Abstract
Glucagon-like peptide-1 (GLP-1) is a gastrointestinal hormone secreted from L cells in the distal small intestine and proximal colon after a meal that acts as an incretin to augment the insulin response, while also inhibiting glucagon and slowing gastric emptying. These characteristics of GLP-1, as well as its ability to reduce islet beta cell apoptosis and expand beta cell mass and its cardioprotective and neuroprotective effects, provide a broad spectrum of actions potentially useful for the management of type-2 diabetes mellitus. GLP-1 also has the added advantage of having its incretin effects dependent on the level of serum glucose, only acting in the presence of hyperglycaemia, and thereby preventing hypoglycemic responses. Although natural GLP-1 has a very short half-life, limiting its therapeutic usefulness, a variety of analogues and formulations have been developed to provide extended actions and to limit side effects. However, all of these peptides require parenteral administration. Potentially orally active small-molecule agonists acting at the GLP-1 receptor are also being developed, but have not yet been approved for clinical use. Recent insights into the molecular nature of the class B G-protein-coupled GLP-1 receptor has provided insights into the modes of binding these types of ligands, as well as providing opportunities for rational enhancement. The advantages and disadvantages of each of these agents and their possible clinical utility will be explored.
Keywords: glucagon-like peptide-1, incretin, type-2 diabetes, G-protein-coupled receptor
Obesity and type-2 diabetes mellitus have reached epidemic levels, affecting populations around the world.1 It is particularly important to expand the available therapeutic armamentarium to control and manage these problems to reduce their very serious co-morbidities, which are associated with substantial expense and suffering. Recent efforts include developing agonists acting at the glucagon-like peptide-1 (GLP-1) receptor, a class B1 G-protein-coupled receptor (GPCR).2 This is the receptor for the most potent endogenous incretin, a gastrointestinal hormone that stimulates the secretion of insulin in a glucose-dependent manner.2 The incretins, GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), are believed to be responsible for up to 70% of the meal-stimulated insulin response.3 However, this incretin effect is reduced in the setting of type-2 diabetes mellitus with hyperglycaemia.4 Importantly, the GLP-1 effect was largely retained, whereas that of GIP was substantially reduced.
GLP-1 peptides
Similar to all of the natural peptide ligands for members of this receptor family, GLP-1 is a moderate-length peptide having a diffuse pharmacophoric domain.5 In addition, similar to the other ligands, it has a tendency to form a helical conformation in its mid-region and carboxyl terminus, particularly when in a solvent that mimics the membrane environment.6 The two-domain hypothesis that has been proposed for binding of members of this family to their receptors suggests that the carboxyl-terminal ends of these peptides provide significant binding energy by interacting with the amino-terminal domain of their receptors, whereas the amino-terminal ends of the peptides are critical for biological activity by interacting with the core domain of their receptors.7 This hypothesis has been based on peptide structure-activity series, receptor mutagenesis and chimeric receptor structures, as well as photoaffinity labelling, and it has been quite consistent across the class B1 family of GPCRs.8
GLP-1 is synthesized in and secreted by open-type neuroendocrine L cells scattered along the mucosa of the distal small intestine and proximal colon. It is derived from the post-translational processing of preproglucagon, a gene that can yield glucagon, as well as four forms of GLP-1 (GLP-1(1-36)NH2, GLP-1(7-36)NH2, GLP-1(1-37) and GLP-1(7-37)), GLP-2, glicentin and oxyntomodulin.9 Of note, all the forms of GLP-1 and oxyntomodulin are able to bind to the GLP-1 receptor with varying affinities and potencies, and even with differential signalling responses. GLP-1 secretion occurs in response to nutrients (particularly carbohydrate and protein) reaching the distal bowel where the L cells reside.10
GLP-1 receptor
The GLP-1 receptor is a member of a small group of GPCRs (class B1) that includes receptors for GIP, glucagon, GLP-2, secretin, vasoactive intestinal polypeptide, pituitary adenylate cyclase-activating polypeptide, calcitonin, calcitonin gene-related peptide, parathyroid hormone, corticotrophin-releasing factor and growth hormone-releasing hormone.11 Many of these are believed to be potentially important drug targets. Of note, in addition to the GLP-1 receptor, the first three listed members of this receptor family have also been implicated as targets for possible treatments for obesity and diabetes. Although the class B1 GPCRs are predicted to possess seven transmembrane helical segments and general topology similar to the extensively studied class A GPCRs, they lack the signature sequences typical of the class A receptors and, based on sequence analysis, have been predicted to exhibit substantial differences in the orientation and packing of their helical bundle.12, 13 Unfortunately, this makes it unclear as to how useful the high-resolution crystal structures that have recently been solved for class A GPCRs12 might be as templates for the class B receptors.
A major advance in our understanding of the class B1 GPCRs has come from the recent structural characterization of their extracellular amino-terminal domain, known to be important for natural ligand binding.13 This domain is typically more than 100 residues in length and includes six highly conserved cysteines that are involved in three intradomain disulfide bonds.14 Several members of this family have had the structures of their isolated amino-terminal domains solved using nuclear magnetic resonance and x-ray crystallography, with a number of such structures also including a bound ligand.13 This has revealed a characteristic folding pattern including two anti-parallel beta sheet regions, three disulfide bonds, several loops and less consistently an amino-terminal alpha helix.13 The ligands occupy a cleft above the stable core and between the helix and loop regions. It is noteworthy that all the ligand-receptor amino terminus structures show this portion of the bound ligands in a helical conformation as docked.13 Although the patterns and positions of the bound ligands have been generally consistent, there have been differences among the members of this family.13
The GLP-1 receptor is expressed on a wide variety of cells. This includes beta, alpha and delta cells of the pancreatic islets, heart, lung, kidney, stomach, small bowel, skin and nerves in the peripheral and central nervous systems.11 There are also responses to GLP-1 in liver, fat and muscle that may be mediated indirectly, rather than by direct binding to GLP-1 receptors on those tissues. Major actions of GLP-1 on these targets include pancreatic effects to increase insulin secretion, increase insulin biosynthesis, increase beta cell proliferation, reduce beta cell apoptosis and reduce glucagon secretion. Other effects that also are relevant to diabetes and weight control include reduced gastric emptying, reduced appetite and the likely indirect effects to reduce glucose production in liver and to increase glucose uptake and storage in muscle and fat. On the basis of this spectrum of actions, the potential value of a GLP-1 receptor agonist in the management of type-2 diabetes mellitus, and even potentially in the setting of obesity, becomes clear. It is important to understand that the satiety effect of GLP-1 requires a higher concentration of this hormone than its effect on insulin. Major advantages of GLP-1 agonists include the glucose-dependent effects on the beta cell, making unlikely the over-stimulation or inappropriate secretion of insulin that could lead to dangerous hypoglycaemia. In addition, in contrast to many agents that reduce blood glucose, GLP-1 does not lead to weight gain, and it may actually contribute to weight reduction. There was a recent report suggesting that GLP-1 could also change food preferences, reducing the craving for sweets, another possible advantage for GLP-1 agonists.15 Other actions of this hormone include cardioprotective and neuroprotective effects.
Peptide drug development
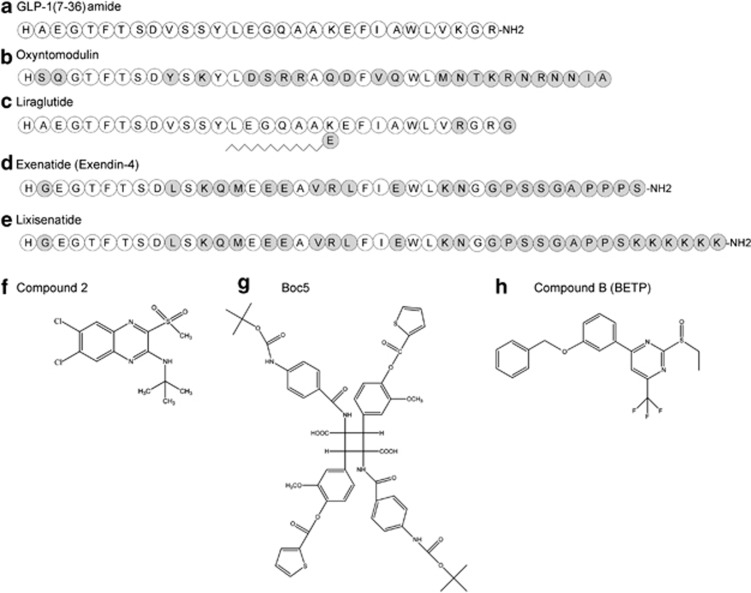
The use of peptides as therapeutic agents is increasingly common (Figure 1). Although this often carries the requirement for parenteral administration, owing to proteolysis of peptides in the gastrointestinal tract and the inability for intact molecule absorption, their specificity and high-affinity interaction with their receptors often overcome this inconvenience. Obviously, the use of the natural peptide ligand brings with it millions of years of evolutionary selection. However, similar to GLP-1 peptides, these often also bring with them endogenous regulatory mechanisms that might limit their action. GLP-1 has a half-life in the circulation of less than 2 minutes, reflecting both proteolytic cleavage by dipeptidyl peptidase-IV (DPP-4) to yield an inactive product and renal clearance.
Figure 1.
Structures of representative GLP-1 receptor agonist ligands. Shown are the primary amino-acid sequences of natural GLP-1 (a), oxyntomodulin (b), liraglutide (c), exenatide (d) and lixisenatide (e). Residues identical to those in GLP-1 are shown in white circles, whereas residues that are different from the natural residues are illustrated in grey circles. Shown also are the chemical structures of three non-peptidyl small-molecule agonist ligands, Compound 2 (ref 28) (f), Boc5 (ref 26) (g) and Compound B29 (h).
This problem was partially overcome by use of a peptide (exendin-4(1-39) or exenatide) discovered in the saliva of the Gila monster that has 53% homology with GLP-1 and is resistant to DPP-4 cleavage.16 Exendin-4(1-39) is a potent full agonist at the GLP-1 receptor that binds with similar affinity to that of GLP-1.17 Its longer plasma half-life (2.4 h) has allowed this peptide to be administered twice a day subcutaneously, although another challenge is the antigenicity of a peptide that is different from normal human GLP-1. The addition of this agent to metformin and a sulfonylurea has been shown to reduce hemoglobin A1c (HbA1c) levels by 0.8% over 30 weeks, as well as leading to weight reduction, whereas the control group increased their HbA1c levels.18 Another study documented the durability of this response over three years.19 Additional benefits to diabetic control and weight loss in these studies included improvements in the serum lipid profile and in blood pressure. The most common side effect was nausea, but this typically improved over time.
Another relatively short-acting GLP-1 receptor agonist, lixisenatide,20 a modified exendin-4(1-39) in which the carboxyl-terminal Pro-Ser dipeptide is removed and replaced with Ser and six Lys residues, is also resistant to DPP-4 degradation and has a half-life of 3–4 hours. The slightly longer half-life allows this drug to be administered once a day, but it still behaves much like exenatide. Both of these relatively short-acting GLP-1-like peptides have their major effects through the slowing of gastric emptying and the reduction in postprandial peaks in glucose delivery and absorption.
Other longer-acting GLP-1-like peptide agonists have been developed, with these having greater effects on insulin secretion, acting through supraphysiological activation of the GLP-1 receptor owing to sustained levels of the drug maintained in the circulation throughout the day. The first of these drugs to be approved was liraglutide, a synthetic analogue of the natural hormone that included changes to reduce DPP-4 cleavage, as well as the addition of a palmitate fatty acid with a gamma-glutamic acid linker to encourage binding to albumin, thus increasing the half-life to 13 h and allowing once-daily administration.21 This agent appears to be more effective in reducing HbA1c than exenatide and it may have less nausea associated with it.21 The 3.0 mg dose of liraglutide has been shown to be better than diet, orlistat or lower doses of liraglutide, resulting in a 10-kg weight loss that was maintained over 2 years.22 Of note, if an 800-kcal diet was initiated at the beginning of the study, this was able to yield a 20-kg weight reduction, competing well with bariatric surgery. This yielded resolution of diabetes mellitus and its cardiovascular effects, while the only significant side effect was nausea, with that resolving in all but about 10% of patients over time. Of note, although the gastric emptying effect of this agent seems to exhibit desensitization, the beneficial effects on serum glucose persist.
Even longer-acting GLP-1-like peptide agonists have also been developed. The strategies to achieve this include the covalent bonding of two molecules of GLP-17, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 having an Ala8-to-Gly modification to albumin (albiglutide), the conjugation of the peptide to an Fc fragment of IgG (dulaglutide), creative formulation such as the addition of zinc to delay absorption (taspoglutide—withdrawn because of hypersensitivity reactions) and the use of poly(lactic-co-glycolic) microspheres for protracted release (exenatide-LAR).23 It is not yet clear whether these will exhibit adequate advantages over the daily forms of this hormone that are now in extensive use. In one study, the control of diabetes was good, but this yielded less weight loss than a shorter-acting agonist such as liraglutide.24
As a class, the GLP-1 agonists all seem to exhibit some level of nausea, probably related most to its effect to slow gastric emptying. When this action is downregulated, particularly in the long-acting forms of GLP-1 agonists, the nausea frequently improves. Other potential complications that have been raised include pancreatitis, trophic effects on cells such as the thyroid C cells and possibly even pancreatic carcinoma.24, 25 Isolated case reports have noted pancreatitis and pancreatic cancer, but the incidence of these problems may reflect the underlying diabetes mellitus, rather than the treatment with GLP-1 agonists. The concern about thyroid C-cell hyperplasia and medullary thyroid cancer reflects observations in rodents, and it has not been observed during human administration. All of these potential problems are being carefully monitored, but they have not been confirmed after human administration of current agents on a larger scale or in epidemiological studies.
DPP-4 inhibitors (such as gliptin, sitagliptin and vildagliptin) have also been used to reduce the cleavage of endogenous GLP-1; however, this enzyme also degrades other natural bioactive peptides and proteins as well. Some of these targets appear to be involved in immune regulation, providing still another concern. In addition, in clinical trials, these agents have not induced the weight reduction that direct administration of GLP-1 receptor agonists has displayed.
Another approach to the GLP-1 receptor that is relevant to the management of obesity, owing to its effects to suppress food intake and to increase energy expenditure but not directly to the control of diabetes, is the use of another secretory product from the intestinal L cells, oxyntomodulin.25 This peptide has agonist actions at both the GLP-1 receptor and the glucagon receptor. It is a less potent agonist at both of these receptors than their natural peptide ligands, GLP-1 and glucagon, respectively. Again, the natural peptide has limited usefulness because of its very short half-life, being degraded by both DPP-4 and neutral endopeptidase 24.11 (refs 25,26). Similar strategies to those described above for GLP-1 analogues have been used to improve plasma half-life, including protease-resistant analogues and conjugation with lipid or polyethylene glycol. Although such agents have not yet been used clinically, they are in development as possible agents for the management of obesity.
Development of small-molecule agonists
As noted above, orally active small-molecule agonists could have the advantage of being more comfortable to administer and practical to use, thus ensuring better compliance. However, such agents have been very difficult to develop for this receptor family. The reasons for this difficulty are not yet apparent, but may relate to differences in the structures of the helical bundle domains of the class B1 GPCRs from the well-characterized structures of the class A GPCRs. Challenges probably relate to not yet having (i) optimal lattices for such drugs, (ii) their mechanisms of binding clearly established and (iii) their target structure well defined. Small-molecule agonists have recently been described.26, 27, 28, 29, 30 Substituted quinoxalines (‘compound 2' has been extensively used and represents N-tert-butyl-6,7-dichloro-3-methylsulfonyl-quinoxalin-2-amine, whereas ‘compound 1' represents 2-[6,7-dichloro-3-(trifluoromethyl)quinoxalin-2-yl]sulfanyl-5-methyl-1,3,4-thiadiazole) appear to represent allosteric agonists, acting both as allosteric enhancers of GLP-1 binding and action, as well as having intrinsic agonist activity.27, 28 The lead compound in that series (compound 2), however, had substantial supramaximal inhibitory activity, making administration of the correct and optimal dose quite challenging. Other compounds in that series do not have this action and might well be useful. Substituted cyclobutanes (‘Boc5' represents 1,3-bis [[4-tert-butoxy-cabonlyamino]benzoyl]amino)-2,4-bis [3-methoxy-4-(thiophene-2-carbonyloxy)phenyl]cyclobutane-1,3-dicarboxylic acid) have also been reported, probably representing orthosteric partial agonist ligands with their action competitively blocked by exendin-4.26, 30 The physicochemical characteristics of this compound and related compounds in this series will probably not be compatible with oral administration. Another promising lead for a small-molecule GLP-1 receptor agonist is ‘Compound B' or BETP, representing 4-(3-(benzyloxy)phenyl)-2-(ethylsulfinyl)-6-(trifluoromethyl)pyrimidine.29 This compound is known to act allosterically, on the basis of its action at the amino-terminally truncated GLP-1 receptor construct. A number of other chemistries, including phenylalanine derivatives, azoanthracenes, pyrazoles, flavinoids and imidazopyridines, have also been described in preliminary form,31 but have not yet been adequately characterized to understand how promising they might be.
Ligand bias
It is becoming clear that drugs can not only mimic or inhibit the signalling characteristics of a natural agonist at its receptor that can often involve activation of multiple pathways, but can also exhibit a subset of those activities, or an alternate profile of activities. This is known as ligand bias, when a ligand might exhibit the G-protein-mediated activities, but not the arrestin-mediated activities (or vice versa), probably by stabilizing slightly distinct molecular conformations. More subtle bias can also be expressed where ligand activation of one or more of the signalling pathways, such as stimulation of cAMP, intracellular calcium and pERK1/2, occurs with different relative potency and/or efficacy from that of the natural agonist. Indeed, such ligand bias has been reported for the GLP-1 receptor.32, 33, 34 It has been proposed that some allosteric ligands can also modulate the activity of different peptide ligands in a selective or differential manner.34
Receptor dimerization
Another feature of the GLP-1 receptor, similar to many members of this family, is the tendency for this receptor to associate within the lipid bilayer with other GLP-1 receptors and perhaps with other receptors in this family in a ligand-independent manner.35 This receptor is believed to have a propensity to form homodimeric complexes.35 Similar to the secretin receptor,36 these complexes contribute towards the high-affinity state of the receptor and are important in mediating negative cooperativity of binding. Dimerization of the GLP-1 receptor also appears to contribute to signalling efficacy, while it has recently been demonstrated that allosteric small-molecule ligands of the GLP-1 receptor act via the same protomer (cis-acting agent) as the natural ligand that binds with high affinity.35 It is not yet known the extent to which clinically developed ligands might affect and be affected by the state of dimerization of this receptor.
Conclusions
There are compelling data to support the usefulness of GLP-1 receptor agonists in the safe and effective management of type-2 diabetes mellitus. Although the most useful such agents currently approved and in clinical use are peptides that are structurally related to the natural agonist, small-molecule agonists are also being developed. The relative advantages and differences between these agents are being actively examined.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (DK046577). PS has received an NHMRC Program grant (#519461) and an NHMRC Project grant (#1002180).
The authors declare no conflict of interest.
Footnotes
This article is published as part of a supplement sponsored by the Université Laval's Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments, and prevention of obesity.
References
- Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011; 34: 1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt W. The incretin concept today. Diabetologia 1979; 16: 75–85. [DOI] [PubMed] [Google Scholar]
- Elrick H, Stimmler L, Hlad Jr CJ, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab 1964; 24: 1076–1082. [DOI] [PubMed] [Google Scholar]
- Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia 1986; 29: 46–52. [DOI] [PubMed] [Google Scholar]
- Adelhorst K, Hedegaard BB, Knudsen LB, Kirk O. Structure-activity studies of glucagon-like peptide-1. J Biol Chem 1994; 269: 6275–6278. [PubMed] [Google Scholar]
- Neidigh JW, Fesinmeyer RM, Prickett KS, Andersen NH. Exendin-4 and glucagon-like-peptide-1: NMR structural comparisons in the solution and micelle-associated states. Biochemistry 2001; 40: 13188–13200. [DOI] [PubMed] [Google Scholar]
- Hoare SR. Mechanisms of peptide and nonpeptide ligand binding to class B G-protein-coupled receptors. Drug Discov Today 2005; 10: 417–427. [DOI] [PubMed] [Google Scholar]
- Dong M, Lam PC, Pinon DI, Hosohata K, Orry A, Sexton PM et al. Molecular basis of secretin docking to its intact receptor using multiple photolabile probes distributed throughout the pharmacophore. J Biol Chem 2011; 286: 23888–23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JW, Saunders GF. Structure of the human glucagon gene. Nucleic Acids Res 1986; 14: 4719–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen K, Christensen LL, Holst JJ, Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul Pept 2003; 114: 189–196. [DOI] [PubMed] [Google Scholar]
- Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B et al. International union of pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev 2003; 55: 167–194. [DOI] [PubMed] [Google Scholar]
- Shoichet BK, Kobilka BK. Structure-based drug screening for G-protein-coupled receptors. Trends Pharmacol Sci 2012; 33: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthier C, Reedtz-Runge S, Rudolph R, Stubbs MT. Passing the baton in class B GPCRs: peptide hormone activation via helix induction? Trends Biochem Sci 2009; 34: 303–310. [DOI] [PubMed] [Google Scholar]
- Lisenbee CS, Dong M, Miller LJ. Paired cysteine mutagenesis to establish the pattern of disulfide bonds in the functional intact secretin receptor. J Biol Chem 2005; 280: 12330–12338. [DOI] [PubMed] [Google Scholar]
- Miras AD, Jackson RN, Jackson SN, Goldstone AP, Olbers T, Hackenberg T et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr 2012; 96: 467–473. [DOI] [PubMed] [Google Scholar]
- Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem 1992; 267: 7402–7405. [PubMed] [Google Scholar]
- Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol 2012; 166: 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005; 28: 1083–1091. [DOI] [PubMed] [Google Scholar]
- Buse JB, Klonoff DC, Nielsen LL, Guan X, Bowlus CL, Holcombe JH et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: an interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007; 29: 139–153. [DOI] [PubMed] [Google Scholar]
- Christensen M, Knop FK, Holst JJ, Vilsboll T. Lixisenatide, a novel GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus. IDrugs 2009; 12: 503–513. [PubMed] [Google Scholar]
- Knudsen LB. Liraglutide: the therapeutic promise from animal models. Int J Clin Pract Suppl 2010; 64: 4–11. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Meier JJ. Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regul Pept 2005; 128: 135–148. [DOI] [PubMed] [Google Scholar]
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 728–742. [DOI] [PubMed] [Google Scholar]
- Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia 2006; 49: 2564–2571. [DOI] [PubMed] [Google Scholar]
- Baggio LL, Huang Q, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology 2004; 127: 546–558. [DOI] [PubMed] [Google Scholar]
- Chen D, Liao J, Li N, Zhou C, Liu Q, Wang G et al. A nonpeptidic agonist of glucagon-like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci USA 2007; 104: 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin N, Flatt PR, Patterson S, Green BD. Insulin-releasing and metabolic effects of small molecule GLP-1 receptor agonist 6,7-dichloro-2-methylsulfonyl-3-N-tert-butylaminoquinoxaline. Eur J Pharmacol 2010; 628: 268–273. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Kiel D, Teng M, Behrens C, Bhumralkar D, Kodra JT et al. Small-molecule agonists for the glucagon-like peptide 1 receptor. Proc Natl Acad Sci USA 2007; 104: 937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloop KW, Willard FS, Brenner MB, Ficorilli J, Valasek K, Showalter AD et al. Novel small molecule glucagon-like peptide-1 receptor agonist stimulates insulin secretion in rodents and from human islets. Diabetes 2010; 59: 3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, He M, Li H, Liu Q, Wang J, Wang Y et al. Boc5, a non-peptidic glucagon-like Peptide-1 receptor agonist, invokes sustained glycemic control and weight loss in diabetic mice. PLoS One 2008; 3: e2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard FS, Bueno AB, Sloop KW. Small molecule drug discovery at the glucagon-like peptide-1 receptor. Exp Diabetes Res 2012; 2012: 709893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole C, Wootten D, Simms J, Miller LJ, Christopoulos A, Sexton PM. Second extracellular loop of human glucagon-like peptide-1 receptor (GLP-1R) has a critical role in GLP-1 peptide binding and receptor activation. J Biol Chem 2012; 287: 3642–3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole C, Wootten D, Simms J, Savage EE, Miller LJ, Christopoulos A et al. Second extracellular loop of human glucagon-like peptide-1 receptor (GLP-1R) differentially regulates orthosteric but not allosteric agonist binding and function. J Biol Chem 2012; 287: 3659–3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole C, Wootten D, Simms J, Valant C, Sridhar R, Woodman OL et al. Allosteric ligands of the glucagon-like peptide 1 receptor (GLP-1R) differentially modulate endogenous and exogenous peptide responses in a pathway-selective manner: implications for drug screening. Mol Pharmacol 2010; 78: 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Wootten D, Pinon DI, Koole C, Ball AM, Furness SGB et al. Glucagon-like peptide-1 receptor dimerization differentially regulates agonist signalling but does not affect small molecule allostery. Proc Natl Acad Sci USA 2012; 109: 18607–18612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Harikumar KG, Dong M, Lam PC, Sexton PM, Christopoulos A et al. Functional importance of a structurally distinct homodimeric complex of the family B G protein- coupled secretin receptor. Mol Pharmacol 2009; 76: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]