Abstract
Excessive consumption of high-energy, palatable food contributes to obesity, which results in the metabolic syndrome, heart disease, type-2 diabetes and death. Current knowledge on the function of the hypothalamus as the brain ‘feeding centre' recognizes this region as the main regulator of body weight in the central nervous system. Because of their intrinsically fast and adaptive activities, feeding-controlling neural circuitries are endowed with synaptic plasticity modulated by neurotransmitters and hormones that act at different hierarchical levels of integration. In the hypothalamus, among the chemical mediators involved in this integration, endocannabinoids (eCBs) are ideal candidates for the fast (that is, non-genomic), stress-related fine-tuning of neuronal functions. In this article, we overview the role of the eCB system (ECS) in the control of energy intake, and particularly in the consumption of high-energy, palatable food, and discuss how such a role is affected in the brain by changes in the levels of feeding-regulated hormones, such as the adipose tissue-derived anorexigenic mediator leptin, as well as by high-fat diets. The understanding of the molecular mechanisms underlying the neuronal control of feeding behaviours by eCBs offers many potential opportunities for novel therapeutic approaches against obesity. Highlights of the latest advances in the development of strategies that minimize central ECS overactivity in ‘western diet'-driven obesity are discussed.
Keywords: CB1, hypothalamus, leptin, high-fat diet, synaptic rewiring, endocannabinoids
Introduction
The hypothalamus is the most extensively interconnected area of the brain, and through its wide web of neural circuits it controls a variety of essential autonomic and somatomotor functions. Neuroanatomical studies have demonstrated direct projections to hypothalamic areas from several brain regions such as cortical/limbic areas and autonomic motor systems of the brainstem. Such extensive connectivity is thought to represent the anatomical basis supporting sleep–wake regulation, energy homeostasis and cognitive and reward-related functions.1 Hormonal and nutrient signals are processed in the hypothalamus and inform the brain about the free and stored levels of fuel available to the organism. In turn, the hypothalamic neuronal circuits use this information to regulate energy intake, energy expenditure and peripheral lipid and glucose metabolism.2 Because of their intrinsic functional activities, and the necessity to adapt to often significant changes in nutritional status, neural feeding circuitries are endowed with synaptic plasticity modulated by neurotransmitters and hormones that act at different hierarchical levels of integration.1 Among the chemical mediators involved in this integration, the endocannabinoids (eCBs) are the master regulators of the fast (that is, non-genomic) and stress-related fine-tuning of energy intake and processing. The eCB system (ECS) is composed of two Gi/o-protein-coupled ‘cannabinoid' CB1 and CB2 receptors, their lipid ligands (that is, the eCBs) and the enzymatic machinery for eCB synthesis and degradation. The most studied eCBs, N-arachidonoyl-ethanolamide (anandamide) and 2-arachidonoylglycerol (2-AG), are members of the fatty acid amide and monoacylglycerol families of neutral lipids, respectively, and are produced from cell membrane phospholipids following cell stimulation, before being released to target the cannabinoid receptors.3 The ECS regulates a wide variety of processes including pain, mood, reward, memory and, as mentioned above, appetite and energy metabolism. Within a given neuronal network, eCB ‘tone' is mostly the result of the regulation of eCB levels as determined by different, often concurring, enzymatic biosynthetic and catabolic cascades, which occur at the pre- and post-synaptic levels of brain circuitries.3, 4
Herein, we review some very recently emerged aspects of eCB control by, and of, leptin signalling and lipid and glucose intake and metabolism, and discuss the possible use of inhibitors of eCB biosynthesis as novel anti-obesity drugs.
New data on eCB action in the hypothalamus: leptin signalling and peripheral lipid and glucose intake and metabolism
During the past years, we and others have helped establish that the ECS has a pivotal role in the regulation of energy balance through interactions with the anorexigenic adipocyte-derived hormone leptin. In particular, it is known that leptin reduces hypothalamic eCB levels5 and attenuates eCB-mediated ‘retrograde' neuromodulatory actions at pre-synaptic CB1 receptors.4, 6, 7 Given the orexigenic effects of eCBs when infused into the hypothalamus,8 these actions of leptin likely contribute to the anorectic effects of the hormone. Accordingly, in the hypothalamus of obese and hyperphagic rodents lacking leptin, such as ob/ob mice, or with defective leptin signalling-that is, db/db mice and Zuckerfa/fa rats-eCB levels are significantly increased, whereas pharmacological or genetic blockade of eCB action at CB1 receptors reduces food intake and body weight in these rodents, and renders wild-type mice resistant to high-fat-diet (HFD)-induced obesity (DIO).5, 9 Furthermore, recent findings show that functional CB1 receptor signalling within the hypothalamus is required by leptin and ghrelin to exert their respective anorexigenic and orexigenic effects, as selective genetic knockout of CB1 receptors in the hypothalamus abolishes the anorectic action of leptin,10 whereas the orexigenic effects of hypothalamic ghrelin are absent in CB1 knockout mice.11
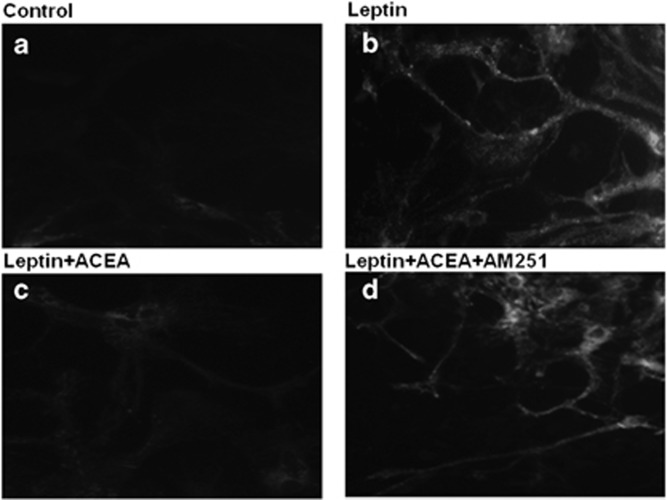
Although leptin controls eCB levels and signalling at CB1 receptors, CB1 receptor activation by eCBs may, in turn, control leptin signalling. Although CB1 activation in adipocytes and pre-junctional sympathetic fibres innervating the adipose tissue stimulates leptin biosynthesis and release, thus possibly contributing to engender leptin resistance,12 we have unpublished evidence that, in the hypothalamus, CB1 receptors may negatively control leptin action. In fact, it was recently shown that leptin might produce its anorexic effects via elevation of reactive oxygen species and subsequent pro-opiomelanocortin neuron activation and neuropeptide Y- and agouti-related peptide-co-producing neuron inhibition.13 We found that primary cultures of mouse arcuate nucleus neurons treated with this hormone produce reactive oxygen species and that this effect is prevented by a selective CB1 receptor agonist (arachidonylchloro ethanolamide) in a manner sensitive to a CB1 receptor antagonist/inverse agonist (AM251; Palomba and Di Marzo, unpublished data, Figure 1). Studies are ongoing to evaluate whether or not this effect occurs tonically in the hypothalamus, either in the early stages of food deprivation, to produce a negative feedback action on leptin-induced food intake inhibition, or in mice with DIO, where an upregulation of hypothalamic 2-AG levels is observed and contributes to preference for high fat (see below).
Figure 1.
Effect of a CB1 receptor agonist on leptin-induced reactive oxygen species formation in primary hypothalamic neurons. Dihydrorhodamine-loaded primary hypothalamic neurons isolated from postnatal day 1 C57BL/6J mice were treated with serum-free medium in the absence (b) or presence (c) of 0.5 μM arachidonylchloro ethanolamide with (d) or without (c) 1 μM AM251. After 30 min, the cells were exposed to 100 ng ml−1 leptin for an additional 30 min and then analysed with a fluorescence microscope. A representative micrograph of control cells is shown in (a). Scale bar: 20 m.
In agreement with their orexigenic action, and their levels being positively regulated by glucocorticoids and ghrelin, and negatively by leptin, hypothalamic eCB, mainly 2-AG, levels are increased after a brief period of food deprivation and then decreased after food consumption.14 On the other hand, rodents undergoing a prolonged food restriction exhibit decreased hypothalamic 2-AG levels,15 whereas DIO mice show instead increased 2-AG levels. This paradoxical deregulation of eCB signalling under these two extreme nutritional conditions can be interpreted, in the former case, as an adaptive response to better cope with the lack of food and to allow the utilization of reserve energy provided by fat, and, instead, as a maladaptive response in obesity, possibly caused by leptin resistance and overactivity of the hypothalamic–pituitary–adrenal axis, and contributing to unrestrained lipogenesis in adipocytes and hepatic glucose production and, eventually, insulin resistance. Indeed, hypothalamic CB1 receptors are now believed to control not only energy intake but also energy processing in the liver and adipose tissue, probably by modulating the activity of the sympathetic nervous system and of hypothalamic insulin. In fact, acute or chronic administration of CB1 receptor antagonists or inverse agonists, or conditional knockout of CB1 receptors in central and sympathetic neurons, increases sympathetic tone and insulin action in the mediobasal hypothalamus.16, 17, 18, 19 On the other hand, central infusion of CB1 agonists, or HFD consumption for a few days, leading to elevated hypothalamic eCB levels, reduces insulin effects in the mediobasal hypothalamus and subsequently disrupts hypothalamic insulin control over hepatic glucose production and adipose tissue lipolysis.19, 20 Perhaps even more importantly, conditional mutant mice lacking hypothalamic CB1 receptors under a normocaloric, standard diet show not only decreased body-weight gain over time but also elevated energy expenditure, as well as increased β3-adrenergic receptor and uncoupling protein-1 mRNA levels in the brown adipose tissue, in the absence of any effect on food intake.10 These effects are required to induce thermogenesis in the brown adipose tissue and lipolysis in the white adipose tissue.
Leptin-mediated hypothalamic eCB/orexin interactions
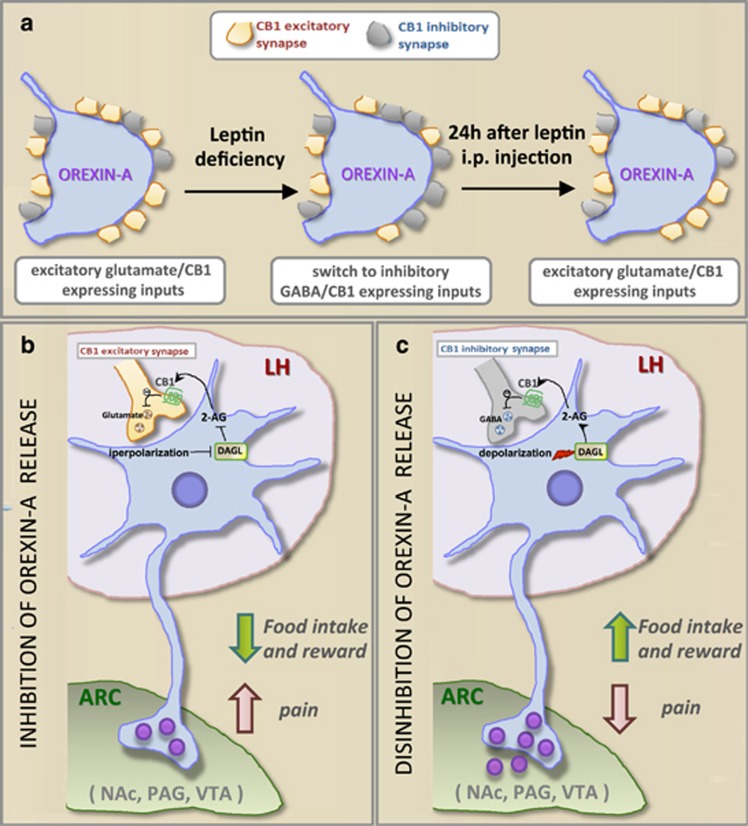
The regulation of food intake by eCBs at the hypothalamic level might be more complex than initially thought because of emerging evidence of a bimodal orexigenic versus anorexigenic effects of CB1 receptors, depending on whether they are activated in glutamatergic or GABAergic terminals, respectively.21 However, in fasted versus ad lib fed, or in lean versus obese, animals the hypothalamic circuitry can be rapidly rewired at the synaptic level, in terms of what and how many neurons are regulated by excitatory versus inhibitory neurotransmission. This rewiring is likely to affect the way CB1 receptors modulate food intake in fasted versus ad lib fed, and in lean versus obese, animals and is effected through changes in feeding-regulated hormones, in particular leptin and glucocorticoids.22 These hormones decrease and increase, respectively, eCB levels within the hypothalamus, and become upregulated during obesity and downregulated during anorexia, thus resulting in changes of hypothalamic ECS tone.5, 7, 11 We recently found that lateral hypothalamus neurons producing orexin-A (also known as hypocretin-1) are also subject to a rewiring during obesity, and instead of being innervated by mostly CB1-expressing glutamatergic fibres, as in lean mice, they establish synapses with CB1-expressing GABAergic fibres, a phenomenon observed after weaning in leptin-deficient ob/ob mice and in adult DIO, leptin-resistant mice (Figure 2a). Furthermore, these orexinergic neurons are able to produce and release 2-AG through the catalytic action of diacylglycerol lipase-α (DAGLα), which is expressed in their somata and dendrites and is upregulated in weaned ob/ob and adult DIO mice (Figure 2b). As a consequence of these alterations, orexinergic neurons are subjected to CB1-mediated disinhibition rather than inhibition, with subsequent increase of orexin-A release in lateral hypothalamus output regions, such as the arcuate nucleus, ventral tegmental area and nucleus accumbens. Both increased orexinergic signalling in these brain areas and the rewiring of orexinergic neurons in the lateral hypothalamus are fully reversed following treatment of ob/ob mice with leptin, indicating that these changes are due to defective leptin signalling in the hypothalamus of obese mice (Figure 2).
Figure 2.
Leptin-mediated hypothalamic endocannabinoid/orexin interactions. (a) A synaptic switch from glutamate/CB1 to GABA/CB1-expressing inputs on orexin-A neurons occurs in leptin-deficient (ob/ob mice) or ARC leptin-resistant DIO mice after prolonged HFD. This is reversed by 24-h leptin injection or after normal-fat diet.48 (b) In lean mice, orexin-A neurons of the lateral hypothalamus receive and project inputs from/to ARC, and project to various nuclei outside the hypothalamus, including the nucleus accumbens (NAc), ventrotegmental area (VTA) and periaqueductal gray area (PAG). In wild-type and leptin-sensitive lean mice, glutamate/CB1-expressing inputs outnumber GABA/CB1-expressing inputs to orexin-A neurons, thus inducing hyperpolarization of orexin-A neuronal activity and inhibition of orexin-A release to target areas. Hyperpolarization then may downregulate the biosynthesis of 2-AG via the DAGL and keep this CB1/2-AG-mediated retrograde regulation of orexin-A neurons under control. (c) Increase in endocannabinoid (mainly 2-AG) tone occurs in orexin-A neurons of leptin-deficient (ob/ob) or leptin-insensitive (DIO) mice, where the GABA/CB1-expressing inputs outnumber glutamate/CB1-expressing inputs to orexin-A neurons, thus inducing depolarization of orexin-A neuronal activity and disinhibition of orexin-A release to target areas. Depolarization may further upregulate the biosynthesis of 2-AG via DAGL, thus possibly creating a vicious circle. The synaptic remodelling of inputs projecting to orexin-A neurons allows modification of the orexin neural circuitry, thus possibly resulting in the modulation of feeding behaviours linked to reward and of pain perception.
Orexins A and B exert their actions via G-protein-coupled receptors known as OX1 and OX2, which can be found in both the central nervous system and peripheral tissues. They have a pivotal role in the regulation of sleep/wakefulness, pain, reward and appetite, and a recent study demonstrates their interaction with the ECS.23 Interestingly, in the rat dorsal raphe nucleus, orexin-B-mediated inhibition of glutamate release can be due to 2-AG release and subsequent activation of CB1 receptors on glutamatergic presynaptic terminals.24 In the rat periaqueductal gray, orexin-A-induced analgesic responses rely on a similar 2-AG/CB1-mediated ‘retrograde' inhibition of Y-aminobutyric acid (GABA) release.25 In an in vitro study, it was demonstrated that the coexpression of CB1 and OX1 receptors results in a higher potentiation of ERK signalling, possibly because of heterodimerization/oligomerization of these two receptors.26 Recently, however, it was also shown that 2-AG produced and released through the activation of OX1 receptor acts as both a paracrine and autocrine messenger via CB1 receptors,23, 27 thus possibly explaining the above-mentioned findings in the rat dorsal raphe nucleus and periaqueductal gray, as well as the potentiation of ERK phoshorylation that follows OX1/CB1 receptor coexpression. These data suggest the involvement of 2-AG and CB1 receptors not only in orexin-A release but also in orexin-mediated regulation of synaptic transmission.
Endocannabinoids and the ‘liking' of palatable foods
Increasing evidence supports the notion that the ECS has a pivotal role in reward/reinforcement circuits of the mesolimbic system. All elements of the ECS are found in the nucleus accumbens and ventral tegmental area, where CB1 activation modulates both dopaminergic and opioidergic pathways, thereby participating in reinforcing both the ‘liking' and ‘wanting' of highly palatable food.28 For example, injection into the nucleus accumbens of the psychotropic plant cannabinoid and CB1 agonist, THC, increases sucrose-induced hedonic activity, as well as dopamine release.29 On the other hand, CB1 antagonists reduce the increase in extracellular dopamine release in the nucleus accumbens mediated by a novel high palatable food,28 which is suggestive of palatable food-induced activation of eCB tone in this area. In mice made obese by a HFD, eCB levels are upregulated in the hippocampus, which is an important anatomical substrate of hedonic eating, indicating that highly palatable foods may be more satisfying under these conditions, resulting in a vicious circle leading to obesity.30 In the hypothalamus, which is involved in the control of reward through its lateral hypothalamus-originating connections with the mesolimbic system, 2-AG is transiently or permanently upregulated following acute or prolonged fat consumption, respectively, thus possibly participating in both the induction and maintenance stage of HFD preference.31 It is not known, however, whether the emerging effects of leptin and ghrelin on the hedonic neural correlates of food intake in the mesolimbic system32 are mediated by eCBs and CB1 signalling, much in the same way that their hypothalamic effects on the homeostatic aspects of food intake are.
The ECS in both central and peripheral tissues might also modulate the way the sensory properties of food are perceived, in a manner to maximize food intake. For example, the levels of 2-AG are elevated in the olfactory epithelium of tadpoles following food deprivation, and the subsequent activation of CB1 in this tissue lowers odour detection thresholds, thus rendering olfactory neurons more sensitive. eCBs in the nose may thus heighten food-seeking behaviour.33 CB1 receptor activation by eCB in type II taste cells, which also express the T1r3 sweet taste receptor, increases gustatory nerve responses to sweeteners without affecting responses to salty, sour, bitter and umami compounds. eCBs increase behavioural responses to sweet–bitter mixtures and electrophysiological responses of taste receptor cells to sweet compounds. Thus, the ECS also participates in enhancing the sensory properties of sweet foods, a role opposite to that of leptin.34 Finally, mouth exposure to fatty food in sham-fed rats leads to vagus nerve-mediated increases of eCB levels in the small intestine, which then, by activating CB1 in this gut region, participate in stimulating fat-food intake.35 Finally, CB1 stimulation in the pontine parabrachial nucleus, a brainstem region gating neurotransmission associated with the gustatory properties of food, enhances the consumption of palatable foods containing fat and/or sugar.36
These observations may help explain why consumption of a favourite food, as compared with normal food, in healthy human volunteers was recently shown to be accompanied by elevated plasma 2-AG levels, which in turn correlated positively with elevated plasma ghrelin levels.37 This effect might be due to either direct or indirect (for example, ghrelin-mediated) effects of palatable food consumption on central and peripheral eCB levels, reviewed above, although this would not explain why 5 min before consuming their favourite food the volunteers, who knew that they would be consuming it, already exhibited higher plasma 2-AG levels.37 This latter observation might rather also suggest that anticipatory mechanisms trigger changes in peripheral eCB levels, which might then participate in both the motivational and rewarding/sensory aspects of palatable food intake.
Inhibition of eCB biosynthesis as a possible strategy for the treatment of ‘western diet'-induced obesity
As outlined in this article, brain and peripheral levels of eCBs, in particular 2-AG, are altered following exposure to palatable and/or fatty foods, and such alterations seem to have a major role in hyperphagia and obesity. However, strong alterations in eCB levels are also found, during obesity, in the liver, adipose tissue, pancreas and skeletal muscle, where they contribute to hepatic steatosis and insulin resistance, adipocyte hypertrophy and inflammation, reduced glucose uptake and oxygen consumption in the muscle and disrupted β-cell function.38 These observations have provided the bases for the development of CB1 inverse agonists for the treatment of obesity and related metabolic disorders, the prototypical compound being rimonabant.39 This first in its class anti-obesity drug reached the market after several successful trials, revealing metabolic benefits along with body-weight reduction in overweight and obese subjects. However, chronic treatment with rimonabant and other CB1 inverse agonists increases the appearance of psychiatric adverse events, such as anxiety, depressive mood disorders and suicidal ideation.40 For this reason, the clinical development and further marketing of these compounds were interrupted. It has been reasoned that, apart from non-brain permeant CB1 inverse agonists, which are currently being developed and tested in preclinical studies, also inhibitors of 2-AG biosynthesis might have therapeutic applications in obesity and metabolic disorders,41 possibly similar to those of ‘neutral' CB1 receptor antagonists, which, by being uncapable of modifying CB1 activity in the absence of elevated EC levels, produce fewer side effects than CB1 receptor inverse agonists.42, 43, 44 In fact, O-5596, a prototypical inhibitor of 2-AG biosynthesis, was reported to only inhibit the stimulated, and not the baseline, levels of 2-AG in intact cells and to transiently reduce the intake of palatable food in mice.45
The biosynthesis of 2-AG originates from diacylglycerol (DAG) precursors through the action of specific selective DAGLs. Two DAGL isozymes (DAGLα and DAGLβ) have been cloned and enzymatically characterized, and a series of inhibitors with reasonable selectivity for these enzymes have been developed, although, unfortunately, they did not appear to be suitable for systemic use in vivo because of their lack of stability and poor permeability through the plasma membrane.46 Recently, we reported the pharmacological characterization of three new selective inhibitors of DAGLα. In particular, we showed that the acute intraperitoneal administration of the compound denoted as O-7460 dose-dependently inhibited the intake of HFD in mice. The most efficacious dose of O-7460 was comparable to those previously shown to be required for CB1 receptor inverse agonists and neutral antagonists to suppress food intake when acutely administered i.p. Interestingly, we also showed that this DAGL inhibitor causes an acute reduction in body weight. In fact, mice treated with O-7460 not only did not gain weight over the observation period but even exhibited a reduction in body weight.47 It is likely that this latter effect is due to the reduction in HFD consumption described above, although other possible mechanisms cannot be ruled out. For example, very recently, it has been demonstrated that 2-AG is involved in the regulation of the activity of forebrain neural circuits implicated in the control of energy dissipation.41 Importantly, the effect of O-7460 on HFD intake and body weight was not accompanied by overt actions on locomotor behaviour or body temperature but instead occurred concomitantly with the reduction in HFD-induced elevation of 2-AG levels in the hypothalamus and liver, levels that, following treatment, became identical to those found in mice given a normal chow. Therefore, it is likely that compounds such as O-7460 might produce their effects on food intake by restoring physiological levels of 2-AG and without lowering them to an extent below that necessary for this mediator to exert its normal functions in the brain.
In conclusion, selective DAGLα inhibitors might be considered a useful pharmacological tool to further investigate the role of 2-AG in the framework of eCB function in the control of palatable food intake and energy homeostasis. They might also be used to treat other conditions determined by pathologically elevated 2-AG levels, with less adverse effects than compounds such as rimonabant.
The authors declared no conflict of interest.
Footnotes
This article is published as part of a supplement sponsored by the Université Laval's Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments, and prevention of obesity.
References
- Zeltser LM, Seeley RJ, Tschöp MH. Synaptic plasticity in neuronal circuits regulating energy balance. Nat Neurosci 2012; 15: 1336–1342. [DOI] [PubMed] [Google Scholar]
- Jordan SD, Könner AC, Brüning JC. Sensing the fuels: glucose and lipid signalling in the CNS controlling energy homeostasis. Cell Mol Life Sci 2010; 67: 3255–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci 2011; 14: 9–15. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 2009; 89: 309–380. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Bátkai S, Járai Z et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001; 410: 822–825. [DOI] [PubMed] [Google Scholar]
- Jo YH, Chen YJ, Chua Jr SC, Talmage DA, Role LW. Integration of endocannabinoid and leptin signaling in an appetite-related neural circuit. Neuron 2005; 48: 1055–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 2006; 26: 6643–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br J Pharmacol 2001; 134: 1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M et al. The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 2003; 112: 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal P, Bellocchio L, Clark S, Cannich A, Klugmann M, Lutz B et al. Hypothalamic CB1 cannabinoid receptors regulate energy balance in mice. Endocrinology 2012; 153: 4136–4143. [DOI] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One 2008; 3: e1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Cinar R, Liu J, Godlewski G, Wesley D, Jourdan T et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab 2012; 16: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diano S, Liu ZW, Jeong JK, Dietrich MO, Ruan HB, Kim E et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med 2011; 71: 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol 2002; 136: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Avraham Y, Ben-Shushan D, Zolotarev O, Berry EM, Mechoulam R. Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res 2003; 983: 144–151. [DOI] [PubMed] [Google Scholar]
- Molhoj S, Hansen HS, Schweiger M, Zimmermann R, Johansen T, Malmlöf K. Effect of the cannabinoid receptor-1 antagonist rimonabant on lipolysis in rats. Eur J Pharmacol 2010; 646: 38–45. [DOI] [PubMed] [Google Scholar]
- Quarta C, Bellocchio L, Mancini G, Mazza R, Cervino C, Braulke LJ et al. CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab 2010; 11: 273–285. [DOI] [PubMed] [Google Scholar]
- Bajzer M, Olivieri M, Haas MK, Pfluger PT, Magrisso IJ, Foster MT et al. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia 2011; 54: 3121–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C. Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 2011; 60: 1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer T, Lindtner C, Zielinski E, O'Hare J, Filatova N, Buettner C. Short term voluntary overfeeding disrupts brain insulin control of adipose tissue lipolysis. J Biol Chem 2012; 287: 33061–33069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafenetre P, Cannich A, Cota D, Puente N, Grandes P et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 2010; 13: 281–283. [DOI] [PubMed] [Google Scholar]
- Crosby KM, Inoue W, Pittman QJ, Bains JS. Endocannabinoids gate statedependent plasticity of synaptic inhibition in feeding circuits. Neuron 2011; 71: 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen PM, Jäntti MH, Kukkonen JP. OX1 orexin/hypocretin receptor signaling through arachidonic acid and endocannabinoid release. Mol Pharmacol 2012; 82: 156–167. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci 2005; 25: 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YC, Lee HJ, Tung LW, Liao YY, Fu SY, Teng SF et al. Activation of orexin 1 receptors in the periaqueductal gray of male rats leads to antinociception via retrograde endocannabinoid (2-arachidonoylglycerol)-induced disinhibition. J Neurosci 2011; 31: 14600–14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Pediani JD, Milligan G. Heteromultimerization of cannabinoid CB(1) receptor and orexin OX(1) receptor generates a unique complex in which both protomers are regulated by orexin A. J Biol Chem 2011; 286: 37414–37428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäntti MH, Putula J, Turunen PM, Nasman J, Reijonen S, Lindqvist C et al. Autocrine endocannabinoid signaling through CB1 receptors potentiates OX1 Orexin receptor signaling. Mol Pharmacol 2013; 83: 621–632. [DOI] [PubMed] [Google Scholar]
- Melis T, Succu S, Sanna F, Boi A, Argiolas A, Melis MR. The cannabinoid antagonist SR 141716A (Rimonabant) reduces the increase of extra-cellular dopamine release in the rat nucleus accumbens induced by a novel high palatable food. Neurosci Lett 2007; 419: 231–235. [DOI] [PubMed] [Google Scholar]
- De Luca MA, Solinas M, Bimpisidis Z, Goldberg SR, Di Chiara G. Cannabinoid facilitation of behavioral and biochemical hedonic taste responses. Neuropharmacology 2012; 63: 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa F, Mancini G, Schmidt H, Steindel F, Mackie K, Angioni C et al. Alterations in the hippocampal endocannabinoid system in diet-induced obese mice. J Neurosci 2010; 30: 6273–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi S, Irie K, Yamaguchi R, Katsuki M, Araki M, Ohji M et al. Hypothalamic 2-arachidonoylglycerol regulates multistage process of high-fat diet preferences. PLoS ONE 2012; 7: e38609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin J, Figlewicz DP, Bennett-Jay J, Kittleson S, Cummings DE. Ghrelin increases the motivation to eat, but does not alter food palatability. Am J Physiol Regul Integr Comp Physiol 2012; 303: R259–R269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig E, Czesnik D, Piscitelli F, Di Marzo V, Manzini I, Schild D. Endocannabinoid modulation in the olfactory epithelium. Results Probl Cell Differ 2010; 52: 139–145. [DOI] [PubMed] [Google Scholar]
- Yoshida R, Ohkuri T, Jyotaki M, Yasuo T, Horio N, Yasumatsu K et al. Endocannabinoids selectively enhance sweet taste. Proc Natl Acad Sci USA 2010; 107: 935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Astarita G, Schwartz G, Li X, Piomelli D. Endocannabinoid signal in the gut controls dietary fat intake. Proc Natl Acad Sci USA 2011; 108: 12904–12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPatrizio NV, Simansky KJ. Activating parabrachial cannabinoid CB1 receptors selectively stimulates feeding of palatable foods in rats. J Neurosci 2008; 28: 9702–9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Matias I, Martiadis V, De Petrocellis L, Maj M, Di Marzo V. Blood levels of the endocannabinoid anandamide are increased in anorexia nervosa and in binge-eating disorder, but not in bulimia nervosa. Neuropsychopharmacology 2005; 30: 1216–1221. [DOI] [PubMed] [Google Scholar]
- Silvestri C, Ligresti A, Di Marzo V. Peripheral effects of the endocannabinoid system in energy homeostasis: adipose tissue, liver and skeletal muscle. Rev Endocr Metab Disord 2011; 12: 153–162. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Després JP. CB1 antagonists for obesity – what lessons have we learned from rimonabant? Nat Rev Endocrinol 2009; 5: 633–638. [DOI] [PubMed] [Google Scholar]
- Christopoulou FD, Kiortsis DN. An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J Clin Pharm Ther 2011; 36: 10–18. [DOI] [PubMed] [Google Scholar]
- Jung KM, Clapper JR, Fu J, D'Agostino G, Guijarro A, Thongkham D et al. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab 2012; 15: 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero DR. Cannabinoid-1 receptor (CB1R) blockers as medicines: beyond obesity and cardiometabolic disorders to substance abuse/drug addiction with CB1R neutral antagonists. Expert Opin Emerg Drugs 2012; 17: 17–29. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Raffa RB. Rimonabant redux and strategies to improve the future outlook of CB1 receptor neutralantagonist/inverse-agonist therapies. Obesity (Silver Spring) 2011; 19: 1325–1334. [DOI] [PubMed] [Google Scholar]
- Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the aetiopathology of metabolic disorders. Cell Metab 2013; 17: 475–490. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Burston JJ, Rai R, Allarà M, Saha B, Mahadevan A et al. Synthesis and pharmacological activity of a potent inhibitor of the biosynthesis of the endocannabinoid 2-arachidonoylglycerol. ChemMedChem 2009; 4: 946–950. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Cascio MG, Saha B, Mahadevan A, Urbani P, Minassi A et al. Development of the first potent and specific inhibitors of endocannabinoid biosynthesis. Biochim Biophys Acta 2006; 1761: 205–212. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Mahadevan A, Coccurello R, Chang JW, Allarà M, Chen Y et al. A novel fluorophosphonate inhibitor of the biosynthesis of the endocannabinoid 2-arachidonoylglycerol with potential anti-obesity effects. Br J Pharmacol 2013; 169: 748–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristino L, Busetto G, Imperatore R, Ferrandino I, Palomba L, Silvestri C et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc Natl Acad Sci USA 2013; 110: E2229–E2238. [DOI] [PMC free article] [PubMed] [Google Scholar]