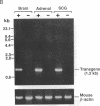
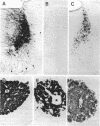
Abstract
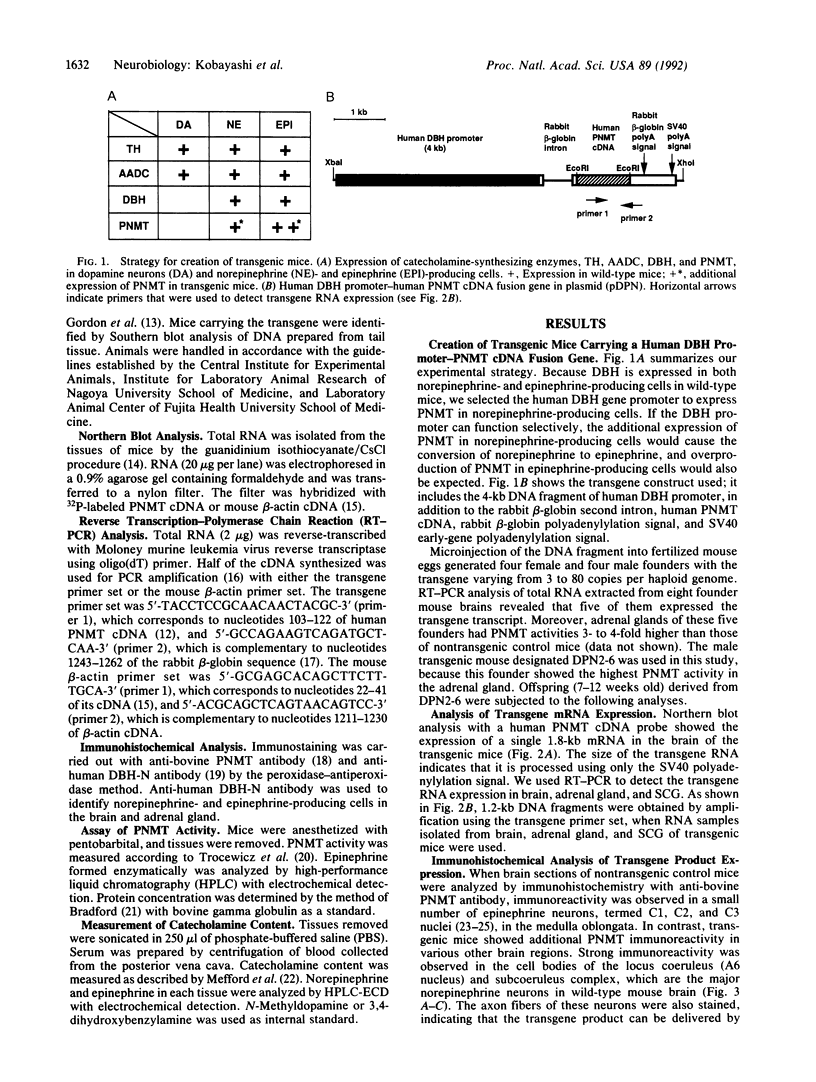
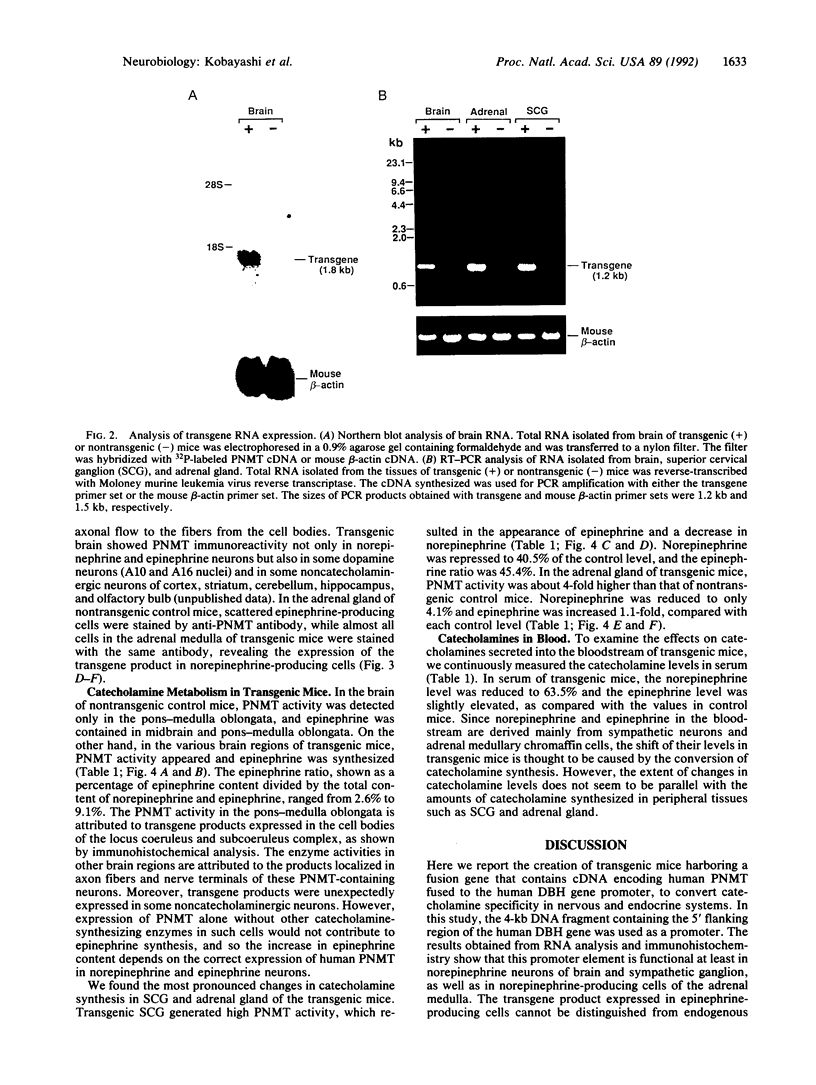
Epinephrine-producing cells are characterized by the presence of phenylethanolamine N-methyltransferase (PNMT), which catalyzes the formation of epinephrine from norepinephrine. We generated a line of transgenic mice carrying a chimeric gene containing human PNMT cDNA fused to the 4-kilobase fragment of the human dopamine beta-hydroxylase (DBH) gene promoter, to switch catecholamine phenotype in the nervous and endocrine systems. Human PNMT transcripts and immunoreactivity were mainly detected in norepinephrine neurons in brain and sympathetic ganglion as well as in norepinephrine-producing cells in adrenal medulla of transgenic mice, indicating that the human DBH gene promoter of 4 kilobases is sufficient to direct expression of the gene in norepinephrine-producing cells. Analysis of catecholamines in the various tissues showed that the expression of human PNMT in transgenic mice induced the appearance of epinephrine in sympathetic ganglion and dramatic changes in norepinephrine and epinephrine levels in brain, adrenal gland, and blood. These results indicate that the additional PNMT expression in norepinephrine-producing cells can convert these cells to the epinephrine phenotype, and suggest that norepinephrine-producing cells normally possess the basic machinery required for the synthesis of epinephrine except for PNMT. Thus it appears that the only major difference between norepinephrine- and epinephrine-producing cells is the expression of PNMT. Our transgenic animals provide an experimental model to investigate the functional differences between norepinephrine and epinephrine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD J. Purification and properties of phenylethanolamine-N-methyl transferase. J Biol Chem. 1962 May;237:1657–1660. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chaplin L., Cohen A. H., Huettl P., Kennedy M., Njus D., Temperley S. J. Reserpic acid as an inhibitor of norepinephrine transport into chromaffin vesicle ghosts. J Biol Chem. 1985 Sep 15;260(20):10981–10985. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Deupree J. D., Weaver J. A. Identification and characterization of the catecholamine transporter in bovine chromaffin granules using [3H]reserpine. J Biol Chem. 1984 Sep 10;259(17):10907–10912. [PubMed] [Google Scholar]
- Friedman S., Kaufman S. 3,4-dihydroxyphenylethylamine beta-hydroxylase. Physical properties, copper content, and role of copper in the catalytic acttivity. J Biol Chem. 1965 Dec;240(12):4763–4773. [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7380–7384. doi: 10.1073/pnas.77.12.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe P. R., Costa M., Furness J. B., Chalmers J. P. Simultaneous demonstration of phenylethanolamine N-methyltransferase immunofluorescent and catecholamine fluorescent nerve cell bodies in the rat medulla oblongata. Neuroscience. 1980;5(12):2229–2238. doi: 10.1016/0306-4522(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Greengard P. Regulation of neurotransmitter receptor desensitization by protein phosphorylation. Neuron. 1990 Nov;5(5):555–567. doi: 10.1016/0896-6273(90)90211-w. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Transgenic animals. Science. 1988 Jun 10;240(4858):1468–1474. doi: 10.1126/science.3287623. [DOI] [PubMed] [Google Scholar]
- Kaneda N., Ichinose H., Kobayashi K., Oka K., Kishi F., Nakazawa A., Kurosawa Y., Fujita K., Nagatsu T. Molecular cloning of cDNA and chromosomal assignment of the gene for human phenylethanolamine N-methyltransferase, the enzyme for epinephrine biosynthesis. J Biol Chem. 1988 Jun 5;263(16):7672–7677. [PubMed] [Google Scholar]
- Kawasaki E. S., Clark S. S., Coyne M. Y., Smith S. D., Champlin R., Witte O. N., McCormick F. P. Diagnosis of chronic myeloid and acute lymphocytic leukemias by detection of leukemia-specific mRNA sequences amplified in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5698–5702. doi: 10.1073/pnas.85.15.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K., Kurosawa Y., Fujita K., Nagatsu T. Human dopamine beta-hydroxylase gene: two mRNA types having different 3'-terminal regions are produced through alternative polyadenylation. Nucleic Acids Res. 1989 Feb 11;17(3):1089–1102. doi: 10.1093/nar/17.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG W., WEISSBACH H., UDENFRIEND S. Aromatic L-amino acid decarboxylase. J Biol Chem. 1962 Jan;237:89–93. [PubMed] [Google Scholar]
- Mefford I. N., Gilberg M., Barchas J. D. Simultaneous determination of catecholamines and unconjugated 3,4-dihydroxyphenylacetic acid in brain tissue by ion-pairing reverse-phase high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1980 May 15;104(2):469–472. doi: 10.1016/0003-2697(80)90101-3. [DOI] [PubMed] [Google Scholar]
- Mishina M., Kurosaki T., Tobimatsu T., Morimoto Y., Noda M., Yamamoto T., Terao M., Lindstrom J., Takahashi T., Kuno M. Expression of functional acetylcholine receptor from cloned cDNAs. Nature. 1984 Feb 16;307(5952):604–608. doi: 10.1038/307604a0. [DOI] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- Nagatsu I., Kobayashi K., Fujii T., Komori K., Sekiguchi K., Titani K., Fujita K., Nagatsu T. Antibodies raised against different oligopeptide segments of human dopamine-beta-hydroxylase. Neurosci Lett. 1990 Dec 11;120(2):141–145. doi: 10.1016/0304-3940(90)90023-3. [DOI] [PubMed] [Google Scholar]
- Nomura Y., Kajiyama H., Segawa T. Hypersensitivity of cardiac beta-adrenergic receptors after neonatal treatment of rats with 6-hydroxydopa. Eur J Pharmacol. 1980 Aug 29;66(2-3):225–232. doi: 10.1016/0014-2999(80)90146-6. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Palkovits M., Brownstein M. J., Axelrod J. Localisation of phenylethanolamine N-methyl transferase in the rat brain nuclei. Nature. 1974 Apr 19;248(5450):695–696. doi: 10.1038/248695a0. [DOI] [PubMed] [Google Scholar]
- Stewart L. C., Klinman J. P. Dopamine beta-hydroxylase of adrenal chromaffin granules: structure and function. Annu Rev Biochem. 1988;57:551–592. doi: 10.1146/annurev.bi.57.070188.003003. [DOI] [PubMed] [Google Scholar]
- Tokunaga K., Taniguchi H., Yoda K., Shimizu M., Sakiyama S. Nucleotide sequence of a full-length cDNA for mouse cytoskeletal beta-actin mRNA. Nucleic Acids Res. 1986 Mar 25;14(6):2829–2829. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trocewicz J., Oka K., Nagatsu T. Highly sensitive assay for phenylethanolamine N-methyltransferase activity in rat brain by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1982 Feb 12;227(2):407–413. doi: 10.1016/s0378-4347(00)80393-x. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Bechtel W. D., Rouot B. M., Snyder S. H. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol Pharmacol. 1979 Jul;16(1):47–60. [PubMed] [Google Scholar]
- UDENFRIEND S., WYNGAARDEN J. B. Precursors of adrenal epinephrine and norepinephrine in vivo. Biochim Biophys Acta. 1956 Apr;20(1):48–52. doi: 10.1016/0006-3002(56)90261-x. [DOI] [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]