Abstract
The central nervous melanocortin system maintains body mass and adiposity within a ‘healthy' range by regulating satiety and metabolic homeostasis. Neural melanocortin-4 receptors (MC4R) modulate satiety signals and regulate autonomic outputs governing glucose and lipid metabolism in the periphery. The functions of melanocortin-3 receptors (MC3R) have been less well defined. We have observed that food anticipatory activity (FAA) is attenuated in Mc3r−/− mice housed in light:dark or constant dark conditions. Mc3r−/− mice subjected to the restricted feeding protocol that was used to induce FAA also developed insulin resistance, dyslipidaemia, impaired glucose tolerance and evidence of a cellular stress response in the liver. MC3Rs may thus function as modulators of oscillator systems that govern circadian rhythms, integrating signals from nutrient sensors to facilitate synchronizing peak foraging behaviour and metabolic efficiency with nutrient availability. To dissect the functions of MC3Rs expressed in hypothalamic and extra-hypothalamic structures, we inserted a ‘lox-stop-lox' (TB) sequence into the Mc3r gene. Mc3rTB/TB mice recapitulate the phenotype reported for Mc3r−/− mice: increased adiposity, accelerated diet-induced obesity and attenuated FAA. The ventromedial hypothalamus exhibits high levels of Mc3r expression; however, restoring the expression of the LoxTB Mc3r allele in this nucleus did not restore FAA. However, a surprising outcome came from studies using Nestin-Cre to restore the expression of the LoxTB Mc3r allele in the nervous system. These data suggest that ‘non-neural' MC3Rs have a role in the defence of body weight. Future studies examining the homeostatic functions of MC3Rs should therefore consider actions outside the central nervous system.
Keywords: melanocortin; metabolism; glucose; lipids; hypothalamus; feeding
The central nervous melanocortin system forms a neural ‘nutrient-sensing' network, connecting signals of metabolic state with centres of the brain that regulate ingestive behaviours and metabolic homeostasis.1 Genetic screens using morbidly obese individuals identified carriers with mutations in the melanocortin system, indicating that studying the melanocortin system has clinical relevance.2 Nonsense mutations in the Proopiomelanocortin (POMC) gene 3 or the melanocortin-4 receptor (MC4R) gene are associated with hyperphagia and obesity.4, 5 MC3R mutations have also been suggested to be a predisposing factor for excessive weight gain,6 although the association between MC3R mutations and obesity is less clear when compared with MC4R mutations.
Data obtained from human genetics and animal models suggested that further study of the central nervous melanocortin system, and particularly MC4Rs, could lead to new therapies against obesity.7 Although this outcome has yet to be realized, the information gathered from investigating this system significantly advanced the understanding of neural systems governing satiety and metabolic homeostasis. The distribution of Mc3r mRNA expression in the hypothalamic and limbic regions of the brain also suggests functions related to the control of metabolic homeostasis and control of complex behaviours related to feeding.8, 9, 10 MC3Rs are presumed to be regulated in parallel with MC4Rs in response to signals of metabolic state, and the actions of both are related to the control of adiposity.11, 12 However, although the initial studies using knockout mice indicated a role for MC3Rs in energy homeostasis, they yielded few insights into the exact functions related to maintaining adiposity and energy balance.11, 12 The purpose of this review is to discuss recent data published by our laboratory and others that have provided some insights into the potential roles of this receptor in metabolic homeostasis.13, 14, 15, 16, 17, 18
Overview of the central nervous melanocortin system
The central nervous melanocortin system comprises neurons expressing the endogenous ligands and the receptors known to be expressed in the brain (MC3R and MC4R).19 POMC neurons are found in the arcuate nucleus of the hypothalamus (ARC), with a smaller population in nucleus tractus solitarius (NTS) in the hindbrain. Post-translational processing of POMC produces several neuropeptides including the melanocyte-stimulating hormones (α-, β- and γ1−3 MSH). POMC neurons are thought to release MSH in response to signals that inhibit food intake, such as for example 5HT2CR agonists20, 21, 22 or leptin.23, 24, 25, 26 Activation of POMC neurons restricts weight gain during periods of energy sufficiency by controlling food intake, although these neurons may also be important in maintaining metabolic homeostasis via control of autonomic and pituitary functions. Central administration of α-MSH reduces food intake and may also increase energy expenditure, resulting in weight loss. β- and γ-MSH have similar effects on food intake when administered intracerebroventricularly, although they are less potent when compared with α-MSH.27 There are also important differences in the pharmacology of the three peptides with respect to melanocortin receptor activation. α-MSH functions as an MC3R and MC4R agonist, stimulating the accumulation of cAMP through Gs.28 β-MSH, although showing species specificity in its presence, is also an agonist for both receptors. In contrast, γ-MSH differs in that it exhibits modest selectivity for MC3Rs.8, 28 However, γ-MSH is still a functional MC4R agonist; the behavioural responses observed to pharmacological doses of this peptide likely involve the activation of both MC3R and MC4R.
Another group of neurons in the ARC express agouti-related peptide (AgRP), which has inverse agonist and antagonist functions for the MC3R and MC4R.19 AgRP neurons also coexpress the potent orexigen NPY,29 and are generally considered to have opposing functions to POMC neurons. The activity of AgRP neurons is essential for feeding behaviour, as ablation of these neurons in adult mice results in death from anorexia.30, 31 AgRP neurons are inhibited by signals that reduce food intake, such as activation of 5HT1BR by serotonin21 or leptin.32 On the other hand, the orexigen ghrelin increases food intake through the stimulation of AgRP neurons.33, 34 Central administration of AgRP increases weight gain, stimulates appetite and may also reduce energy expenditure.
The orexigenic activity of AgRP neurons is generally thought to be increased by nutrient scarcity.29, 35, 36, 37 These neurons may thus function to mediate an adaptive response, increasing the motivation to seek food and attenuating satiety signals to allow consumption of large meals. Outputs from these neurons may also regulate metabolic adaptation, reducing energy expenditure and promoting deposition of ingested energy into fat reserves.38, 39, 40 In ‘normal' physiological conditions where activation of these neurons occurs, weight gain would not be expected. However, the attenuated response of AgRP (and POMC) neurons to normal regulatory inputs in the obese state41 could contribute to the metabolic syndrome associated with weight gain. On the other hand, AgRP neurons may have functions related to maintaining homeostasis in fed conditions. Insulin receptors expressed by AgRP neurons have an important role in the suppression of hepatic glucose output by insulin.42, 43
POMC and AgRP neurons are heterogeneous populations (for example, the expression of insulin and leptin receptors is not universal44) that integrate signals from nutrient-sensing systems in the periphery with outputs governing ingestive behaviours and peripheral metabolic activity.1 The two populations of ‘primary' or first-order neurons in the melanocortin system respond to endocrine signals (‘inputs') from nutrient-sensing systems in the gut and adipose tissues. The main ‘outputs' that are known to be regulated by these primary neurons include brain regions that regulate complex ingestive behaviours, including the mesolimbic dopaminergic system. They influence metabolism via links with preganglionic autonomic neurons coupled to the sympathetic and parasympathetic nervous system, and hypophysiotropic neurons that send projections to the pituitary to control thyroid function. It is not the intent of this review to provide a comprehensive description of POMC and AgRP neurons; the reader is directed to several recent review articles for a comprehensive overview of their neuroanatomy, role and regulation.45, 46
The actions of melanocortin ligands released in the central nervous system are mediated by two melanocortin receptors (MC3R, MC4R) that belong to a family of five rhodopsin-like seven transmembrane domain G-protein-coupled receptors (GPCR). In heterologous cell-based assays, the application of α- or γ-MSH stimulates cAMP accumulation by increasing adenylyl cyclase activity via coupling to Gs.47 The analysis of receptor activity using cell-based assays suggests that MC3Rs also use other signalling pathways, including β-arrestins, the phosphoinotisol-3 kinase pathway, extracellular signalling-regulated kinase (ERK) and mobilizing intracellular calcium stores.9 We have observed increased ERK phosphorylation in a heterologous cell-based assay, and observed an increase in the number of neurons staining for phosphorylated ERK in the dorsomedial hypothalamus of mice treated intracerebroventricularly with γ-MSH.13
AgRP was initially proposed to function as an antagonist/inverse agonist at the MC3R and MC4R.35, 36 Recent observations suggest that α-MSH and AgRP function as biased agonists for these receptors. Both AgRP and α-MSH stimulate β-arrestin recruitment and receptor internalization; however, although α-MSH stimulates coupling with the stimulatory subunit (Gs), AgRP stimulates coupling with the inhibitory subunit of the heterotrimeric G-protein complex (Gi/o).48 In addition, recent data have suggested the possibility that the MC3R and MC4R form heterodimers with other GPCRs. For example, MC3R may form heterodimers with the ghrelin receptor (GHSR).49 These findings add complexity to the pharmacology of the system, and also suggest additional points of interaction between melanocortins and other ligand–receptor couplings with roles in metabolic homeostasis.
Targeted deletion of the mouse Mc4r gene and loss-of-function mutations in the human MC4R gene are associated with severe obesity that primarily results from hyperphagia, although reduced energy expenditure and nutrient partitioning favouring fat storage may contribute to the phenotype.38, 50, 51, 52, 53, 54 The LoxTB Mc4r model, where transcription is blocked by insertion of a ‘lox-stop-lox' sequence, has been very useful for investigating the functions of the central nervous melanocortin system. Reactivation of the LoxTB Mc4r allele in the brain is sufficient to restore a normal phenotype.55 Although not excluding a role for MC4Rs expressed in non-neural tissues, a large body of literature exists suggesting a mechanism for the regulation of metabolic activity in the periphery. MC4Rs expressed by preganglionic autonomic neurons have an important role in regulating glucose homeostasis,56, 57, 58, 59 although earlier studies indicated that functional MC4Rs are necessary for the stimulation of the autonomic nervous system and energy expenditure by melanocortins.11, 53, 60, 61 MC4Rs also appear to be the dominant receptor involved in the stimulation of thyroid-stimulating hormone synthesis in the paraventricular nucleus by α-MSH.62
A role for melanocortin-3 receptors in the defence of body weight and regulation of ‘nutrient partitioning'
The use of mouse genetics has also had an important role in experiments investigating the functions of melanocortin-3 receptors (MC3Rs). Mc3r−/− mice fed standard rodent chow exhibited very modest differences in body weight; there was an increase in adiposity due to reduced lean mass and increased fat mass.11, 12 Mc3r−/− mice also exhibit an accelerated diet-induced obese phenotype when fed high-fat diets.12, 50, 51, 63, 64, 65 One study reported evidence of a mild hyperphagia in Mc3r−/− mice fed a high-fat diet;51 however, the general consensus is that obesity results from abnormal metabolism and/or reduced physical activity. Reduced fat oxidation may also contribute to the nutrient partitioning phenotype.50, 51 However, MC3Rs in normal conditions do not appear to be required for glucose homeostasis. In chow-fed conditions, Mc3r−/− mice exhibit a very mild metabolic disorder when compared with the severe insulin-resistant phenotype observed in Mc4r−/− mice (for example, fasting hyperinsulinaemia, impaired glucose tolerance, hepatomegaly, severe hepatic steatosis and dyslipidaemia).11, 12, 16, 18, 50, 53, 64, 65, 66, 67 On the other hand, a therapeutic role for melanocortin receptors other than MC4Rs in improving insulin sensitivity may be possible, as treatment of obese Mc4r−/− mice with melanocortin analogues significantly improves fasting hyperinsulinaemia.18
As discussed above, the potential mechanisms that link neural MC3Rs with metabolic pathways in the periphery are unclear. The experimental evidence available supports a role for MC4R in the brain in controlling autonomic outputs involved in metabolic homeostasis. MC3Rs also cannot compensate for the loss of MC4Rs, and activation of MC3Rs by centrally administered melanocortin analogues in mice lacking MC4Rs does not result in altered autonomic nervous activity.59, 60, 63
Melanocortin-3 receptors as modulators of the anticipatory response to nutrients
Our interest in food anticipatory activity (FAA) stemmed from unpublished observations obtained while one of the authors (Butler) was a post-doctoral fellow in the laboratory of Dr Roger Cone at the Vollum Institute in Portland, OR. The Mc3r−/− strain had recently been generated and the initial characterization of the obesity phenotype published by Cone's laboratory11 coincided with a report from the Merck Research Laboratories in Rahway, NJ in which essentially the same phenotype was observed.12 The mild obesity phenotype compared with Mc4r−/− mice11, 12, 53 and the retention of acute feeding response to melanocortin agonists led us to explore alternative hypotheses for MC3R function. Work dating back to the 1970s by Dr Friedrich Stephan at Florida State University in Tallahassee, FL suggested that circadian rhythms are influenced by photic and caloric cues, and that the response to caloric cues was not dependant on the ‘master clock' found to reside in the suprachiasmatic nucleus of the hypothalamus.68 Mechanisms for timing FAA have been proposed to exist that would ‘optimize foraging and metabolic efficiency' with nutrient availability,69 in effect synchronizing peaks in capacity for foraging and the metabolic processing of nutrients with the time when food resources are available. Around this time, Dr Mary Dallman had speculated that the ventromedial hypothalamus (VMH) might contain a ‘food entrainable oscillator' (FEO).70 As the VMH is one of the few areas in the brain exhibiting very high levels of Mc3r expression (8), we examined whether Mc3r−/− mice would express FAA, a readout used frequently to investigate entrainment to food presentation.69
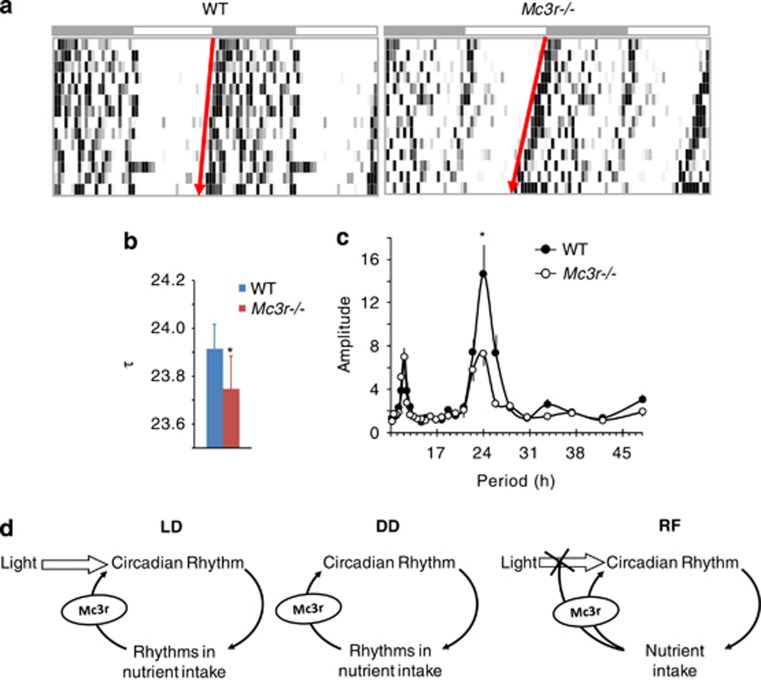
FAA is a progressive increase in locomotor activity before the presentation of food in a restricted feeding (RF) regime. Our RF regime involved feeding mice a reduced amount of energy (70% of normal intake), with food presented every 24 hours at some time in the lights-on period [typically 1300 h in mice housed in a 12-hour light–dark cycle, with lights on from 0600 to 1800 h). Using Mc3r−/− and wild-type littermate controls on a mixed 129;B6 background housed in wheel cages, we observed attenuated FAA in Mc3r−/− mice. Although the outcomes of this experiment were not published, they were later replicated by experiments using the same Mc3r−/− mice backcrossed onto the C57BL/6J background. These mice also exhibit attenuated FAA when subjected to RF in a light–dark and constant dark settings.13, 14, 17 Mc3r−/− mice also lost more weight when food access was limited to a 4-hour interval in the lights-on period, primarily because of reduced food intake.13 The studies using mice housed in light–dark and constant dark settings also found that the activity of the circadian oscillators that govern the expression of circadian rhythms was compromised in the cortex of Mc3r−/− mice during RF.14, 16, 17 We also observed shortened period length (τ) and reduced rhythm amplitude, suggesting a weakened rhythm, in free-running Mc3r−/− mice housed in constant dark (Figures 1a–c). Moreover, actions involving MC3Rs may also affect the sensitivity of the circadian clock to light cues, as loss of Mc3r results in a faster resynchronization to a new light–dark cycle following a 5-h phase advance.17 However, this hypothesis requires further investigation, as the shortened τ observed in Mc3r−/− mice (Figures 1a–c) could also affect the response to a phase advance in the light–dark cycle.
Figure 1.
Altered circadian rhythms in Mc3r−/− mice housed in constant dark conditions. (a) Free-running rhythms of a representative WT and Mc3r−/− mice housed for 2 weeks in constant dark conditions. (b) The period length (τ) of free-running Mc3r−/− mice is significantly shorter compared with WT controls (*P<0.05 by Student's t-test). (c) The amplitude in the rhythm of free-running Mc3r−/− mice is significantly reduced compared with WT controls (*P<0.05). (d) Schematic showing the proposed role of MC3Rs as modulators of the circadian rhythm. In light–dark conditions, light is the dominant zeitgeber that establishes the circadian rhythm in arousal and nutrient intake. Signals from nutrient sensors that are received by MC3R-expressing neurons act to ‘reinforce' rhythms; the absence of inputs from MC3Rs results in a weakened rhythm, and it also results in attenuated entrainment during periods of restricted feeding (RF). N=18–20 per group.
Collectively, these data suggest that Mc3r−/− mice are a form of circadian mutant with an attenuated response of the oscillators governing circadian rhythms to caloric cues and exaggerated dependency on light cues. MC3Rs may thus act as central modulators of the circadian rhythm, ‘reinforcing' rhythms in response to signals from nutrient sensors during normal conditions and having a more pronounced role in situations of nutrient scarcity where entrainment to nutrient availability would have a selective advantage (Figure 1d).
To investigate whether MC3Rs expressed in the VMH are involved in regulating the anticipatory response, we used a genetic approach allowing selective restoration of Mc3r expression. The approach was the same used for developing the LoxTB Mc4r mouse.55 A ‘lox-stop-lox' sequence was inserted into the 5′ untranslated region of the MC3R gene using C57BL/6J embryonic stem cells. Homozygous carriers of the ‘blocked' allele (Mc3rTB/TB) exhibit undetectable levels of Mc3r expression in the brain and have the same obesity phenotype previously reported for Mc3r−/− mice: increased fat mass, reduced lean mass and accelerated diet-induced obesity.15 VMH Mc3r expression was rescued using the steroidogenic factor-1 (SF-1) Cre mouse used by several laboratories to target this nucleus. Both Sf1-Cre; Mc3rTB/TB and Mc3rTB/TB mice exhibited attenuated FAA, indicating that the VMH MC3Rs are not sufficient to restore the expression of this complex group of behaviours.15
In retrospect, this outcome is not that surprising, as data published over the past 10 years indicate that the VMH does not contain a ‘FEO'.71 Indeed, although some studies suggest that the anticipatory response to feeding involves the dorsomedial hypothalamus,71, 72, 73, 74, 75 it has been debated whether the FEO resides in a single, definable structure.76 Clocks responding to caloric cues have been observed in many organ systems, and are now thought to be distributed throughout the body.77 It is therefore possible that the expression of the anticipatory response is an emergent feature involving the collective actions of a distributed system. The LoxTB Mc3r model will allow us to determine whether the expression of FAA can be restored by reactivating Mc3r expression in a few areas of the central nervous system or whether it requires the actions of a distributed network of neurons (and perhaps other cell types) expressing this receptor.
When the experiments investigating FAA in Mc3r−/− mice were performed in Dr Roger Cone's laboratory, the physiological significance and relevance of rhythms in behaviour and metabolism to obesity were not clear. However, interest in the role of circadian rhythms in metabolic homeostasis increased markedly following the report that C57BL/6J mice that are homozygous carriers of a mutation in the Clock gene that disrupts rhythms exhibit an obese phenotype.78 The proteins that comprise the core circadian oscillator are now known to regulate, and be regulated by, transcription factors that regulate the expression of genes involved in metabolic homeostasis.77, 79 The importance of rhythms in human health was also indicated by several clinical studies showing that misalignment of circadian rhythms rapidly led to a deterioration in metabolic homeostasis.80 The central nervous melanocortin system has an important role in metabolic homeostasis.1 In addition, Mc3r mRNA expression is observed in hypothalamic regions known to be involved in metabolic homeostasis.8, 9 We were therefore interested in determining the metabolic response of Mc3r−/− mice to RF.
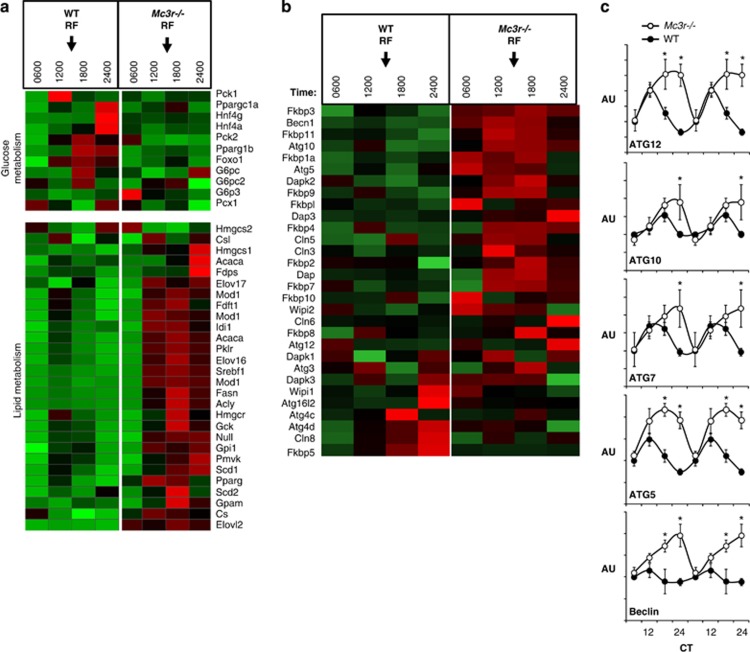
Our studies investigating FAA in the Mc3r−/− (and Mc3rTB/TB) models use a purified low-fat, high-carbohydrate diet (Research Diets 12450B; 10% kJ/fat, 20% kJ/protein and 70% kJ/carbohydrate). The rationale for using this diet is that it can be difficult for mice to obtain sufficient energy when using rodent chows. On this diet, Mc3r−/− mice can exhibit a mild form of hyperinsulinism that is commensurate with a modest increase in fat mass; however, the metabolic phenotype in general is mild.50 We have also observed normal glucose tolerance in Mc3r−/− mice fed low-fat or chow diets (unpublished data).50 Indeed, the control group for the study examining the metabolic response to RF exhibited normal insulin and blood glucose levels throughout most of the day.16 However, Mc3r−/− mice subjected to RF rapidly develop hyperinsulinaemia, hyperglycaemia, impaired glucose tolerance and dyslipidaemia. We proposed that Mc3r−/− mice subjected to RF were developing a ‘mixed insulin-resistant' phenotype, as the liver appeared to be exhibiting a normal response to elevated insulin. Compared with wild-type controls, the liver of Mc3r−/− mice exhibited increased expression of enzymes involved in lipogenesis and triglyceride synthesis and suppression of gluconeogenic enzymes. Microarray analysis of liver gene expression using the same protocol previously reported by our laboratory81 produced a similar outcome (Figure 2a). The microarray data also suggested the upregulation of a cellular stress response in the liver of Mc3r−/− mice, with increased expression of genes involved in autophagy (Figures 2b and c), and in genes expressing heat-shock proteins and ubiquitination (our unpublished observations). The combination of hyperinsulinaemia and increased expression of genes involved in autophagy is unique. Insulin is generally considered to inhibit autophagy, although autophagy has been suggested to regulate the release of fatty acids from lipids stores in hepatocytes.82 These differences were not observed in previous studies using microarray for the analysis of gene expression in the liver of Mc3r−/− mice in ad libitum-fed conditions (our unpublished data). Although further research is required to investigate these responses at the protein level, these observations nevertheless suggest that Mc3r−/− mice are not able to maintain metabolic homeostasis when fed during the daytime, resulting in the development of a diabetic phenotype and metabolic stress.
Figure 2.
Heatmap showing the expression of genes involved in glucose and lipid metabolism (a) and autophagy (b); the expression profile of selected genes involved in autophagy was further analysed using quantitative RT–PCR (c). The microarray and RT–PCR used total RNA isolated from the liver of wild-type (WT) and Mc3r−/− mice subjected to restricted feeding (RF); the expression of genes involved in clock activity, carbohydrate metabolism and lipid metabolism using these samples was reported in Sutton et al. (2010).16 Time of feeding is indicated by arrows in a and b; mice were presented food daily at 1300 h for 7 days. On the day of tissue collection, mice were not provided with food and they were euthanized at the times indicated. Insulin stimulates lipogenesis via increasing the activity of the transcription factor SREBP1,93 and the increased expression levels of SREBP1a and 1c correlate with increases in serum insulin levels in Mc3r−/− mice for the 1200-, 1800- and 2400-h time points reported previously.16 The results shown in b suggest that the amplitude of rhythms in the expression of genes involved in autophagy, which are under the control of clock genes,94 is also amplified in Mc3r−/− mice subjected to RF. *P<0.05 vs wild type.
Another recent observation that is worthy of mention here is the finding of abnormal metabolic adaptation of Mc3r−/− mice to fasting.83 Renquist et al. found that fasted Mc3r−/− mice do not exhibit increased lipolysis and also exhibit reduced activation of the hypothalamo–pituitary–adrenal axis. The increase in the expression of orexigenic neuropeptides in the hypothalamus is also attenuated (including AgRP). These findings are relevant to our studies, as RF involves periodic bouts of fasting for at least twenty hours. Indeed, we have also observed reduced food intake of Mc3r−/− mice when food access is restricted using a mechanical barrier to 4-h periods in the middle of the light cycle.13 It would be interesting to investigate whether increased AgRP expression in the ARC occurs in anticipation of food access during RF. Indeed, several groups have reported a role for ghrelin in the expression of FAA,84, 85, 86, 87 whereas the release of AgRP and NPY from these neurons is critical for the orexigenic response to this gut hormone.33
One criticism of the FAA phenotype observed in Mc3r−/− mice is whether it is secondary to the metabolic consequence of obesity. However, the Mc3r−/− mice fed the low-fat diet ad libitum exhibited very subtle differences in adiposity and minor differences in indicators of glucose homeostasis.16, 17 Moreover, although the SF1-Cre transgene improved metabolic homeostasis, it did not improve FAA.15 What was also remarkable about the metabolic phenotype of the Mc3r−/− mice used in our studies was that severe metabolic disturbances develop after the mice are subjected to RF. Weight loss associated with RF actually improved the metabolic phenotype of the lethal yellow Ay/a strain, which develops obesity and severe glucose intolerance owing to ubiquitous expression of the agouti protein, an MC1R and MC4R antagonist.16 To the best of our knowledge, Mc3r−/− mice are thus unique in showing evidence of increased lipogenesis, hyperinsulinaemia and impaired glucose tolerance in situations of negative energy balance. Our findings may therefore suggest a specific deficit in the ability of Mc3r−/− mice to adapt metabolically to daytime feeding.
Another critique stems from the observation of FAA in Mc3r−/− mice crossed onto the leptin-deficient Lepob/Lepob background. Lepob/LepobMc3r−/− mice exhibit an exaggerated FAA when compared with Lepob/Lepob mice.88 However, the hypothalamic neural circuitry involved in maintaining homeostasis fails to develop normally in Lepob/Lepob mice. A postnatal surge in serum leptin levels has a trophic effect on the development of projections from the arcuate nucleus to other hypothalamic (and perhaps extra-hypothalamic) nuclei, with the absence of the surge in Lepob/Lepob mice leading to marked abnormalities in the development of projections from the arcuate nucleus.89 When this point is taken into consideration, along with the severe neuroendocrinological deficits in Lepob/Lepob mice (for example, hypogonadotropic hypogonadism, severe hypercorticosteronaemia), the interpretation of the use of this model to study the neural circuitry involved in mediating the anticipatory responses to RF is questionable.
Restoring the LoxTB MC3R allele in the nervous system does not rescue obesity—are we looking in the right places?
When investigating the role of melanocortins in metabolic homeostasis and obesity, melanocortin receptors expressed in the brain have received most if not all of the attention. Indeed, reactivation of the LoxTB Mc4r allele either globally by deleting the lox-stop-lox sequence from the germ line or the nervous system of LoxTB Mc4r mouse using the Nestin-Cre transgene has a similar effect to completely rescue the obesity phenotype.55 For the LoxTB Mc3r mouse, germ-line reactivation rescued the obese phenotype. However, restoring expression in the nervous system using the Nestin-Cre transgene only partially rescued the obese phenotype in chow-fed conditions and did not rescue the accelerated diet-induced obese phenotype.15 The published results used female mice; our unpublished data suggest a similar outcome in male mice, whereas preliminary observations of male mice where the Nestin-Cre transgene was used to restore the expression of the LoxTB Mc3r allele in the nervous system suggest that FAA is rescued. These intriguing results suggest a functional divergence between neural and non-neural MC3Rs. MC3Rs expressed in the brain may have an important role in the expression of food anticipatory behaviours, whereas MC3Rs in non-neural tissues may have an important role in metabolic homeostasis particularly in situations where dietary fat intake is high.
Of course, another possibility is that the Nestin-Cre transgene has failed to restore the expression of the LoxTB Mc3r allele in a small subset of neurons and that the normal activity of this subset of MC3R-expressing neurons is critical for homeostasis. Another pitfall is the phenotype of the Nestin-Cre transgenic strain reported by Galichet et al.90 Nestin-Cre mice exhibit mild hypopituitarism with reductions in the levels of pituitary hormones, including growth hormone, adrenocorticotrophic hormone and thyroid-stimulating hormone. The authors proposed that the phenotype results from compromised hypothalamic development, as Cre-activity was not observed in the pituitary. We and others have also reported an adiposity phenotype, with reduced lean mass and increased fat mass.15, 91, 92 We are currently determining whether restoring the expression of the LoxTB Mc3r allele using another neural-selective Cre strain results in similar outcomes.
Concluding comments
The results from our investigations suggest that Mc3r−/− mice are a form of circadian mutant exhibiting an attenuated response to caloric cues. More recent studies using the LoxTB Mc3r mouse suggest that signaling via receptors expressed in non-neural tissues may be important for protecting against diet-induced obesity. The LoxTB Mc3r model will be a valuable tool to identify subpopulations of MC3R-expressing neurons (and perhaps non-neural cell types) that modulate the anticipatory response to nutrients and further explore the role of peripheral MC3Rs in protecting against diet-induced obesity.
Acknowledgments
The research described in this article has been supported by the NIDDK (DK0730189) and TSRI-Florida.
RAK has received grant support from Zafgen. AAB has received grant support through a Novo Nordisk Diabetes Innovation Award. The remaining authors declare no conflict of interest.
Footnotes
This article is published as part of a supplement sponsored by the Université Laval's Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments, and prevention of obesity.
References
- Garfield AS, Lam DD, Marston OJ, Przydzial MJ, Heisler LK. Role of central melanocortin pathways in energy homeostasis. Trends Endocrinol Metab 2009; 20: 203–215. [DOI] [PubMed] [Google Scholar]
- O'Rahilly S. Human genetics illuminates the paths to metabolic disease. Nature 2009; 462: 307–314. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 1998; 19: 155–157. [DOI] [PubMed] [Google Scholar]
- Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 1998; 20: 111–112. [DOI] [PubMed] [Google Scholar]
- Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet 1998; 20: 113–114. [DOI] [PubMed] [Google Scholar]
- Tao YX. Mutations in the melanocortin-3 receptor (MC3R) gene: impact on human obesity or adiposity. Curr Opin Investig Drugs 2010; 11: 1092–1096. [PubMed] [Google Scholar]
- MacNeil DJ, Howard AD, Guan X, Fong TM, Nargund RP, Bednarek MA et al. The role of melanocortins in body weight regulation: opportunities for the treatment of obesity. Eur J Pharmacol 2002; 450: 93–109. [DOI] [PubMed] [Google Scholar]
- Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB et al. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 1993; 90: 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renquist BJ, Lippert RN, Sebag JA, Ellacott KL, Cone RD. Physiological roles of the melanocortin-3 receptor. Eur J Pharmacol 2011; 660: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG. Distribution and function of melanocortin receptors within the brain. Adv Exp Med Biol 2011; 681: 29–48. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J et al. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology 2000; 141: 3518–3521. [DOI] [PubMed] [Google Scholar]
- Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet 2000; 26: 97–102. [DOI] [PubMed] [Google Scholar]
- Begriche K, Marston OJ, Rossi J, Burke LK, McDonald P, Heisler LK et al. Melanocortin-3 receptors are involved in adaptation to restricted feeding. Genes Brain Behav 2012; 11: 291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begriche K, Sutton GM, Butler AA. Homeostastic and non-homeostatic functions of melanocortin-3 receptors in the control of energy balance and metabolism. Physiol Behav 2011; 104: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begriche K, Levasseur PR, Zhang J, Rossi J, Skorupa D, Solt LA et al. Genetic dissection of the functions of the melanocortin-3 receptor, a seven-transmembrane g-protein coupled receptor, suggests roles for central and peripheral receptors in energy homeostasis. J Biol Chem 2011; 286: 40771–40781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Begriche K, Kumar KG, Gimble JM, Perez-Tilve D, Nogueiras R et al. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J 2010; 24: 862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD et al. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci 2008; 28: 12946–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KG, Sutton GM, Dong JZ, Roubert P, Plas P, Halem HA et al. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides 2009; 30: 1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 2005; 8: 571–578. [DOI] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ et al. 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 2008; 60: 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Jobst EE, Sutton GM, Zhou L, Borok E, Thornton-Jones Z et al. Serotonin reciprocally regulates melanocortin neurons to modulate food intake. Neuron 2006; 51: 239–249. [DOI] [PubMed] [Google Scholar]
- Heisler LK, Cowley MA, Kishi T, Tecott LH, Fan W, Low MJ et al. Central serotonin and melanocortin pathways regulating energy homeostasis. Ann N Y Acad Sci 2003; 994: 169–174. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 2001; 411: 480–484. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest 2008; 118: 1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology 1997; 138: 4489–4492. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 1997; 46: 2119–2123. [DOI] [PubMed] [Google Scholar]
- Tung YC, Piper SJ, Yeung D, O'Rahilly S, Coll AP. A comparative study of the central effects of specific proopiomelancortin (POMC)-derived melanocortin peptides on food intake and body weight in POMC null mice. Endocrinology 2006; 147: 5940–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adan RA, Cone RD, Burbach JP, Gispen WH. Differential effects of melanocortin peptides on neural melanocortin receptors. Mol Pharmacol 1994; 46: 1182–1190. [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1998; 1: 271–272. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005; 310: 683–685. [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 2005; 8: 1289–1291. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 1999; 23: 775–786. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 2004; 145: 2607–2612. [DOI] [PubMed] [Google Scholar]
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z et al. Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J 2005; 19: 1680–1682. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 1997; 278: 135–138. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Graham M, Kinsey AC, Scully S, Luthy R, Stark KL. Hypothalamic expression of ART, a novel gene related to agouti, is up-regulated in obese and diabetic mutant mice. Genes Dev 1997; 11: 593–602. [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 1999; 140: 814–817. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Wiedmer P, Perez-Tilve D, Veyrat-Durebex C, Keogh JM, Sutton GM et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest 2007; 117: 3475–3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG et al. Effects of chronic central nervous system administration of agouti-related protein in pair-fed animals. Diabetes 2001; 50: 248–254. [DOI] [PubMed] [Google Scholar]
- Small CJ, Liu YL, Stanley SA, Connoley IP, Kennedy A, Stock MJ et al. Chronic CNS administration of Agouti-related protein (Agrp) reduces energy expenditure. Int J Obes Relat Metab Disord 2003; 27: 530–533. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 2007; 5: 181–194. [DOI] [PubMed] [Google Scholar]
- Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007; 5: 438–449. [DOI] [PubMed] [Google Scholar]
- Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E et al. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in AgRP and POMC neurons. Diabetes 2010; 59: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci 2010; 30: 2472–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metab 2012; 16: 144–152. [DOI] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 2012; 15: 1350–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev 2006; 27: 736–749. [DOI] [PubMed] [Google Scholar]
- Breit A, Buch TR, Boekhoff I, Solinski HJ, Damm E, Gudermann T. Alternative G protein coupling and biased agonism: new insights into melanocortin-4 receptor signalling. Mol Cell Endocrinol 2011; 331: 232–240. [DOI] [PubMed] [Google Scholar]
- Rediger A, Piechowski CL, Habegger K, Gruters A, Krude H, Tschop MH et al. MC4R dimerization in the paraventricular nucleus and GHSR/MC3R heterodimerization in the arcuate nucleus: is there relevance for body weight regulation. Neuroendocrinology 2012; 95: 277–288. [DOI] [PubMed] [Google Scholar]
- Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ et al. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology 2006; 147: 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA. The melanocortin system and energy balance. Peptides 2006; 27: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW et al. Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology 2004; 145: 243–252. [DOI] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 2001; 4: 605–611. [DOI] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA 2000; 97: 12339–12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005; 123: 493–505. [DOI] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 2003; 457: 213–235. [DOI] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM et al. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 2003; 23: 7143–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 1994; 8: 1298–1308. [DOI] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 2011; 13: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 2003; 23: 5998–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res 2000; 9: 145–154. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. Role of melanocortin signaling in the regulation of the hypothalamic-pituitary-thyroid (HPT) axis. Peptides 2006; 27: 310–325. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kilroy GE, Henagan TM, Prpic-Uhing V, Richards WG, Bannon AW et al. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB J 2005; 19: 1482–1491. [DOI] [PubMed] [Google Scholar]
- Trevaskis JL, Gawronska-Kozak B, Sutton GM, McNeil M, Stephens JM, Smith SR et al. Role of adiponectin and inflammation in insulin resistance of Mc3r and Mc4r knockout mice. Obesity (Silver Spring) 2007; 15: 2664–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellacott KL, Murphy JG, Marks DL, Cone RD. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology 2007; 148: 6186–6194. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997; 88: 131–141. [DOI] [PubMed] [Google Scholar]
- Perez-Tilve D, Hofmann SM, Basford J, Nogueiras R, Pfluger PT, Patterson JT et al. Melanocortin signaling in the CNS directly regulates circulating cholesterol. Nat Neurosci 2010. ## [DOI] [PMC free article] [PubMed]
- Stephan FK. The ‘other' circadian system: food as a Zeitgeber. J Biol Rhythms 2002; 17: 284–292. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Food-anticipatory circadian rhythms: concepts and methods. Eur J Neurosci 2009; 30: 1718–1729. [DOI] [PubMed] [Google Scholar]
- Choi S, Wong LS, Yamat C, Dallman MF. Hypothalamic ventromedial nuclei amplify circadian rhythms: do they contain a food-entrained endogenous oscillator? J Neurosci 1998; 18: 3843–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci 2006; 9: 398–407. [DOI] [PubMed] [Google Scholar]
- Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci 2003; 23: 10691–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science 2008; 320: 1074–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Richardson JA, Tanaka K, Yanagisawa M. The dorsomedial hypothalamic nucleus as a putative food-entrainable circadian pacemaker. Proc Natl Acad Sci USA 2006; 103: 12150–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Galvan G, Yi CX, van der Vliet J, Jhamandas JH, Panula P, Angeles-Castellanos M et al. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc Natl Acad Sci USA 2011; 108: 5813–5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Neurobiology of food anticipatory circadian rhythms Physiol Behav 2011; 4: 535–545. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science 2010; 330: 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005; 308: 1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer 2009; 9: 886–896. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009; 106: 4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KG, Trevaskis JL, Lam DD, Sutton GM, Koza RA, Chouljenko VN et al. Identification of adropin as a secreted factor linking dietary macronutrient intake with energy homeostasis and lipid metabolism. Cell Metab 2008; 8: 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M et al. Autophagy regulates lipid metabolism. Nature 458: 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renquist BJ, Murphy JG, Larson EA, Olsen D, Klein RF, Ellacott KL et al. Melanocortin-3 receptor regulates the normal fasting response. Proc Natl Acad Sci USA 2012; 109: E1489–E1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci USA 2009; 106: 13582–13587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL et al. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience 2009; 164: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JF, Choi DL, Clegg DJ, Benoit SC. Signaling through the ghrelin receptor modulates hippocampal function and meal anticipation in mice. Physiol Behav 2011; 103: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen LA, Egecioglu E, Luijendijk MC, Hillebrand JJ, Adan RA, Dickson SL. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur Neuropsychopharmacol 2011; 21: 384–392. [DOI] [PubMed] [Google Scholar]
- Ribeiro AC, Ceccarini G, Dupre C, Friedman JM, Pfaff DW, Mark AL. Contrasting effects of leptin on food anticipatory and total locomotor activity. PLoS One 2011; 6: e23364## [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004; 304: 108–110. [DOI] [PubMed] [Google Scholar]
- Galichet C, Lovell-Badge R, Rizzoti K. Nestin-Cre mice are affected by hypopituitarism, which is not due to significant activity of the transgene in the pituitary gland. PLoS One 2010; 5: e11443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Zhao L, Donato Jr J, Kohno D, Xu Y, Elias CF et al. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci USA 2011; 108: 10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briancon N, McNay DE, Maratos-Flier E, Flier JS. Combined neural inactivation of suppressor of cytokine signaling-3 and protein-tyrosine phosphatase-1B reveals additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes 2010; 59: 3074–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest 2002; 109: 1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Li S, Molusky MM, Lin JD. Circadian autophagy rhythm: a link between clock and metabolism? Trends Endocrinol Metab 2012; 23: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]