Abstract
Epicardial adipose tissue is a unique fat depot around the heart that shares a close anatomic proximity and vascular supply with the myocardium and coronary arteries. Its accumulation around the heart, measured using various imaging modalities, has been associated with the onset and progression of coronary artery disease in humans. Epicardial adipose tissue is also the only fat depot around the heart that is known to express uncoupling protein 1 at both mRNA and protein levels in the detectable range. Recent advances have further indicated that human epicardial fat exhibits beige fat-like features. Here we provide an overview of the physiological and pathophysiological relevance of human epicardial fat, and further discuss whether its thermogenic properties can serve as a target for the therapeutic management of coronary heart disease in humans.
Human heart is surrounded by multiple layers of fat, which can be divided into fat depots that lie inner to the visceral pericardium and the ones lying outside the parietal pericardium. Inconsistencies in the definition of these depots have generated various commonly used terms such as epicardial fat,1, 2 pericardial fat,3, 4, 5 mediastinal fat,6, 7 paracardial fat,8 intrathoracic fat,9, 10 perivascular fat11, 12 and periadventitial fat.8, 13 Although some of them by and large represent the same depot, significant differences do exist in the origin, anatomical location and hence in the plausible physiological relevance of other fat depots around the heart. This understanding underscores the growing need to standardize these definitions such that the relative importance of each fat depot can be appreciated. The epicardial adipose tissue represents the fat surrounding the heart that lies inner to the visceral pericardium, whereas the terms ‘paracardial', ‘intrathoracic' or ‘mediastinal' adipose tissue refer to the fat that lies outside the parietal pericardium but sits within the thoracic cavity.14 The term ‘pericardial fat' is often used interchangeably with epicardial fat, however, there is growing consensus for this term to be used as a representative of the total fat around the heart, that is, epicardial and paracardial fat combined.15, 16 The terms ‘perivascular' and ‘periadventitial' adipose tissue are used to represent fat depots that cover various blood vessels including coronaries and the peripheral vasculature,12 leading to the occasional use of the term ‘pericoronary fat' to represent epicardial fat.17
Despite the discrepancies in the nomenclature, it has become clear that epicardial fat, which is the subject of the current review, is capable of influencing cardiac physiology and function in a unique manner. Burgeoning evidence also points toward its association with the pathophysiology of coronary artery disease (CAD) in humans.18, 19 Here we provide a brief overview of the current perspectives on epicardial fat and its association with the development of CAD in humans. In addition, we have identified gaps in the current understanding of epicardial fat, and further discuss a potential role for its thermogenic properties in the management of cardiovascular risk in humans.
Physiological relevance of human epicardial fat
Epicardial fat is derived from the splanchnopleuric mesoderm and is considered to be the true visceral fat depot of the heart.8 A recent study further demonstrates that the origin of human and mouse epicardial fat lies in the epicardium itself.20 In terms of its distribution, postmortem studies have revealed that epicardial fat can cover up to 80% of the heart surface and contribute up to 20% of total ventricular weight under normal conditions, while being capable of covering the heart completely and even extending up to 2 cm in thickness during obesity in humans.21, 22 Anatomically, epicardial fat is not separated from the underlying myocardium with any fascia-like structure and is also known to share coronary blood supply with the latter, thereby supporting the idea of plausible ‘vasocrine' or ‘paracrine' cross talk between the two. However, a direct microcirculatory interconnection between the two has not been established so far.23, 24 Nonetheless, owing to its unique anatomic juxtaposition, numerous plausible physiological roles have been offered to epicardial fat including, but not limited to, that (1) it serves as the anatomic site for the cardiac nervous system,25 (2) it serves to cushion the coronary arteries against pulse wave torsion,14 (3) it serves as a heating blanket to protect the myocardium during hypothermia,26, 27 (4) it is an immunological tissue that serves to protect the myocardium and coronaries against pathogens and inflammatory activators28 and (5) it serves to maintain fatty acid homeostasis in the coronary microcirculation.5
In a seminal study looking at the anatomy, histology and biochemical properties of epicardial fat across a spectrum of species, epicardial fat was reported to exhibit a higher capacity for breakdown, synthesis and release of fatty acids relative to other intra-abdominal fat depots in guinea pigs.23 In addition, epicardial fat exhibited higher rates of fatty acid incorporation, basal- as well as insulin-induced lipogenesis in a subsequent study involving guinea pigs.5 Based on these observations, Marchington et al.5 proposed that epicardial fat could serve as a buffer to protect the myocardium from lipid toxicity during times of energy excess by absorbing excess fatty acids, whereas it could also serve to provide fuel for myocardial contraction during times of energy restriction by undergoing fatty acid release. Considering that fatty acids are the major source of fuel for contracting heart muscle, this proposal is highly plausible and therefore widely accepted.8, 22, 27 However, it is important to note that these observations have not been repeated in other animal models or validated for human epicardial fat. Considering that rodents do not necessarily possess epicardial fat under normal conditions and even when observed (that is, due to obesity or aging), such as in the case of guinea pigs, epicardial fat distribution differs from that in humans.20, 29 Thus, species–specific differences in the biochemical, molecular as well as physiological relevance of epicardial fat can be expected, and hence it can be stated that our understanding of the human epicardial fat is quite limited at this point. By demonstrating that human and mouse epicardial adipocytes originate from ventricular epicardium that constitutively express peroxisome proliferator-associated receptor-γ in case of humans, but not in case of mice, Yamaguchi et al.20 have pinpointed such differences. Additional studies looking at the characterization of biochemical and molecular properties of epicardial fat and other cardiac fat stores in humans as well as in other animal models are thus required to clearly understand the biology of this unique fat depot.
Pathophysiological relevance of human epicardial fat
Despite our limited understanding of human epicardial fat under physiological conditions, there is no dearth of evidence that points toward a definite role for epicardial fat under pathophysiological conditions, especially in the development of CAD in humans. Clinical assessment of epicardial fat mass/volume using various imaging modalities has clearly revealed that epicardial fat accumulation shares a strong association with the onset and development of CAD in humans. Initial studies, including the Framingham Heart Study and Multi-Ethnic Study of Atherosclerosis, identified pericardial fat as an independent predictor of cardiovascular risk.4, 10 In fact, in comparison with the Framingham risk score (where smoking and type 2 diabetes mellitus only account for 1.6- and 3-fold risk of having CAD, respectively), pericardial fat volume was identified as the strongest predictor of developing CAD (odds ratio of 4.1).30, 31 In addition, a case–control study identified pericardial fat volume to be a strong predicator of myocardial ischemia in patients without CAD.32
The question of whether such predictive capabilities can be attributed specifically to epicardial fat has been addressed by recent studies such as the Heinz Nixdorf Recall study, where epicardial fat volume was significantly associated with coronary events over the course of 8 years, even after adjusting for traditional cardiovascular risk factors.33 Another recent study indicated that epicardial fat volume could serve as a marker for the presence and severity of atherosclerosis burden in asymptomatic patients.34 Bachar et al.35 further suggested that an epicardial fat thickness of 2.4 mm can be used as an optimal cutoff to predict the occurrence of CAD in asymptomatic patients. Epicardial fat volume has also been associated with obstructive CAD and non-calcified plaque burden, or, in other words, with the plaque characteristics in patients with CAD.1 In addition, epicardial fat volume has been shown to independently predict thin-cap fibroatheromas, which can in turn predict the recurrence of adverse cardiovascular events in patients with advanced CAD.36 It can therefore be concluded that epicardial fat accumulation shares a distinct and robust association with the onset, as well as with various stages of CAD progression in humans, thereby supporting the idea that its assessment should become a part of the routine clinical practice to aide with the risk stratification and appropriate management of CAD.
Evidently, these observations further underscore the possibility of a causal association between epicardial fat accumulation and development of CAD, thereby raising yet another important question of whether epicardial adipose tissue should be targeted therapeutically to manage CAD in humans. Weight loss in association with caloric restriction,37 bariatric surgery38 and physical activity39 has been shown to result in the reduction of epicardial fat volume, and at least in one study, this reduction was shown to correlate with improvements in the insulin resistance status of obese men with metabolic syndrome.40 However, whether a reduction in epicardial fat would directly translate into a reduction of CAD burden in humans remains to be seen. In a recent study involving mini-pigs serving as their own controls, selective surgical resection of epicardial fat surrounding the left anterior descending artery was shown to be associated with slower progression of coronary atherosclerosis over a period of 3 months.41 In this particular study, McKenney et al. 41 first fed an atherogenic diet to Ossabaw miniature swines for a period of 6 months in order to induce CAD, following which selective coronary adipectomy was performed on their left anterior descending artery and animals were allowed to recover while continuing on the atherogenic diet. Three months later, no plaque progression was observed in the surgically manipulated section of left anterior descending artery relative to the baseline as opposed to other coronary arteries that exhibited plaque progression. Despite its limitations, this study supports the hypothesis that epicardial fat contributes to the development of coronary atherosclerosis locally, and further makes the case that targeting its reduction can help with the progression of CAD. Such experimental setups can also be utilized to explore whether reduction in epicardial fat can lead to a reversal of existing CAD in the future.
Association between epicardial fat and CAD: is it simply a proxy for obesity-induced CAD?
Obesity is an independent risk factor for the development of cardiovascular disease and CAD. Indeed, epicardial fat mass and volume are known to exhibit strong associations with visceral fat accumulation,42 although such associations are not always reported for total body fat mass. Increased epicardial fat mass has also been shown to correlate with most of the common risk factors for metabolic syndrome and cardiovascular disease including low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, diastolic blood pressure, plasma insulin, fasting glucose, insulin resistance as well as the presence of CAD in humans. These associations have led to the paradigm that an increase in epicardial fat mass due to obesity, in particular visceral obesity, leads to the development of CAD in humans.42 As a result, epicardial fat has been discussed as a therapeutic target for the management of obesity-related cardiovascular disease.43
Strong associations between epicardial fat mass and CAD, however, have also been reported independent of body adiposity measures,34 including abdominal visceral fat mass.44 In the Framingham Offspring Heart Study, epicardial fat volume was significantly associated with prevalent cardiovascular disease, even after adjustments for body mass index (BMI) and waist circumference.10, 33 Recently, epicardial fat volume was reported to associate positively with coronary atherosclerosis burden as assessed by coronary artery calcium score independent of abdominal visceral fat.44 In addition, non-obese patients with CAD have been shown to exhibit higher epicardial fat volume compared with non-obese patients without CAD.45, 46 In one of these studies, epicardial fat volume was compared for both obese and non-obese patients with and without CAD, and the differences in epicardial fat volume among CAD and non-CAD patients were found to be specific for non-obese patients.45 Indeed, obese individuals had higher epicardial fat volume relative to non-obese patients, raising the question of whether presence of obesity can mask the direct effects of epicardial fat on the development of CAD. Alternatively, it has been postulated that the mechanisms underlying the association between epicardial fat and development of CAD might be different for lean and obese individuals.31
Mechanisms underlying the pathophysiological association between epicardial fat and CAD
A plausible role for direct involvement of epicardial fat in the development of CAD is supported by the observations that its absence from certain sections of coronary arteries protects them against atherosclerosis.30 It has been observed in both animal and human studies that segments of coronary arteries that are covered by intramyocardial bridges instead of epicardial fat do not develop atherosclerosis.30, 47 Indeed, epicardial fat has been proposed to influence the myocardium and coronary arteries via paracrine and vasocrine mechanisms.48
Human epicardial fat is known to be a source of various bioactive molecules including adipokines and cytokines that are altered under CAD conditions. Mazurek et al.49 reported elevated inflammatory infiltrate in epicardial relative to subcutaneous fat in patients with CAD. Elevated expression of most classic inflammatory cytokines including tumor necrosis factor-α, monocyte chemoattractant protein 1, interleukin (IL)-1, IL6, IL receptor 2-α, IL8, IL10, IL1β and C-reactive protein has been observed in epicardial fat of CAD patients.8, 50 In addition, epicardial fat obtained from patients with CAD has been shown to exhibit a pro-inflammatory polarization of M1/M2 macrophages.51 A potential role of nuclear factor-κB and c-Jun N-terminal kinase pathways in the enhancement of inflammation observed in epicardial fat under CAD conditions has also been proposed.2 In addition, higher oxidative stress levels, including reactive oxygen species production and higher levels of enzymes involved in regulating oxidative stress such as catalase and glutathione S-transferase P, have been reported in epicardial fat relative to subcutaneous fat in patients with CAD.52
In addition to the studies looking at gene expression of the inflammatory and oxidative stress markers, proteomic and secretome analyses of epicardial fat obtained from CAD patients have also revealed that it is capable of secreting factors that can induce atherogenic changes in monocytes and endothelial cells,53 as well as induce cardiomyocyte dysfunction.54 Conditioned media derived from epicardial fat of guinea pigs was also reported to induce insulin resistance in rat cardiomyocytes.55 These observations tend to support the ‘outside to inside' signaling hypothesis, which suggests that secretory products of dysfunctional epicardial fat or adventitia can lead to the development of intimal atherosclerotic lesions in coronary arteries and other blood vessels.22 If this hypothesis was to be accepted, it would raise a series of other important questions such as to what initiates or precedes epicardial fat dysfunction? Does epicardial fat undergo its transformation into a dysfunctional depot concomitantly with other fat stores, or does it parallel systemic inflammation?
Epicardial fat volume has been shown to associate with systemic inflammation as measured by C-reactive protein, IL6 and monocyte chemoattractant protein 1 previously.56 In addition, epicardial fat volume was found to be significantly associated with circulating C-reactive protein levels.57 However, a direct role for inflammation in the onset of epicardial adipocyte dysfunction remains to be demonstrated. Alternatively, the idea that endothelial dysfunction within vessel wall could induce epicardial adipocyte dysfunction in an ‘inside to outside' signaling model cannot be ruled out either.43
Human epicardial fat: a thermogenic tissue?
Recent advances in the field of adipose tissue biology have clearly revealed that brown adipocytes, which are characterized by the presence of multiple elongated mitochondria and the expression of a unique mitochondrial protein named uncoupling protein 1 (UCP1), are not obsolete cells that were once thought to be carried by human newborns only.58, 59 With the aid of imaging studies looking at the uptake of radiolabelled fluorodeoxyglucose (18FDG) to identify metabolically active areas, thermogenic brown adipocytes have been identified in adult humans throughout most ages, their presence and metabolic activity being affected by the age, sex, BMI and diabetes status of the individual.60, 61
Fat depots around the heart in the pericardial–mediastinal region were reported to possess multilocular brown adipocytes as early as in 1972.62 However, presence of UCP1 mRNA in human epicardial fat was not demonstrated until 2009.26 In their seminal work, Sacks et al.26 further reported that UCP1 expression associated with the age and BMI of the study participants, and also hypothesized that the thermogenic activity of epicardial brown adipocytes could serve to protect the underlying myocardium from hypothermia during cold challenge. We confirmed the presence of UCP1 mRNA in human epicardial fat in a cohort of CAD patients, and further proposed a more metabolically active role for its brown adipocytes.6 Epicardial fat exhibited a gene expression profile that was consistent with this depot having a higher capacity for oxidizing fatty acids relative to subcutaneous fat. In addition, epicardial fat expression of genes involved in beta oxidation and thermogenesis correlated positively with circulating HDL cholesterol and negatively with circulating triglyceride (TG) levels in a depot-specific manner, as no such associations were seen for paired biopsies of mediastinal fat.6 Indeed, one cannot establish a cause and effect relationship in these settings; however, our observations clearly suggest that the thermogenic activity of brown adipocytes could contribute to the systemic lipid metabolism in humans, as previously reported in case of rodents.63
It is important to acknowledge that in the field of brown adipose tissue biology, thermogenic capacity and thermogenic activity are two closely related yet clearly distinct terms. Indeed, thermogenic capacity does not necessarily equate with the thermogenic activity of a fat depot, especially considering that the sympathetic nervous system exerts a strict control over the phenomenon of thermogenesis.64 Most studies including ours have only looked at the thermogenic capacity of epicardial fat. Whether UCP1 expression truly translates into epicardial fat having a higher capacity for thermogenesis remains to be demonstrated. Furthermore, the question of whether thermogenesis in epicardial fat is regulated by sympathetic nervous system remains to be addressed.
Human epicardial fat: a classic brown or beige depot?
Owing to significant interest in unlocking the thermogenic potential of brown adipocytes to treat metabolic conditions in humans, this field has seen exponential growth in the recent years. Parallel advances in our understanding of both human and rodent brown fat biology further revealed that all brown adipocytes are not alike. Much similar to the anatomical and functional disparity between subcutaneous and visceral white adipocytes, differences in the anatomic location of brown adipocyte populations were found to associate with distinct developmental origins as well as molecular signatures in rodents.58 Two specific brown adipocyte subtypes were identified: the classic brown and beige/brite/recruitable adipocytes. Although classical brown adipocytes are known to be clustered at specific anatomic locations, such as interscapular brown fat, and share their developmental origins with myocytes, brite adipocytes are found diffused among white adipocytes and share their ontogeny with white adipocytes. Furthermore, it has been demonstrated that the basal UCP1 expression in brite adipocytes is very low (that is, close to white adipocytes); however, upon stimulation with β-adrenergic agonists, these cells upregulate their UCP1 expression as well as the thermogenic capacity to levels comparable to classic brown adipocytes.65 Indeed, this understanding called for deciphering the nature of human brown fat, and, as a result, multiple studies reported the presence of both classic brown and brite cells in humans.66, 67, 68, 69 However, human adipose organ was found to be even more complex than once expected, as, unlike rodents, classic brown and brite adipocytes were found sitting right next to each other anatomically in the neck region of adult humans.69 The physiological or metabolic context for the anatomic juxtaposition of classic brown and brite adipocytes in humans remains to be explored.
A relevant question of whether human epicardial adipose tissue carries classic brown or brite adipocytes have been looked at by Sacks et al.70 who reported that in middle-aged humans epicardial fat exhibits features of beige fat, that is, the expression of UCP1 protein and beige marker CD137 mRNA. We have observed the presence of UCP1-positive multilocular cells in the epicardial fat depot of patients with CAD, and also observed clear upregulation of beige markers TMEM26, TBX1 and CD137 in this depot, indicating that epicardial fat is certainly beige in nature (unpublished observations). However, whether epicardial fat is capable of upregulating its thermogenic machinery and activity upon stimulation, similar to classic brown adipocytes, remains to be seen.
As a cardiac fat depot, are thermogenic properties exclusive to epicardial fat?
Besides epicardial fat, there is some evidence linking mediastinal fat as yet another cardiac fat store with the development of insulin resistance, hypertension and metabolic syndrome in humans.7, 71 Furthermore, all of the studies looking at pericardial fat that do not demarcate between epicardial and paracardial/mediastinal fat may indirectly support a role for this depot in the development of CAD as well. However, as previously highlighted, epicardial and paracardial are not the same fat depots. Besides the anatomic distinction, paracardial and epicardial fat depots are also known to have separate developmental origins. Although epicardial fat originates from mesothelial cells migrating from the septum transversum, the paracardial fat depot originates from primitive thoracic mesenchymal cells.72 Furthermore, unlike epicardial fat, this fat depot gets its blood supply from non-coronary sources.72 Based on these differences, a difference in the metabolic profile and oxidative capacity of epicardial and paracardial/mediastinal fat can be easily expected. We and others have reported that UCP1 mRNA is detectable in the human mediastinal depot; however, this expression is at a much lower level relative to epicardial fat.6, 26, 73 In addition, UCP1 protein does not seem to be found in abundance in human mediastinal fat depot so far.
In a recent study, Cheung et al.74 compared the gene expression data of the human mediastinal fat depot with murine brown and white fat depots using microarrays, and found gene set enrichments that pointed toward a higher mitochondrial activity for this depot relative to subcutaneous fat depot. They further reported that the human mediastinal depot expresses UCP1 mRNA and contains multilocular UCP1-immunopositive adipocytes, thereby exhibiting brown fat-like features. However, neither UCP1 protein (at the tissue level) nor any of the beige markers were detectable in this depot.74 In contrast, Sacks et al.70 noticed higher expression of beige marker CD137 in paracardial fat (even higher than epicardial fat); however, as they also could not locate UCP1 protein in this depot, they attributed the beige phenotype only to epicardial adipose tissue. We have observed gene expression of various beige fat markers in the mediastinal fat depot similar to the epicardial fat depot, however at lower expression levels (unpublished observations), leading to the suggestion that the mediastinal fat depot is likely to be a beige fat depot that possesses higher mitochondrial activity. Whether this higher mitochondrial activity translates into higher UCP1-mediated uncoupling under basal or stimulated conditions remains to be seen.
It is important to note that Cheung et al.74 did not observe any associations between brown-like features of human mediastinal fat and any of the physiological parameters, similar to our observations where associations among circulating lipids and brown fat gene expression were exclusive to epicardial fat.6 Future studies would be required to either completely dismiss or acknowledge a role for mediastinal fat and its thermogenic properties in terms of physiological relevance in humans.
Thermogenesis and atherosclerosis: can the former be activated to reduce the development of the latter?
Thermogenesis has always been considered to be a tightly regulated phenomenon that is predominantly involved in thermoregulation. The observations that activation of brown fat could also burn signficant calories and stored energy further paved the way to our massive interest in understanding brown fat thermogenesis. However, the realization that activation of thermogenesis could also help reduce atherosclerosis came only in 2011, when Bartelt et al.63 demonstrated that cold exposure turned brown adipose tissue into a major TG-rich lipoprotein uptake and catabolism site in mice. The net effect of brown fat activation in this study was a reduction in circulating TG levels and increase in HDL cholesterol levels that reflected a plasma lipid profile long known to be beneficial in terms of atherosclerosis. Indeed, this study did not identify any direct reciprocal relationship between thermogenesis and atherosclerosis.
A direct role for UCP1-mediated lipolysis and thermogenesis in the onset and progression of atherosclerosis was reported in mice recently; however, contrary to what one might have expected, this relationship was found to be deleterious in nature. Using LDL receptor and apolipoprotein-E knock-out mice and persistent cold exposure (periods of 4–8 weeks) during which these mice were pair-fed to mice kept at thermoneutrality, Dong et al.75 reported that UCP1-mediated lipolysis caused massive hypercholesterolemia and an upregulation in plaque-growth and -instability. In our opinion, and as rightly speculated by the authors themselves, much of their observations stemmed from the absence of the lipoprotein clearance receptors or apolipoproteins in these mice. Thus, we believe that these observations cannot be used to represent the relationship between cold-induced thermogenesis and atherosclerosis under normal conditions. In normal mice, cold exposure would result in the upregulation of lipolysis as well as lipoprotein metabolism such that peripheral TGs can be transported to brown fat in order to fuel thermogenesis driven by sympathetic stimulation of UCP1. After the lipoproteins are depleted of TGs, their cholesterol remnants would undergo catabolism in the liver via clearance receptors that would be upregulated to meet the demands of a cold-challenged system. Indeed, Dong et al. observed an upregulation in the lipoprotein clearance receptors and hence could not observe cold-induced hyperlipidemia in normal mice within the same study.
It is well known that atherosclerosis cannot be induced in normal mice until lipoprotein receptors are knocked off, which results in a plasma lipoprotein profile carrying significantly higher levels of atherogenic LDL cholesterol. As demonstrated by Bartelt et al.,63 lipoprotein metabolism is tightly linked to the metabolic adaptations of a thermogenically stimulated system. Absence of lipoprotein clearance receptors per se would directly interfere with the regular function of the thermogenic machinery. Thus, in our opinion, LDL receptor or apolipoprotein-E knock-out mice do not represent an optimal model to study the relationship between thermogenesis and atherosclerosis. Animal models such as mini-pigs that can develop atherosclerosis in response to high-fat-diet feeding,41 but still retain a working lipoprotein metabolism cycle, would serve as a better model to assess this relationship. Indeed, a recent study using APOE*3-Leiden.CETP mice, which retain a functional hepatic lipoprotein remnant clearance route and a human-like lipoprotein metabolism, demonstrated that brown fat activation can result in amelioration of hyperlipidemia and protection from atherosclerosis in mice.76
Can brown adipocytes of epicardial serve as a therapeutic target for CAD?
Besides the association between whole-body thermogenesis that could occur as a result of the activation of total brown fat in the body, the question of whether brown adipocytes within the epicardial fat depot could have a specific role to play in the onset and progression of atherosclerosis has been addressed to a certain extent recently.
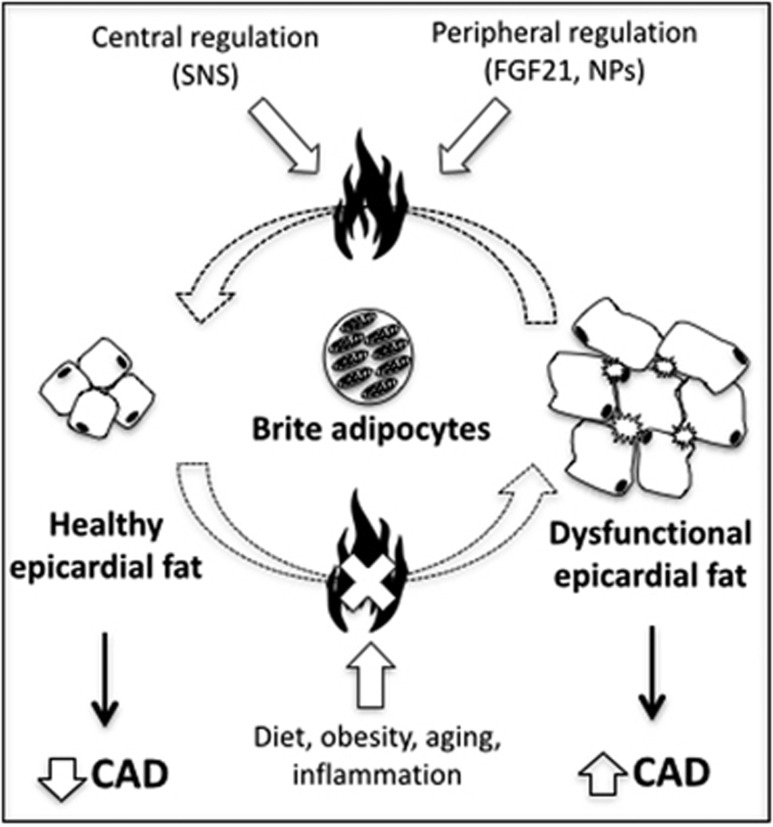
Epicardial fat has previously been reported to express both pro- and anti-inflammatory factors, an observation that led Iacobellis et al.77 to propose that yet-to-be identified factors could tilt the balance between pro- and anti-inflammatory properties of epicardial fat, which could eventually alter its association with the onset and progression of CAD. Based on our own unique observations for epicardial fat, we had proposed that brown adipocytes of epicardial fat could serve as ‘the factor' whose presence and absence could alter the inflammatory profile and nature of epicardial fat, and hence its relationship with CAD Figure 1.6 A recent study demonstrated that increased reactive oxygen species production from epicardial fat of CAD patients was associated with brown to white transdifferentiation of adipocytes within epicardial fat.78 Yet another study revealed that an increase in brown epicardial fat was associated with a prediction of lack of progression of coronary atherosclerosis in humans.79 Indeed, these observations support the hypothesis that brown adipocytes within epicardial fat could serve as a therapeutic target for altering the proximal environment around the heart that could eventually alter the development and progression of CAD; however, a greater understanding of the thermogenic properties and its association with the atherogenic profile of epicardial fat would have to be established before a conclusive role can be offered to these adipocytes.
Figure 1.
Thermogenic/oxidative potential of epicardial adipose tissue. We propose that epicardial adipocyte dysfunction occurs as a result of dietary, metabolic and environmental factors, which contribute to the loss of its brite adipocytes and progression of CAD in humans. Thermogenic activation of brite adipocytes, thus, represents a plausible target to alter the metabolic health of epicardial adipose tissue. However, regulation of the their oxidative and thermogenic capacity by well-known central and peripheral regulators such as SNS, FGF21 and NPs remains unknown. SNS, sympathetic nervous system; FGF21, fibroblast growth factor 21; NPs, natriuretic peptides; CAD, coronary artery disease.
Conclusions and future perspectives
In conclusion, there is a clear evidence reinforcing that epicardial fat holds a unique anatomic position around the heart that likely translates into a unique physiological relevance for this depot. In addition, an increase in the accumulation of epicardial fat either due to, or independent of, obesity does result in an increased propensity not only for the onset but also for the progression and severity of CAD in humans.18, 22, 27 Although there are numerous gaps in our understanding of epicardial fat and its association with CAD, it is clear that epicardial adipocyte dysfunction may affect the development of CAD, and hence this depot represents a therapeutic target for the management of CAD.
Among many other approaches, presence of UCP1 and its thermogenic capabilities may represent a unique target for therapeutic intervention that could lead to a loss of excess epicardial fat on one hand and/or correction of epicardial adipose tissue dysfunction on the other Figure 1. Either way, such an approach would depend upon our total understanding of the thermogenic properties of epicardial fat and its regulation by the central nervous system and peripheral circulating factors. Factors such as fibroblast growth factor 21, irisin and natriuretic peptides have been shown to induce browning of white fat in rodents.58 Could these factors also affect the thermogenic capacity and thermogenic activity of human epicardial fat? If so, is it possible that heart exerts its own control over the thermogenic properties of epicardial fat through natriuretic peptides. Moreover, how does sympathetic nervous system mediated regulation factor into this heart–epicardial fat cross talk. Indeed, much work is needed to complete our understanding of the thermogenic potential of the epicardial fat as well as its regulation. Furthermore, the very location of epicardial fat poses a unique challenge of finding safe ways that could lead to sustained yet carefully controlled activation of its thermogenic potential without compromising the temperature or function of heart itself.
Acknowledgments
We thank Dr Yves Deshaies for his feedback on the paper. KC is a recipient of the Banting postdoctoral fellowship from Canadian Institute of Health Research.
Disclaimer
This article is published as part of a supplement sponsored by the Université Laval's Research Chair in Obesity, in an effort to inform the public on the causes, consequences, treatments and prevention of obesity.
The authors declare no conflict of interest.
References
- Alexopoulos N, McLean DS, Janik M, Arepalli CD, Stillman AE, Raggi P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 2010; 210: 150–154. [DOI] [PubMed] [Google Scholar]
- Baker AR, Harte AL, Howell N, Pritlove DC, Ranasinghe AM, da Silva NF et al. Epicardial adipose tissue as a source of nuclear factor-κb and c-jun n-terminal kinase mediated inflammation in patients with coronary artery disease. J Clin Endocrinol Metab 2009; 94: 261–267. [DOI] [PubMed] [Google Scholar]
- Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation 2008; 117: 605–613. [DOI] [PubMed] [Google Scholar]
- Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2009; 90: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes (Lond) 1990; 14: 1013–1022. [PubMed] [Google Scholar]
- Chechi K, Blanchard PG, Mathieu P, Deshaies Y, Richard D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int J Cardiol 2012; 167: 2264–2270. [DOI] [PubMed] [Google Scholar]
- Sharma AM. Mediastinal fat, insulin resistance, and hypertension. Hypertension 2004; 44: 117–118. [DOI] [PubMed] [Google Scholar]
- Gaborit B, Abdesselam I, Dutour A. Epicardial fat: more than just an ‘epi' phenomenon? Horm Metab Res 2013; 45: 991–1001. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Leonetti F, Di Mario U. Images in cardiology: massive epicardial adipose tissue indicating severe visceral obesity. Clin Cardiol 2003; 26: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: the Framingham Heart Study. Eur Heart J 2009; 30: 850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer RI, Serne EH, Smulders YM, van Hinsbergh VWM, Yudkin JS, Eringa EC. Perivascular adipose tissue and its role in type 2 diabetes and cardiovascular disease. Curr Diab Rep 2011; 11: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Després JP, Koh KK. Perivascular adipose tissue in the pathogenesis of cardiovascular disease. Atherosclerosis 2013; 230: 177–184. [DOI] [PubMed] [Google Scholar]
- Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: a meta-analysis. Coron Artery Dis 2012; 23: 227–233. [DOI] [PubMed] [Google Scholar]
- Rabkin SW. Epicardial fat: properties, function and relationship to obesity. Obes Rev 2007; 8: 253–261. [DOI] [PubMed] [Google Scholar]
- Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 2009; 17: 625–627. [DOI] [PubMed] [Google Scholar]
- Talman AH, Psaltis PJ, Cameron JD, Meredith IT, Seneviratne SK, Wong DTL. Epicardial adipose tissue: far more than a fat depot. Cardiovasc Diagn Ther 2014; 4: 416–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kälsch H, Seibel RM et al. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: a segment analysis. Atherosclerosis 2010; 211: 195–199. [DOI] [PubMed] [Google Scholar]
- Raggi P, Alakija P. Epicardial adipose tissue: a long-overlooked marker of risk of cardiovascular disease. Atherosclerosis 2013; 229: 32–33. [DOI] [PubMed] [Google Scholar]
- Raggi P. Epicardial adipose tissue as a marker of coronary artery disease risk. J Am Coll Cardiol 2013; 61: 1396–1397. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Cavallero S, Patterson M, Shen H, Xu J, Kumar SR et al. Adipogenesis and epicardial adipose tissue: a novel fate of the epicardium induced by mesenchymal transformation and PPARγ activation. Proc Natl Acad Sci USA 2015; 112: 2070–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradi D, Maestri R, Callegari S, Pastori P, Goldoni M, Luong TV et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc Pathol 2004; 13: 313–316. [DOI] [PubMed] [Google Scholar]
- Iacobellis G, Bianco AC. Epicardial adipose tissue: emerging physiological, pathophysiological and clinical features. Trends Endocrinol Metab 2011; 22: 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B 1989; 94: 225–232. [DOI] [PubMed] [Google Scholar]
- Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 2015; 11: 363–371. [DOI] [PubMed] [Google Scholar]
- Arora RC, Waldmann M, Hopkins DA. Porcine intrinsic cardiac ganglia. Anat Rec A Discov Mol Cell Evol Biol 2003; 27: 249–258. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN, Holman B, Cheema P, Chary A, Parks F et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J Clin Endocrinol Metab 2009; 94: 3611–3615. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN. Human epicardial fat: what is new and what is missing? Clin Exp Pharmacol Physiol 2011; 38: 879–887. [DOI] [PubMed] [Google Scholar]
- Fain JN, Sacks HS, Buehrer B, Bahouth SW, Garrett E, Wolf RY et al. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int J Obes 2008; 32: 810–815. [DOI] [PubMed] [Google Scholar]
- Swifka J, Weiss J, Addicks K, Eckel J, Rösen P. Epicardial fat from guinea pig: a model to study the paracrine network of interactions between epicardial fat and myocardium? Cardiovasc Drugs Ther 2008; 22: 107–114. [DOI] [PubMed] [Google Scholar]
- Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler Thromb Vasc Biol 2009; 29: 781–786. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Denis GV, Nikolajczyk BS. Immune regulators of inflammation in obesity-associated type 2 diabetes and coronary artery disease. Curr Opin Endocrinol Diabetes Obes 2014; 21: 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging 2010; 3: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahabadi AA, Berg MH, Lehmann N, Kälsch H, Bauer M, Kara K et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf Recall Study. J Am Coll Cardiol 2013; 61: 1388–1395. [DOI] [PubMed] [Google Scholar]
- Yerramasu A, Dey D, Venuraju S, Anand DV, Atwal S, Corder R et al. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2012; 220: 223–230. [DOI] [PubMed] [Google Scholar]
- Bachar GN, Dicker D, Kornowski R, Atar E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am J Cardiol 2012; 110: 534–538. [DOI] [PubMed] [Google Scholar]
- Echavarría-Pinto M, Hernando L, Alfonso F. From the epicardial adipose tissue to vulnerable coronary plaques. World J Cardiol 2013; 5: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snel M, Jonker JT, Hammer S, Kerpershoek G, Lamb HJ, Meinders AE et al. Long-term beneficial effect of a 16-week very low calorie diet on pericardial fat in obese type 2 diabetes mellitus patients. Obesity 2012; 20: 1572–1576. [DOI] [PubMed] [Google Scholar]
- Khalaf KI, Taegtmeyer H. Slimming the heart with bariatric surgery. J Am Coll Cardiol 2013; 61: 990–991. [DOI] [PubMed] [Google Scholar]
- Kim MK, Tomita T, Kim MJ, Sasai H, Maeda S, Tanaka K. Aerobic exercise training reduces epicardial fat in obese men. J Appl Physiol 2009; 106: 5–11. [DOI] [PubMed] [Google Scholar]
- Liang KW, Tsai IC, Lee WJ, Lin SY, Lee WL, Lee IT et al. Correlation between reduction of superior interventricular groove epicardial fat thickness and improvement of insulin resistance after weight loss in obese men. Diabetol Metab Syndr 2014; 6: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney ML, Schultz KA, Boyd JH, Byrd JP, Alloosh M, Teague SD et al. Epicardial adipose excision slows the progression of porcine coronary atherosclerosis. J Cardiothorac Surg 2014; 9: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J Clin Endocrinol Metab 2003; 88: 5163–5168. [DOI] [PubMed] [Google Scholar]
- Payne GA, Kohr MC, Tune JD. Epicardial perivascular adipose tissue as a therapeutic target in obesity-related coronary artery disease. Br J Pharmacol 2012; 165: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt N, Toschke AM, Leite D, Rocha J, Carvalho M, Sampaio F et al. Epicardial adipose tissue is an independent predictor of coronary atherosclerotic burden. Int J Cardiol 2012; 158: 26–32. [DOI] [PubMed] [Google Scholar]
- Iwayama T, Nitobe J, Watanabe T, Ishino M, Tamura H, Nishiyama S et al. Role of epicardial adipose tissue in coronary artery disease in non-obese patients. J Cardiol 2014; 63: 344–349. [DOI] [PubMed] [Google Scholar]
- Yong HS, Kim EJ, Seo HS, Kang EY, Kim YK, Woo OH et al. Pericardial fat is more abundant in patients with coronary atherosclerosis and even in the non-obese patients: evaluation with cardiac CT angiography. Int J Cardiovasc Imaging 2010; 26: 53–62. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Ishii T, Asuwa N, Masuda S. Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol-fed rabbits. Virchows Arch 1997; 430: 163–171. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, Stehouwer C. ‘Vasocrine' signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 2005; 365: 1817–1820. [DOI] [PubMed] [Google Scholar]
- Mazurek T. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003; 108: 2460–2466. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Chu CS, Lee KT, Lin TH, Hsieh CC, Chiu CC et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. Int J Obes 2007; 32: 268–274. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Tabata M, Kurobe H, Motoki T, Akaike M, Nishio C et al. Coronary atherosclerosis Is associated with macrophage polarization in epicardial adipose tissue. J Am Coll Cardiol 2011; 58: 248–255. [DOI] [PubMed] [Google Scholar]
- Salgado-Somoza A, Teijeira-Fernández E, Fernández AL, González-Juanatey JR, Eiras S. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am J Physiol Heart Circ Physiol 2010; 299: H202–H209. [DOI] [PubMed] [Google Scholar]
- Karastergiou K, Evans I, Ogston N, Miheisi N, Nair D, Kaski JC et al. Epicardial adipokines in obesity and coronary artery disease induce atherogenic changes in monocytes and endothelial cells. Arterioscler Thromb Vasc Biol 2010; 30: 1340–1346. [DOI] [PubMed] [Google Scholar]
- Greulich S, Maxhera B, Vandenplas G, de Wiza DH, Smiris K, Mueller H et al. Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012; 126: 2324–2334. [DOI] [PubMed] [Google Scholar]
- Greulich S, de Wiza DH, Preilowski S. Secretory products of guinea pig epicardial fat induce insulin resistance and impair primary adult rat cardiomyocyte function. J Cell Mol Med 2011; 15: 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros TM, Massaro JM, Rosito GA, Hoffmann U, Vasan RS, Larson MG et al. Pericardial fat volume correlates with inflammatory markers: the Framingham Heart Study. Obesity 2010; 18: 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granér M, Seppälä-Lindroos A, Rissanen A, Hakkarainen A, Lundbom N, Kaprio J et al. Epicardial fat, cardiac dimensions, and low-grade inflammation in young adult monozygotic twins discordant for obesity. Am J Cardiol 2012; 109: 1295–1302. [DOI] [PubMed] [Google Scholar]
- Chechi K, Carpentier AC, Richard D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol Metab 2013; 8: 408–420. [DOI] [PubMed] [Google Scholar]
- Richard D, Monge-Roffarello B, Chechi K, Labbé SM, Turcotte EE. Control and physiological determinants of sympathetically mediated brown adipose tissue thermogenesis. Front Endocrinol (Lausanne) 2012; 3: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V, Routhier-Labadie A, Bellemare W, Lakhal-Chaieb L, Turcotte E, Carpentier AC et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 2011; 96: 92–199. [DOI] [PubMed] [Google Scholar]
- Chechi K, Nedergaard J, Richard D. Brown adipose tissue as an anti-obesity tissue in humans. Obes Rev 2014; 15: 92–106. [DOI] [PubMed] [Google Scholar]
- Heaton JM. The distribution of brown adipose tissue in the human. J Anat 1972; 11: 35–39. [PMC free article] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med 2011; 17: 200–205. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 2004; 84: 277–359. [DOI] [PubMed] [Google Scholar]
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M et al. Evidence for two types of brown adipose tissue in humans. Nat Med 2013; 19: 631–634. [DOI] [PubMed] [Google Scholar]
- Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med 2013; 19: 631–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homøe P, Loft A et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab 2013; 17: 798–805. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. How brown is brown fat? It depends where you look. Nat Med 2013; 19: 540–541. [DOI] [PubMed] [Google Scholar]
- Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H et al. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab 2013; 98: E1448–E1455. [DOI] [PubMed] [Google Scholar]
- Atalar F, Gormez S, Caynak B, Akan G, Tanriverdi G, Bilgic-Gazioglu S et al. Mediastinal adipose tissue expresses a pathogenic profile of 11 β-hydroxysteroid dehydrogenase type 1, glucocorticoid receptor, and CD68 in patients with coronary artery disease. Cardiovasc Pathol 2013; 22: 183–188. [DOI] [PubMed] [Google Scholar]
- Iozzo P. Myocardial, perivascular, and epicardial fat. Diabetes Care 2011; 34: S371–S379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks HS, Fain JN, Cheema P, Bahouth SW, Garrett E, Wolf RY et al. Depot-specific overexpression of proinflammatory, redox, endothelial cell, and angiogenic genes in epicardial fat adjacent to severe stable coronary atherosclerosis. Metab Syndr Relat Disord 2011; 9: 433–439. [DOI] [PubMed] [Google Scholar]
- Cheung L, Gertow J, Werngren O, Folkersen L, Petrovic N, Nedergaard J et al. Human mediastinal adipose tissue displays certain characteristics of brown fat. Nutr Diabetes 2013; 3: e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Yang X, Lim S, Cao Z, Honek J, Lu H et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab 2013; 18: 118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbée JF, Boon MR, Khedoe PP, Bartelt A, Schlein C, Worthmann A et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat Commun 2015; 6: 6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobellis G, Barbaro G. The double role of epicardial adipose tissue as pro- and anti-inflammatory organ. Horm Metab Res 2008; 40: 442–445. [DOI] [PubMed] [Google Scholar]
- Dozio E, Vianello E, Briganti S, Fink B, Malavazos AE, Scognamiglio ET et al. Increased reactive oxygen species production in epicardial adipose tissues from coronary artery disease patients is associated with brown-to-white adipocyte trans-differentiation. Int J Cardiol 2014; 174: 413–414. [DOI] [PubMed] [Google Scholar]
- Ahmadi N, Nabavi V, Hajsadeghi F, Zeb I, Flores F, Ebrahimi R et al. Aged garlic extract with supplement is associated with increase in brown adipose, decrease in white adipose tissue and predict lack of progression in coronary atherosclerosis. Int J Cardiol 2013; 168: 2310–2314. [DOI] [PubMed] [Google Scholar]