Abstract
We sought to determine the role of functionally selective dopamine (DA) signalling pathways (G protein or β-arrestin) in DA-dependent behaviours. Mice that were globally deficient for β-arrestins or mice deficient in GSK3β in D2 receptor (D2R)-expressing neurons were used to investigate the role of functional selectivity in DA-dependent behaviours such as locomotor activity and conditioned place preference (CPP). Wild-type or knockout mice were injected with drugs such as morphine and amphetamine, which are known to increase DA levels in the brain and to induce a hyper-locomotor response and CPP. Unlike β-arrestin1 (βarr1)-deficient mice, mice globally deficient for β-arrestin2 (βarr2) mount a reduced hyperlocomotor response to either morphine or amphetamine. However, mice deficient in GSK3β in D2R-expressing neurons show a significantly reduced locomotor response to only amphetamine but not morphine. Interestingly, all mice tested show a normal CPP response to either morphine or amphetamine. β-arrestin-mediated DA receptor signalling has an important role in the locomotor response, but not CPP, to drugs such as morphine and amphetamine, demonstrating a functional selectivity of DA-dependent behaviours in mice. It is likely that G-protein-dependent signalling through DA receptors mediates the CPP response.
Keywords: biased signalling; Parkinson's disease; schizophrenia; drug addiction
INTRODUCTION
G-protein-coupled receptors (GPCRs) are seven-transmembrane proteins that represent the largest and most diverse family of membrane receptors in biology and affect virtually every aspect of cellular and organ function in the body from developmental programs to homeostatic control of cellular metabolism.1 Classically, GPCRs were shown to control physiological processes through their activation of heterotrimeric G proteins and they are tightly regulated by one of the common mechanisms that limit the extent and duration of signalling in a process termed desensitization, which is mediated by GPCR kinase (GRK)-mediated phosphorylation of activated receptors and subsequent interactions with arrestin proteins.2 This latter process serves not only to turn off the G-protein-dependent signalling but it also represents the trigger for internalization and recycling of competent receptors back to the plasma membrane.3 However, we now realize that the ability of the activated phospho-GPCR to interact with arrestins endows the receptor with an additional function through the ability of arrestins to scaffold intracellular proteins to elicit an additional wave of signalling distinct from the G-protein-dependent signalling.2, 4 Interestingly, arrestin-dependent signalling displays a slightly later onset but it is much more sustained than G-protein-dependent signalling, and importantly ligands for the same receptor that act as agonists at one pathway can be antagonists at the other or vice versa.5 Growing evidence indicates that these different signalling modes control different cellular and physiological functions. As GPCRs represent the largest class of pharmacotherapy targets, this phenomenon, which is commonly referred to as functional selectivity or biased signalling, provides a previously unappreciated rationale for the development of more selective and effective therapeutic agents. Studies with the nicotinic acid receptor GPR109A and chemokine receptor CXCR7 have shown the importance of functional selectivity at GPCRs and their potential to regulate a subset of behavioural outcomes.6, 7 Specifically, the studies with GPR109A have shown that different behaviours can be regulated by G-protein- or β-arrestin-dependent pathways, suggesting a functional selectivity at the physiological level.8
Dopamine (DA) is a monoamine neurotransmitter implicated in normal physiological functions such as movement, reward, cognition and affect, and deregulation of DA homeostasis is presumably implicated in disease states such as schizophrenia, Parkinson's disease and ADHD. DA binds to and activates GPCRs that belong to two subclasses, the D1 receptor (D1 and D5) class and the D2 receptor (D2, D3 and D4) class, with their highest expression being in the striatum. The G-protein-dependent cAMP/PKA/DARPP32 pathway for DA receptors has been well described.9, 10, 11 We have previously demonstrated that in addition to this canonical G-protein-dependent signalling, DA-dependent signalling downstream of D2R activation can also be mediated through a β-arrestin 2 (βarr2)-dependent signalling complex comprising βarr2/AKT/PP2A that leads to the activation of GSK3β.12, 13 In addition, we have shown that D1Rs can activate a βarr2/ERK signalling complex that can mediate the hyper-locomotor response upon morphine exposure.14
In this study, using genetically altered mouse models, we show that β-arrestin-dependent signalling mediates only certain DA-dependent behaviours and not others, suggesting a functional selectivity of behaviours.
Methods
Animals and drugs
All mouse studies were conducted in accordance with the NIH guidelines for animal care and use and with an approved animal protocol from the Duke University Animal Care and Use Committee. The D2 (DRD2) Cre mice were obtained from Drs. Nathaniel Heintz and Charles Gerfen (The Gene Expression Nervous System Atlas (GENSAT) Project, NINDS Contracts N01NS02331 & HHSN271200723701C to The Rockefeller University (New York, NY, USA)). The Cre mice were backcrossed on to a C57BL6/J background for at least five generations and were maintained on this background. The D2GSK3β−/− mice and their littermate controls were generated by crossing the D2Cre mice to the GSK3β Flx/Flx mouse (BL6/129 background), a generous gift from Dr. James Woodgett (Samuel Lunenfeld Research Institute, Toronto, ON, Canada).15 In addition, all mouse lines were crossed to a Rosa26-stop-EYFP reporter mouse line 16 obtained from The Jackson laboratory (B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J) to confirm the expression pattern of all the Cre lines and to confirm deletion of GSK3β in specific neurons.17 The β-arrestin1 (βarr1; C57Bl6) and β-arr2 (C57/129 mix) knockout mice have been described previously.18, 19 Amphetamine (Amph) and morphine (Sigma, St Louis, MO) were dissolved in saline (Sal). Appropriate dissolving solutions were used as vehicle controls. All drugs were injected at a volume of 10 ml kg−1 animal weight.
Locomotor activity
Locomotor activity was measured in an Accuscan activity monitor (Accuscan Instruments, Columbus, OH, USA) as described previously.14 Briefly, locomotor activity was measured at 5-min intervals, and data were analysed for the total distance traveled in 5-min increments for 150 min or as mentioned in figures. All mice were allowed to acclimatize to the activity monitor for 30 min before any drug treatments, unless mentioned otherwise. Drugs were administered at various time points depending on the experiment, and they are described in the figures and figure legends.
Conditioned place preference
A conditioned place preference (CPP) apparatus from Med Associates (St Albans, VT, USA) was used to analyse place preference to morphine in mice and was performed as described previously.20 Briefly, the CPP procedure consisted of day 1 of preconditioning (pretest), where the mice were allowed to freely move between all chambers of the CPP apparatus and the time spent (basal preference) in each chamber was recorded for 30 min. In the conditioning phase, the mice were injected with morphine (3 or 6 mg kg−1) on days 2, 4 and 6 and Sal on days 3, 5 and 7, and the drug was randomly paired with alternating compartments, such that half of the mice received drug in the black compartment and the other half in the white compartment. On day 8, the test day (test), the mice were handled similar to the preconditioning day and were allowed to move freely between all chambers, and the time spent (drug-induced preference) in each chamber was recorded for 30 min. The data were analyzed by calculating the difference (Δ) in time spent in the drug-paired chamber on the pretest and test days.
Statistical analyses
All data are presented as mean±s.e.m. Data were analyzed by a standard one-way or two-way analysis of variance test for comparison between genotypes, treatments or doses. Individual genotypes, treatments or doses were compared using a post hoc Bonferroni's test.
Results
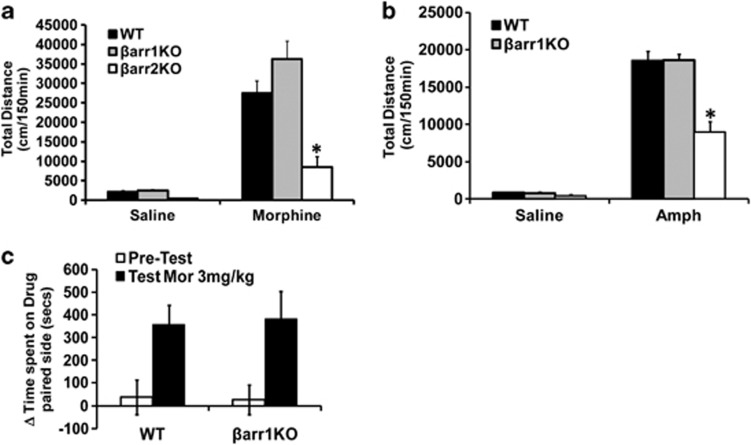
A common property of drugs of abuse is that they increase DA levels in the striatum of mice.21 Drugs such as morphine and amphetamine are known to increase DA levels in mice, activate DA receptors and induce behaviours such as hyperlocomotion and CPP.22 DA receptors activate both G-protein- and β-arrestin-mediated signalling pathways,10, 12, 14 and to determine the role of these individual pathways in mediating functionally selective behaviours we used globally deficient βarr1 or βarr2 mice and mice with a deletion of GSK3β in DA D2 receptor (D2R)-expressing neurons. To assess functional selectivity at the behavioural level, we used two well characterized DA-dependent behavioural tests, hyperlocomotion and CPP, and two commonly used drugs that increase extracellular brain DA, morphine and amphetamine. As shown in Figures 1a and b, βarr2-deficient (βarr2KO) but not βarr1-deficient (βarr1KO) mice show a significantly reduced (P<0.05) hyperlocomotor response to morphine and amphetamine. However, CPP to morphine is intact in the βarr1KO mice (Figure 1c) but enhanced in the βarr2KO mice20 compared with respective controls.
Figure 1.
Role of β-arrestins in locomotion but not CPP. Wild-type (WT), βarr1KO and βarr2KO mice were placed in an activity monitor and the distance was recorded every 5 min for 30 min, and then they were injected with (a) morphine (20 mg kg−1, subcutaneous) or (b) amphetamine (Amph, 3 mg kg−1, intraperitoneal) and the distance was recorded every 5 min for 120 min. βarr2KO but not βarr1KO mice show significantly less cumulative distance traveled (150 min) than WT mice upon (a) morphine or (b) amphetamine treatment. n=8 for all genotype and treatment groups, *P<0.05 comparing WT and βarr2KO. (c) βarr1KO mice spend similar amount of time in the morphine-paired chamber as the WT mice. n=10 mice per genotype per group.
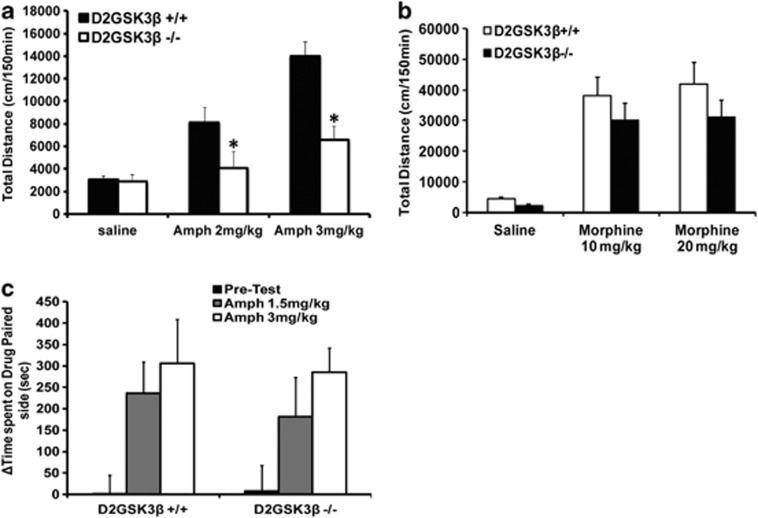
We have previously shown that under hyperdopaminergic conditions GSK3β is activated in a D2R-dependent manner in the striatum of mice through a βarr2/AKT/PP2A signalling complex.12 Therefore, we would expect that a genetic deletion of GSK3β should mimic the βarr2KO mice. To test this hypothesis, we generated mice with a selective deletion of GSK3β in D2R-expressing neurons using Cre/LoxP technology. To test the functional selectivity of this D2R/βarr2/GSK3β pathway in DA-dependent behaviours, we tested these mice for hyper-locomotion and CPP upon stimulation with morphine or amphetamine. As shown in Figure 2, the D2GSK3β−/− mice show a reduced, dose-dependent response to amphetamine (Figure 2a) but not to morphine (Figure 2b) compared with controls. However, these mice show a similar CPP response to amphetamine (Figure 2c) and morphine (data not shown).
Figure 2.
Role of β-arrestin-dependent GSK3β pathway in D2R neurons in locomotion and CPP. Mice with deletion of GSK3β in D2R neurons (D2GSK3β−/−) and controls (D2GSK3β+/+) were placed in an activity monitor and the distance was recorded every 5 min for 30 min, and then they were injected with (a) amphetamine (Amph, 2 or 3 mg kg−1, intraperitoneal) or (b) morphine (10 or 20 mg kg−1, subcutaneous) and the distance was recorded every 5 min for 120 min. D2GSK3β−/− mice show significantly less cumulative distance traveled (150 min) than D2GSK3β+/+ mice upon (a) amphetamine (both doses) but not (b) morphine treatment. n=8 for all genotype and treatment groups, *P<0.05, comparing D2GSK3β−/− and D2GSK3β+/+ Amph-treated groups. (c) D2GSK3β−/− mice spend similar amount of time in the Amph-paired chamber as the D2GSK3β+/+ mice at both doses tested. n=10 mice per genotype per group.
Discussion
Hyperdopaminergia is known to induce several behaviours in mice that are mediated by activation of a combination of DA receptors. However, each DA receptor signals through both G-protein- and β-arrestin-dependent pathways, but the contribution of these individual pathways to DA-dependent behavioral outcomes is not known. The data presented here address this question by assessing DA-dependent behaviours in mice that have deletion of genes that are part of the β-arrestin signalling pathway presumably under D1 or D2 receptors.
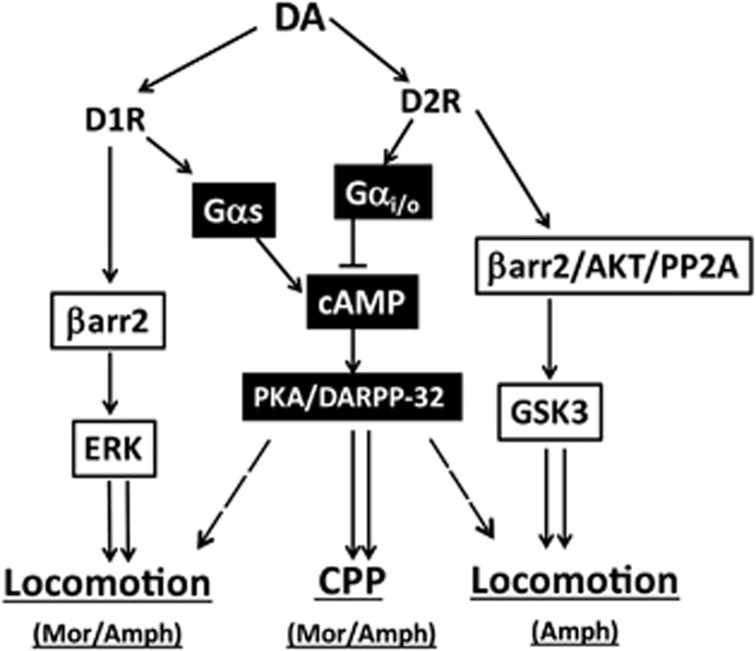
In this study, we show that, under hyperdopaminergic conditions induced by morphine or amphetamine, βarr2 but not βarr1 regulates only locomotion but not CPP. We and Smith et al.23 have shown previously that this locomotor response to morphine is predominantly mediated by D1Rs and not by D2Rs, but interestingly the CPP response is predominantly mediated by D2Rs and not by D1Rs.14, 23 However, G-protein-dependent signalling does contribute to hyperlocomotor responses to DA-ergic drugs, as deleting DARPP-32 in mice does result in a small but significant reduction in hyperlocomotion10, 24 when compared with the drastic reduction in the βarr2KO mice. Moreover, our conclusions are based upon studies conducted with mice that are globally deficient for βarr2, and to further refine our study we used mice wherein we deleted GSK3β (downstream of βarr2 signalling) in D2R neurons (D2GSK3β−/−). Similar to the results with the βarr2KO mice, we observed that in the D2GSK3β−/− mice the hyperlocomotor response to amphetamine is reduced compared with controls, suggesting an important role for β-arrestin pathway in locomotion. However, as expected, the locomotor response to morphine is similar to controls, as we have already shown that morphine-induced locomotion is D1R-dependent. In addition, CPP to amphetamine in the D2GSK3β−/− mice is intact, again suggesting that this behaviour is predominantly G-protein-mediated. We have summarized these findings in the model shown in Figure 3, which shows the role of the G protein or the β-arrestin signalling pathways in DA-dependent behaviours and thereby functional selectivity in the DA system.
Figure 3.
Contribution of functionally selective signalling pathways in DA-dependent behaviours. ↓, Normal activation;  , mild activation; ↓↓, strong activation; ⊥, inhibition. Mor (morphine), Amph (amphetamine).
, mild activation; ↓↓, strong activation; ⊥, inhibition. Mor (morphine), Amph (amphetamine).
We have shown previously that morphine-induced locomotion is ERK-dependent14 and that amphetamine-induced locomotion can also be ERK-dependent.25 Therefore, although morphine-dependent locomotion is solely D1R-dependent, it appears that amphetamine-induced locomotion requires activation of both D1R/βarr2/ERK and D2/βarr2/GSK3β signalling pathways. Amphetamine-induced hyperlocomotion is one of the validated models for psychosis in schizophrenia, and the data above suggest that βarr2-dependent signalling predominantly regulates this behaviour. Therefore, generating functionally selective ligands that target the β-arrestin pathway might be useful therapies for schizophrenia, which give the desirable effects without inducing side effects. Indeed, such a biased ligand has been generated that shows functional selectivity to the β-arrestin pathway at the D2 receptor and has shown specificity in targeting the βarr2 pathway.26 It remains to be determined whether such newly identified biased ligands show clinical efficacy.
In our laboratory, we are using in vitro and in vivo integrated physiological approaches to elucidate the actions of DA in the CNS that appear to be mediated by both modes of signalling downstream of D1 and D2 types of DA receptors. As in the CNS, the DA system is implicated in both neurological and psychiatric disorders such as Parkinson's disease and schizophrenia, the notion of functional selectivity can be leveraged to understand and develop new concepts of therapeutic interventions. The combination of in vivo genetic approaches with high-throughput sequencing should identify yet more selective targets downstream of these signalling modes that can be validated as potential therapies.
As mentioned above, GPCRs represent the largest family of receptors in the human genome with close to 400 such non-olfactory receptors capable of interacting with selective ligands. Of this large number of GPCRs, roughly 200 have been paired with their presumed endogenous ligand. GPCRs are also targets for an estimated 30–50% of drugs used in the clinic practice, but these therapeutic drugs engage as few as 40–50 different receptors. Therefore, understanding the biological function of the remaining orphan GPCRs should lead to a much better comprehension of human physiology. Interestingly, discovering functionally selective ligands to exploit the concept of GPCR functional signalling for known or orphan GPCRs will undoubtedly provide therapeutic approaches with increased efficacy and selectivity. Such strategies should be applicable to every facet of human biology.
Acknowledgments
This work was supported by the National Institutes of Health grants RO1-MH-073853 and U-19-MH-082441. The authors thank Wendy Roberts, Xiuqin Zhang and Benjamin Phillips for maintenance of the mouse colony. GSK3β floxed mice were generously provided by Dr James Woodgett at the Samuel Lunenfeld Research Institute, Toronto, Canada.
MGC has received consulting fees from Lundbeck A/G, owns equity in in USD Acadia Pharmaceutical and has received grant support from Hoffmann LaRoche. NMU has declared no conflicts of interest.
Footnotes
This article is published as part of a supplement sponsored by the Université Laval's Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments, and prevention of obesity.
References
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 1998; 78: 189–225. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science 2005; 308: 512–517. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey III WE, Colapietro AM, Barak LS, Ménard L, Caron MG. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 1996; 271: 363–366. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol 2007; 69: 483–510. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther 2007; 320: 1–13. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Kim J, Ahn S, Craig S, Lam CM, Gerard NP et al. Beta-arrestin- but not G protein-mediated signaling by the ‘decoy' receptor CXCR7. Proc Natl Acad Sci USA 2010; 107: 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman JG, Kanemitsu-Parks M, Gaidarov I, Cameron JS, Griffin P, Zheng H et al. Nicotinic acid receptor agonists differentially activate downstream effectors. J Biol Chem 2007; 282: 18028–18036. [DOI] [PubMed] [Google Scholar]
- Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM et al. Beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest 2009; 119: 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Rognoni F, Fredholm BB, Greengard P, Fisone G. Activation of adenosine A2A and dopamine D1 receptors stimulates cyclic AMP-dependent phosphorylation of DARPP-32 in distinct populations of striatal projection neurons. Neuroscience 1998; 84: 223–228. [DOI] [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N et al. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 2008; 11: 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science 2001; 294: 1024–1030. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 2005; 122: 261–273. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR et al. Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci 2007; 27: 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Daigle TL, Caron MG. A dopamine D1 receptor-dependent beta-Arrestin signaling complex potentially regulates morphine-induced psychomotor activation but not reward in mice. Neuropsychopharmacology 2011; 36: 551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol 2008; 28: 6314–6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 2001; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3beta in D2R-expressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci USA 2012; 109: 20732–20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science 1999; 286: 2495–2498. [DOI] [PubMed] [Google Scholar]
- Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE et al. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res 1997; 81: 1021–1026. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA et al. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci 2003; 23: 10265–10273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 1988; 85: 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol 2009; 49: 327–347. [DOI] [PubMed] [Google Scholar]
- Smith JW, Fetsko LA, Xu R, Wang Y. Dopamine D2L receptor knockout mice display deficits in positive and negative reinforcing properties of morphine and in avoidance learning. Neuroscience 2002; 113: 755–765. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M et al. Diverse psychotomimetics act through a common signaling pathway. Science 2003; 302: 1412–1415. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem 2006; 281: 32072–32080. [DOI] [PubMed] [Google Scholar]
- Allen JA, Yost JM, Setola V, Chen X, Sassano MF, Chen M et al. Discovery of beta-arrestin-biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA 2011; 108: 18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]