009; 10: 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int J Obes (Lond) 2010; 34: S7–S16. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Nakamura K. Central neural pathways for thermoregulation. Front Biosci (Landmark Ed) 2011; 16: 74–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KL, Tseng YH. Brown adipose tissue: recent insights into development, metabolic function, and therapeutic potential. Adipocyte 2012; 1: 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Merkel M, Heeren J. A new, powerful player in lipoprotein metabolism: brown adipose tissue. J Mol Med (Berl) 2012; 90: 887–893. [DOI] [PubMed] [Google Scholar]
- Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 2011; 14: 272–279. [DOI] [PubMed] [Google Scholar]
- Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem 2011; 286: 12983–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med 2011; 17: 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Rivada A, Castelao C, Senin LL, Landrove MO, Baltar J, Belen CA et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One 2013; 8: e60563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burysek L, Houstek J. beta-Adrenergic stimulation of interleukin-1alpha and interleukin-6 expression in mouse brown adipocytes. Growth Regul 1997; 411: 83–86. [DOI] [PubMed] [Google Scholar]
- Villarroya J, Cereijo R, Villarroya F. An endocrine role for brown adipose tissue? Am J Physiol Endocrinol Metab 2013; 305: E567–E572. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444–E452. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508. [DOI] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525. [DOI] [PubMed] [Google Scholar]
- Zingaretti MC, Crosta F, Vitali A, Guerrieri M, Frontini A, Cannon B et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J 2009; 23: 3113–3120. [DOI] [PubMed] [Google Scholar]
- Celi FS. Brown adipose tissue—when it pays to be inefficient. N Engl J Med 2009; 360: 1553–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov 2010; 9: 465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle AJ, Lopez M, Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends Mol Med 2011; 17: 405–411. [DOI] [PubMed] [Google Scholar]
- Enerback S. The origins of brown adipose tissue. N Engl J Med 2009; 360: 2021–2023. [DOI] [PubMed] [Google Scholar]
- Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19: 1252–1263. [DOI] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic PPARgamma activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classical brown adipocytes. J Biol Chem 2010; 285: 7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 1998; 102: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B, Rim JS, Hogan JC, Coulter AA, Koza RA, Kozak LP. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res 2007; 48: 41–51. [DOI] [PubMed] [Google Scholar]
- Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci USA 2007; 104: 2366–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab 2010; 11: 253–256. [DOI] [PubMed] [Google Scholar]
- Richard D, Monge-Roffarello B, Chechi K, Labbe SM, Turcotte EE. Control and physiological determinants of sympathetically mediated brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 2012; 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 2012; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA 2011; 108: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan CH, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from brown fat and sensory denervation alters its thermogenic responses. Am J Physiol Regul Integr Comp Physiol 2012; 302: R1049–R1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Arch JR, Ashwell M. Brown adipose tissue in the parametrial fat pad of the mouse. Growth Regul 1984; 167: 10–14. [DOI] [PubMed] [Google Scholar]
- Loncar D, Bedrica L, Mayer J, Cannon B, Nedergaard J, Afzelius BA et al. The effect of intermittent cold treatment on the adipose tissue of the cat. Apparent transformation from white to brown adipose tissue. J Ultrastruct Mol Struct Res 1986; 97: 119–129. [DOI] [PubMed] [Google Scholar]
- Loncar D, Afzelius BA, Cannon B. Epididymal white adipose tissue after cold stress in rats. II. Mitochondrial changes. J Ultrastruct Mol Struct Res 1988; 101: 199–209. [DOI] [PubMed] [Google Scholar]
- Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992; 103: 931–942. [DOI] [PubMed] [Google Scholar]
- Cousin B, Casteilla L, Lafontan M, Ambid L, Langin D, Berthault MF et al. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology 1993; 133: 2255–2262. [DOI] [PubMed] [Google Scholar]
- Champigny O, Ricquier D, Blondel O, Mayers RM, Briscoe MG, Holloway BR. Beta 3-adrenergic receptor stimulation restores message and expression of brown-fat mitochondrial uncoupling protein in adult dogs. Proc Natl Acad Sci USA 1991; 88: 10774–10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Cui J, Danforth E Jr, Taatjes DJ, Lang SS, Waters BL et al. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol 1994; 266: R1371–R1382. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol 2014; 10: 24–36. [DOI] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012; 122: 1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 2010; 12: 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of PPARgamma. Cell 2012; 150: 620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab 2011; 14: 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellayah D, Bharaj P, Sikder D. Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab 2011; 14: 478–490. [DOI] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 2012; 26: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Estall JL, Adams AC, Antonellis PJ, Bina HA, Flier JS et al. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 2011; 152: 2996–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454: 1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle AJ, Carobbio S, Martins L, Slawik M, Hondares E, Vazquez MJ et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012; 149: 871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab 2011; 14: 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian SW, Tang Y, Li X, Liu Y, Zhang YY, Huang HY et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc Natl Acad Sci USA 2013; 110: E798–E807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T, Miyoshi H, Shimada K, Suzuki A, Okamatsu-Ogura Y, Perfield JW et al. Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype. PLoS One 2010; 5: e14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D et al. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem 2003; 278: 33370–33376. [DOI] [PubMed] [Google Scholar]
- Auffret J, Viengchareun S, Carre N, Denis RG, Magnan C, Marie PY et al. Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity. FASEB J 2012; 26: 3728–3737. [DOI] [PubMed] [Google Scholar]
- De MR, Lucertini F, Guescini M, Polidori E, Zeppa S, Stocchi V et al. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr Metab Cardiovasc Dis 2013; 23: 582–590. [DOI] [PubMed] [Google Scholar]
- Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab 2011; 13: 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K, Wernstedt Astrerholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci USA 2012; 109: 5874–5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer FW, Vernochet C, O'Brien P, Spoerl S, Brown JD, Nallamshetty S et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med 2012; 18: 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med 2001; 7: 1128–1132. [DOI] [PubMed] [Google Scholar]
- Hansen JB, Jorgensen C, Petersen RK, Hallenborg P, De Matteis R, Boye HA et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci USA 2004; 101: 4112–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker MG. RIP140-targeted repression of gene expression in adipocytes. Mol Cell Biol 2005; 25: 9383–9391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang Y, Yehuda-Shnaidman E, Medvedev AV, Kumar N, Daniel KW et al. Liver X receptor alpha is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Mol Cell Biol 2008; 28: 2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 2001; 106: 563–573. [DOI] [PubMed] [Google Scholar]
- Picard F, Gehin M, Annicotte J, Rocchi S, Champy MF, O'Malley BW et al. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 2002; 111: 931–941. [DOI] [PubMed] [Google Scholar]
- Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME et al. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab 2005; 2: 283–295. [DOI] [PubMed] [Google Scholar]
- Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem 2009; 284: 36213–36222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM et al. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol 2011; 13: 958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol 2012; 10: e1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Pasut A, Soleimani VD, Bentzinger CF, Antoun G, Thorn S et al. MicroRNA-133 controls brown adipose determination in skeletal muscle satellite cells by targeting Prdm16. Cell Metab 2013; 17: 210–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zuo J, Zhang Y, Xie Y, Hu F, Chen L et al. Identification of miR-106b-93 as a negative regulator of brown adipocyte differentiation. Biochem Biophys Res Commun 2013; 438: 575–580. [DOI] [PubMed] [Google Scholar]
- Chen Y, Siegel F, Kipschull S, Haas B, Frohlich H, Meister G et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun 2013; 4: 1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovski M, Lodish H. MicroRNA networks regulate development of brown adipocytes. Trends Endocrinol Metab 2013; 24: 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol 2000; 279: C670–C681. [DOI] [PubMed] [Google Scholar]
- Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest 2002; 25: 823–835. [DOI] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010; 298: E1244–E1253. [DOI] [PubMed] [Google Scholar]
- Hull D, Segall MM. Distinction of brown from white adipose tissue. Nature 1966; 212: 469–472. [DOI] [PubMed] [Google Scholar]
- Kozak LP, Koza RA, Anunciado-Koza R, Mendoza T, Newman S. Inherent plasticity of brown adipogenesis in white fat of mice allows for recovery from effects of post-natal malnutrition. PLOS One 2012; 7: e30392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15: 659–667. [DOI] [PubMed] [Google Scholar]
- Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 2012; 15: 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 2013; 15: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19: 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lopez N, Athonvarangkul D, Sahu S, Coletto L, Zong H, Bastie CC et al. Autophagy in Myf5+ progenitors regulates energy and glucose homeostasis through control of brown fat and skeletal muscle development. EMBO Rep 2013; 14: 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P, Sacco J, Adeli K. Autophagy: Emerging roles in lipid homeostasis and metabolic control. Biochim Biophys Acta 2013; 1831: 819–824. [DOI] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454: 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 2013; 495: 379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB. Sympathetic denervation of one white fat depot changes norepinephrine content and turnover in intact white and brown fat depots. Obesity (Silver Spring) 2012; 20: 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010; 34: S36–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab 2012; 16: 348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Rane SG. TGF-beta/Smad3 Signaling Regulates Brown Adipocyte Induction in White Adipose Tissue. Front Endocrinol (Lausanne) 2012; 3: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: Tracing back the origins of fat. Biochim Biophys Acta 2014; 1842: 340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP et al. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci USA 2013; 110: 12480–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama S, Ohno S, Ohta N, Inoue Y, Fukuoka H, Ishijima A et al. Thermosensing function of the Escherichia coli redox sensor Aer. J Bacteriol 2010; 192: 1740–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972; 112: 35–39. [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Leinhard OD, Heglind M, Elander L, Slawik M et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013; 19: 631–634. [DOI] [PubMed] [Google Scholar]
- Walden TB, Hansen IR, Timmons JA, Cannon B, Nedergaard J. Recruited vs. nonrecruited molecular signatures of brown, "brite," and white adipose tissues. Am J Physiol Endocrinol Metab 2012; 302: E19–E31. [DOI] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 2013; 27: 234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes 2013; 62: 1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest 2013; 123: 3404–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lans AA, Hoeks J, Brans B, Vijgen GH, Visser MG, Vosselman MJ et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest 2013; 123: 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013; 19: 635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 2013; 17: 798–805. [DOI] [PubMed] [Google Scholar]
- Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLOS One 2012; 7: e49452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler JG. Targeting energy expenditure via fuel switching and beyond. Diabetologia 2011; 54: 237–244. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab. 2011; 13: 238–240. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med 2011; 17: 200–205. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Thermoregulation in the diabetic-obese (db/db) mouse. The role of non-shivering thermogenesis in energy balance. Pflugers Arch 1979; 380: 227–232. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Kahn CR. Brown fat as a therapy for obesity and diabetes. Curr Opin Endocrinol Diabetes Obes 2010; 17: 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshiro T, Ogawa T, Okamoto N, Matsushita M, Aita S, Kameya T et al. Impact of UCP1 and beta3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int J Obes (Lond) 2013; 37: 993–998. [DOI] [PubMed] [Google Scholar]
- Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012; 122: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. ß-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front Endocrin (Lausanne) 2012; 2: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krief S, Lonnqvist F, Raimbault S, Baude B, Van Spronsen A, Arner P et al. Tissue distribution of beta 3-adrenergic receptor mRNA in man. J Clin Invest 1993; 91: 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijers SL, Schrauwen P, van Baak MA, Saris WH, Marken Lichtenbelt WD. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab 2011; 96: E598–E605. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK et al. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 2002; 297: 843–845. [DOI] [PubMed] [Google Scholar]
- Jimenez M, Leger B, Canola K, Lehr L, Arboit P, Seydoux J et al. Beta(1)/beta(2)/beta(3)-adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Lett 2002; 530: 37–40. [DOI] [PubMed] [Google Scholar]
- Ueta CB, Fernandes GW, Capelo LP, Fonseca TL, Maculan FD, Gouveia CH et al. beta(1) Adrenergic receptor is key to cold- and diet-induced thermogenesis in mice. J Endocrinol 2012; 214: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Sakane N, Wakabayashi Y, Umekawa T, Kondo M. Anti-obesity effect of CL 316,243, a highly specific beta 3-adrenoceptor agonist, in mice with monosodium-L-glutamate-induced obesity. Eur J Endocrinol 1994; 131: 97–102. [DOI] [PubMed] [Google Scholar]
- de Souza CJ, Hirshman MF, Horton ES. CL-316,243, a beta3-specific adrenoceptor agonist, enhances insulin-stimulated glucose disposal in nonobese rats. Diabetes 1997; 46: 1257–1263. [DOI] [PubMed] [Google Scholar]
- Arch JR. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from beta3-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol 2008; 378: 225–240. [DOI] [PubMed] [Google Scholar]
- Clapham JC, Arch JR. Thermogenic and metabolic antiobesity drugs: rationale and opportunities. Diabetes Obes Metab 2007; 9: 259–275. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in brite/beige adipose tissue mitochondria is functionally thermogenic. Cell Rep 2013; 5: 1196–1203. [DOI] [PubMed] [Google Scholar]