Abstract
The catecholamines and the adrenergic receptors have been long known to be vital components in the regulation of fat cell metabolism. Whether in response to stress, cold temperature or diet, the β-adrenergic receptors (βARs) respond to epinephrine/norepinephrine to activate a signalling cascade that drives triglyceride hydrolysis to free fatty acids for use as fuel for skeletal and cardiac muscle work. The βARs also are well-established activators of brown fat for the conversion of substrate energy to generate heat from the oxidation of glucose and fatty acids. Long thought to be irrelevant to the biology of adult humans, the realization that there is indeed functional brown fat in humans has now created great interest and enthusiasm over the possibility that recruiting brown fat to target obesity and metabolic disease could represent a viable therapeutic option. Coupled with newer evidence that various stimuli independent of the βARs may also be able to increase active brown adipocytes, including the cardiac natriuretic peptides, it is an exciting time to be working in this area. This review will focus on the catecholamines and natriuretic peptides as cooperative actors in promoting fat metabolism, and will consider areas in need of further research.
Keywords: adipose, adrenergic receptors, brown fat, cyclic nucleotides, natriuretic peptides, uncoupling
The adrenergic system helps us respond to environmental stresses (Table 1). For humans during the ‘cave-days', when on the run from the saber-toothed tiger, the activities of the β-adrenergic receptors (βARs) within tissues increased cardiac output and mobilized fuel from the liver and adipose tissue so that skeletal muscles and heart could use this metabolic fuel in order to whisk them away from danger. Humans of the cave-days also had to be able to handle variations in temperature, particularly how to cope with cold temperature. Again, the adrenergic system helps promote muscle shivering and the mobilization of metabolic fuel for this energetically expensive (and uncomfortable) process. Importantly, activating the βARs in adipocytes can not only stimulate lipolysis of stored triglycerides but can also ramp up the process of non-shivering thermogenesis in brown adipocytes. As will be discussed, the links between the heart and adipose tissue to regulate whole-body fuel metabolism include the obvious role of the sympathetic nervous system (SNS) shown in Table 1, but also consist of direct hormonal ‘communication' between these organs.
Table 1. The adrenergic system helps us cope with environmental challenges such are predators and cold temperature.
| Predators | Cold temperatures |
|---|---|
| Increase cardiac output | Increase muscle shivering for heat |
| Mobilize fuel from liver and adipose tissue | Mobilize fuel from liver and adipose tissue |
| Vasodilatation: deliver fuel to skeletal (and cardiac) muscle and promote oxidation | Vasodilatation: deliver fuel to skeletal (and cardiac) muscle and promote oxidation |
| Activate and expand the capacity for non-shivering thermogenesis in brown adipocytes |
The adipose organ consists of many different depots, located in subcutaneous and intra-abdominal and intra-thoracic areas. The purpose of these adipose depots is to store excess food energy in times of plenty, as well as to secrete hormones that inform other organs—including the brain—on the status of such energy reserves. In addition, the purpose of brown adipocytes—‘brown' because they have a very high mitochondrial content—is to generate heat from this stored food energy. Heat is created by the regulated passage of protons across the mitochondrial inner membrane through the unique brown adipocyte protein UCP1, further necessitating the import and oxidation of glucose and fatty acids. To maintain sufficient ATP generation in the brown adipocyte, the cell must import glucose and fatty acids for oxidation, resulting in net energy consumption. Although it is considered likely that early humans living in temperate climates used brown adipose tissue as one mechanism by which to keep warm, during the past many decades it has been largely assumed that modern adult humans do not possess brown adipocytes. There is general agreement that brown adipose tissue exists in newborn infants;1 it has been by and large considered to be a vestigial tissue that disappears soon after birth. Owing to the radiology literature, we now know that adult humans without a doubt contain functionally active brown adipocytes (see refs 2,3 and references within). The clinical interest surrounding the existence of brown adipocytes in adult humans stems from numerous studies in laboratory animals that have consistently shown an inverse correlation between the amounts of brown adipocytes—particularly those within white fat depots—and resistance to obesity and metabolic disease.4, 5, 6, 7 Thus, there is renewed interest in ‘brown fat' and non-shivering thermogenesis in adult humans as a possible means to counteract the epidemics of obesity and metabolic syndrome. This idea is supported by recent reports that these pockets of brown adipocytes exist in adult humans and can respond to cold stimuli by increasing their uptake and utilization of metabolic fuels,8 and that activated brown fat in subjects is inversely correlated with body mass index (BMI) and percent body fat.9, 10, 11
Links between the heart and adipose tissue to regulate whole-body metabolism not only include the obvious role of the SNS but have also been shown to include direct hormonal ‘communication' from the heart to adipose tissue, through the cardiac natriuretic peptides. These peptides, atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP), were originally discovered as factors derived from cardiac extracts that could lower blood pressure by stimulating renal sodium and water excretion. The originally understood purpose of these peptides was to protect the heart from the physical trauma of elevated blood pressure. For example, frequent increases in blood pressure can have a negative impact on the integrity and function of cardiac muscle, eventually leading to severely impaired cardiac output. To ameliorate elevated blood pressure, ANP and BNP are released to reflexively reduce blood volume and hence lower this pressure.
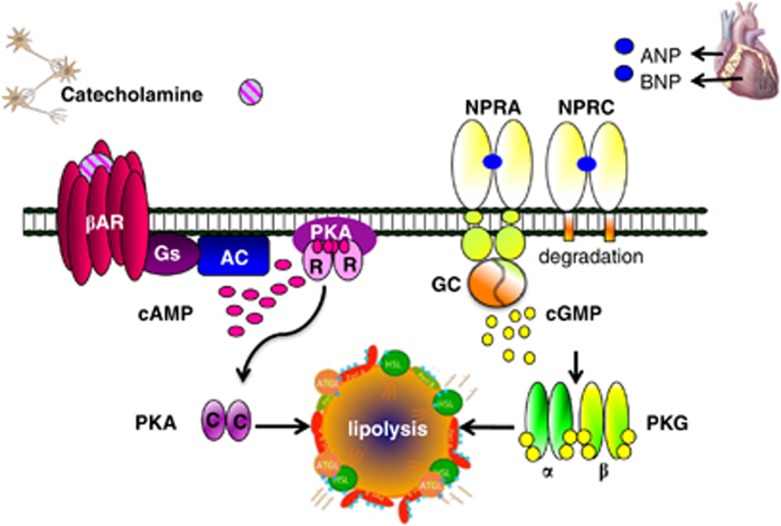
Evidence that ANP and BNP have metabolic actions beyond their ability to regulate blood volume via diuresis/natriuresis has been slowly accumulating. Two decades ago, Sarzani et al.12 observed that the receptors for ANP/BNP—NPRA and NPRC—were expressed in adipose tissue of rats and humans.13 As shown in the upper right portion of Figure 1, NPRA is the ‘signalling' receptor, which contains an intracellular guanylyl cyclase domain that is formed from its homodimeric structure. NPRC is the ‘clearance' receptor. It binds and internalizes the natriuretic peptdies (NPs), removing them from circulation and promoting their degradation (see review by Potter14). A link between the NP receptors and obesity was made when it was observed that obese humans express significantly more of the NPRC clearance receptor in their adipose tissue, as well as reduced circulating NPs and biological efficacy for blood pressure control.15, 16, 17, 18 These studies led to a suggestion that adipose tissue might be a site of natriuretic peptide uptake and degradation in obese subjects. Indeed, obesity and hypertension have been known for many years to be closely associated.19, 20, 21
Figure 1.
The adipocyte expresses the three βAR subtypes. Their activation by catecholamines leads to protein kinase A (PKA)-dependent lipolysis of stored triglycerides. The natriuretic peptide receptors NPRA and NPRC are also in the adipocyte, and the cardiac hormones ANP and BNP can increase lipolysis through a parallel pathway using NPRA and protein kinase G (PKG). Republished from Bordicchia et al.,24 with the permission from the American Society for Clinical Investigation.
Work over the past decade has shown that ANP and BNP can stimulate lipolysis in cultured human adipocytes22, 23 with potency similar to catecholamines (as illustrated in Figure 1). In addition to white adipocyte lipolysis, our recent findings also point to a role for ANP and BNP in brown adipocytes to increase mitochondrial density and thermogenesis via uncoupled mitochondrial respiration.24 NPs stimulate NPRA to activate a signalling pathway through PKG that is parallel to the activation of βAR and PKA. Interestingly, PKG and PKA drive downstream pathways that both converge at p38α MAPK. We previously showed that p38α was a key factor for activating the transcription factors and co-regulators that increase transactivation of the Ucp1 and Pgc-1α genes to propel the process of mitochondrial biogenesis and the metabolic events to increase uncoupled respiration in brown/beige/brite adipocytes and energy expenditure.25, 26
The ability of the adipocyte to respond to catecholamines and natriuretic peptides can be greatly affected by the levels of their respective receptors. Obesity can be accompanied by significant decreases in the expression of βAR subtypes27, 28, 29, 30, 31 and also in the relative ratio of the natriuretic peptide receptors. As already noted, increases in the levels of NPRC have been observed in obese human subjects.15 In rodents, obesity induced by high-fat feeding can shift the relative expression of NPRA and NPRC in adipose tissue, reducing the ability to activate NPRA and to favour clearance by NPRC (Figure 2). Conversely, fasting has been associated with a reduction in the expression of NPRC.32 An interesting finding came from studies in mice placed at 5 °C, a manoeuver that is well known to produce robust increases in sympathetic nerve activity to adipose tissues. The cold-challenged mice exhibited a shift in their relative expression of NPRA and NPRC in white and brown adipose tissues as to now favour NP activation of metabolism, and this was accompanied by increases in circulating BNP levels and ANP and BNP gene expressions in the heart (24 and unpublished observations). These findings, together with the earlier studies in humans and mice, indicate that the expression of the NP receptors is dynamically regulated. Although there has been some work in rodents and kidney cell models on the expression of NPRA,33 it is limited and there is essentially nothing known about the transcriptional control of NPRC.
Figure 2.
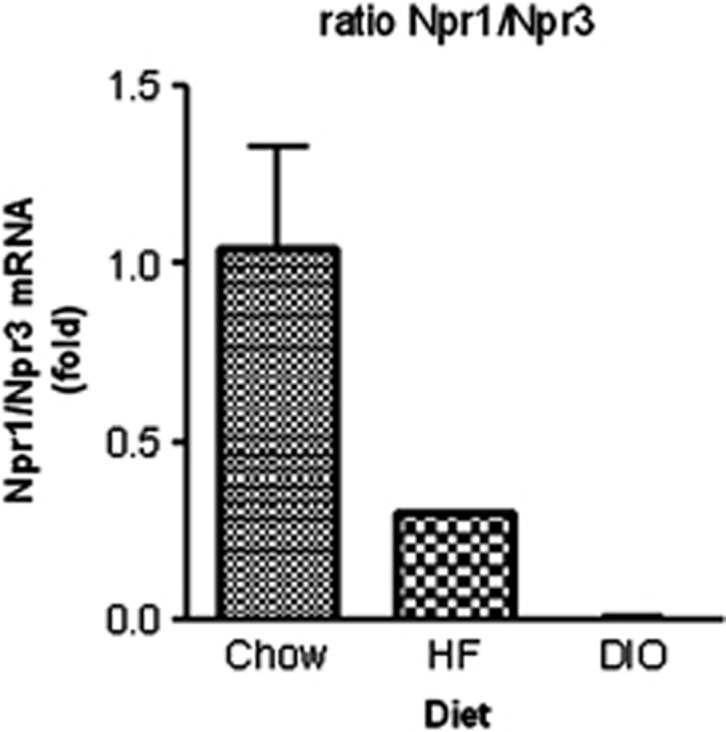
Reciprocal changes in the expression of NPRA (also referred to as Npr1) and NPRC (also referred to as Npr3) in adipose tissue in response to high-fat diet feeding. C57BL/6J male mice were fed either a standard chow diet 8–10% of calories from fat;HF=high-fat diet (60% calories from fat) for 3 weeks (HF), or for 12 weeks, resulting in diet-induced obesity (DIO). White adipose tissue (inguinal) was harvested and analysed for Npr1 and Npr3 transcripts. Ratio of Npr1/Npr3 mRNA. All values are presented relative to the chow control.
This is an obvious area in need of further study and, given our newer understanding of the roles of the NPs in energy partitioning, it is particularly important to define the regulation of NPRA and NPRC expression in metabolic tissues such as adipose, skeletal muscle and heart.
When considering approaches to study the natriuretic peptide receptor system in metabolic disease, it is always important to recognize the similarities and differences between humans and laboratory models. In the case of the natriuretic peptide system, there are significant differences, especially in terms of the receptors. In the first early reports of the ability of NPs to stimulate lipolysis in human adipocytes, it was concluded that this biology was exclusively a primate signalling mechanism34 because it was not detected in mice. This turns out to be largely owing to the fact that the expression of NPRC in rodent adipocytes is massively higher than in humans (http://biogps.org/goto=genereport&id=4883). The importance of the NPRA/NPRC ratio in adipose tissue in mice for determining the response to NPs is underscored by observations in mice with targeted deletions of NPRA and NPRC.35, 36, 37 Mice lacking NPRA are more obese, whereas mice lacking NPRC are very lean, with increased ‘browning' of adipocytes, and they are resistant to diet-induced obesity.24 The role of NPs to increase metabolism may be not only owing to increased uncoupled respiration in brown adipocytes but from other tissues as well. For example, it was recently reported that human skeletal muscle myocytes show increased expression of NPRA with exercise, and treatment of these myocytes with BNP can modestly but significantly increase the expression of mitochondrial protein markers, as well as oxygen consumption.38 Tissue-specific deletions of NPRC in the mice will be valuable in understanding this biology, as well as the regulation of these receptors. Nevertheless, these findings also underscore the fact that the human is the best ‘model organism', whereas the mouse is useful only under defined circumstances.
Efforts in the laboratory are now underway to determine whether manipulating the natriuretic peptide system is a potential target for treating metabolic disease. If increases in both ‘browning' of adipocytes and skeletal muscle fatty acid oxidation can be enhanced through some forms of selective NPRA activation, might we be able to promote energy expenditure and weight loss/maintenance? Given the correlations between obesity and hypertension, if there are reductions in blood pressure, this ‘side effect' might be quite acceptable. Until then, we still have many questions to answer and experiments to perform.
SC has received consulting fees from Merck and Astra-Zeneca and received grant support through the Novo Nordisk Diabetes Innovation Award. The remaining authors declare no conflict of interest.
Footnotes
This article is published as part of a supplement sponsored by the Université Laval's Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments, and prevention of obesity.
References
- Aherne W, Hull D. The site of heat production in the newborn infant. Proc R Soc Med 1964; 57: 1172–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 2007; 293: E444–E452. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Three years with adult human brown adipose tissue. Ann NY Acad Sci 2010; 1212: E20–E36. [DOI] [PubMed] [Google Scholar]
- Collins S, Petro AE, Surwit RS. Strain-specific response to β3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology 1997; 138: 405–413. [DOI] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 1998; 102: 412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes 2004; 53: 3274–3285. [DOI] [PubMed] [Google Scholar]
- Auffret J, Viengchareun S, Carre N, Denis RG, Magnan C, Marie PY et al. Beige differentiation of adipose depots in mice lacking prolactin receptor protects against high-fat-diet-induced obesity. FASEB J 2012; 26: 3728–3737. [DOI] [PubMed] [Google Scholar]
- Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 2012; 122: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009; 58: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med 2009; 360: 1500–1508. [DOI] [PubMed] [Google Scholar]
- Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab 2012; 97: E1229–E1233. [DOI] [PubMed] [Google Scholar]
- Sarzani R, Paci VM, Dessi-Fulgheri P, Espinosa E, Rappelli A. Comparative analysis of atrial natriuretic peptide receptor expression in rat tissues. J Hypertens Suppl 1993; 11: S214–S215. [PubMed] [Google Scholar]
- Sarzani R, Dessi-Fulgheri P, Paci VM, Espinosa E, Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest 1996; 19: 581–585. [DOI] [PubMed] [Google Scholar]
- Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J 2011; 278: 1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessi-Fulgheri P, Sarzani R, Tamburrini P, Moraca A, Espinosa E, Cola G et al. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens 1997; 15(12 Part 2): 1695–1699. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Wilson PW et al. Impact of obesity on plasma natriuretic peptide levels. Circulation 2004; 109: 594–600. [DOI] [PubMed] [Google Scholar]
- Khan AM, Cheng S, Magnusson M, Larson MG, Newton-Cheh C, McCabe EL et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab 2011; 96: 3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisawa T, Kishimoto I, Kokubo Y, Makino H, Miyamoto Y, Yoshimasa Y. Association of plasma B-type natriuretic peptide levels with obesity in a general urban Japanese population: the Suita Study. Endocr J 2010; 57: 727–733. [DOI] [PubMed] [Google Scholar]
- Modan M, Halkin H, Almog S, Lusky A, Eshkol A, Shefi M et al. Hyperinsulinemia. A link between hypertension obesity and glucose intolerance. J Clin Invest 1985; 75: 809–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med 1986; 61: 1081–1090. [PubMed] [Google Scholar]
- Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev 2012; 17: 81–96. [DOI] [PubMed] [Google Scholar]
- Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J 2000; 14: 1345–1351. [PubMed] [Google Scholar]
- Galitzky J, Sengenes C, Thalamas C, Marques MA, Senard JM, Lafontan M et al. The lipid-mobilizing effect of atrial natriuretic peptide is unrelated to sympathetic nervous system activation or obesity in young men. J Lipid Res 2001; 42: 536–544. [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012; 122: 1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Medvedev AV, Daniel KW, Collins S. Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein-1 (UCP1) gene requires p38 MAP kinase. J Biol Chem 2001; 276: 27077–27082. [DOI] [PubMed] [Google Scholar]
- Cao W, Robidoux J, Puigserver P, Daniel KW, Medvedev AV, Bai X et al. p38 MAP kinase is the central regulator of cAMP-dependent transcription of the brown fat uncoupling protein-1 gene. Mol Cell Biol 2004; 24: 3057–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynisdottir S, Wahrenberg H, Carlström K, Rössner S, Arner P. Catecholamine resistance in fat cells of women with upper-body obesity due to decreased expression of beta2-adrenoceptors. Diabetolgia 1994; 37: 428–435. [DOI] [PubMed] [Google Scholar]
- Reynisdottir S, Ellerfeldt K, Wahrenberg H, Lithell H, Arner P. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J Clin Invest 1994; 93: 2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW. Impaired expression and functional activity of the β3- and β1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Mol Endocrinol 1994; 8: 518–527. [DOI] [PubMed] [Google Scholar]
- Collins S, Daniel KW, Rohlfs EM. Depressed expression of adipocyte beta-adrenergic receptors is a common feature of congenital and diet-induced obesity in rodents. Int J Obes Relat Metab Disord 1999; 23: 669–677. [DOI] [PubMed] [Google Scholar]
- Soloveva V, Graves R, Rasenick M, Spiegelman B, Ross S. Transgenic mice overexpressing the β1-adrenergic adipose tissue are resistant to obesity. Mol Endocrinol 1997; 11: 27–38. [DOI] [PubMed] [Google Scholar]
- Sarzani R, Paci VM, Zingaretti CM, Pierleoni C, Cinti S, Cola G et al. Fasting inhibits natriuretic peptides clearance receptor expression in rat adipose tissue. J Hypertens 1995; 13: 1241–1246. [DOI] [PubMed] [Google Scholar]
- Garg R, Oliver PM, Maeda N, Pandey KN. Genomic structure, organization, and promoter region analysis of murine guanylyl cyclase/atrial natriuretic peptide receptor-A gene. Gene 2002; 291: 123–133. [DOI] [PubMed] [Google Scholar]
- Sengenes C, Zakaroff-Girard A, Moulin A, Berlan M, Bouloumie A, Lafontan M et al. Natriuretic peptide-dependent lipolysis in fat cells is a primate specificity. Am J Physiol Regul Integr Comp Physiol 2002; 283: R257–R265. [DOI] [PubMed] [Google Scholar]
- Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL et al. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA 1997; 94: 14730–14735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF et al. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci USA 1998; 95: 2547–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa N, Grzesik WJ, Takahashi N, Pandey KN, Pang S, Yamauchi M et al. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA 1999; 96: 7403–7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest 2012; 122: 4675–4679. [DOI] [PMC free article] [PubMed] [Google Scholar]