Abstract
Objective
The objective was to test the proposed mechanism of action of a task-specific motor learning intervention by examining its effect on measures of the motor control of gait.
Design
Single blinded randomized clinical trial.
Setting
University research laboratory.
Participants
Forty older adults 65 years of age and older, with gait speed >1.0 m/s and impaired motor skill (Figure of 8 walk time > 8 secs).
Interventions
The two interventions included a task-oriented motor learning and a standard exercise program. Both interventions lasted 12 weeks, with twice weekly one hour physical therapist supervised sessions.
Main Outcome Measures
Two measure of the motor control of gait, gait variability and smoothness of walking, were assessed pre and post intervention by assessors masked to treatment arm.
Results
Of 40 randomized subjects; 38 completed the trial (mean age 77.1±6.0 years). Motor control group improved more than standard group in double support time variability (0.13 vs. 0.05 m/s; adjusted difference, AD=0.006, p=0.03). Smoothness of walking in the anterior/posterior direction improved more in motor control than standard for all conditions (usual: AD=0.53, p=0.05; narrow: AD=0.56, p=0.01; dual task: AD=0.57, p=0.04).
Conclusions
Among older adults with subclinical walking difficulty, there is initial evidence that task-oriented motor learning exercise results in gains in the motor control of walking, while standard exercise does not. Task-oriented motor learning exercise is a promising intervention for improving timing and coordination deficits related to mobility difficulties in older adults, and needs to be evaluated in a definitive larger trial.
Keywords: motor control, gait, aging, exercise
Exercise interventions to improve mobility and prevent functional decline in older adults primarily target strength, flexibility and endurance but rarely address the motor control of walking. These exercise programs which overlook the motor control of walking have resulted in only modest improvements in mobility.1–6
To specifically address the learning/relearning of the motor control of walking, an intervention was developed based on motor learning principles and focused on the practice of the smooth, coordinated aspects of walking throughout the gait cycle (i.e. task-oriented motor learning intervention).7–10 We previously showed that in older adults with walking difficulty (slow and variable gait), the task-oriented motor learning intervention promoted greater gains in clinical measures of gait (gait efficiency, gait speed and self-perceived walking ability) compared to a standard exercise program.11 Likewise, in older adults with subclinical walking difficulty (i.e. near normal gait speed > 1.0 m/s but impaired skill in walking, figure 8 walk time > 8 seconds), the task-oriented motor learning program promoted greater gains in mobility (gait speed and walking skill) than the standard exercise program.7 Having shown the clinical effect of the task-oriented motor learning program, the current goal is to test the proposed mechanism of action of the intervention (e.g. improved motor control of walking). Therefore, the purpose of this study is to assess the impact of a motor learning versus a standard exercise program on the motor control of gait (i.e. gait variability and smoothness of walking) in community-dwelling older adults with subclinical gait deficits. Given that the motor learning program focuses on the smooth, coordinated aspects of gait timing, we expect that individuals in the motor learning exercise group will have greater improvements in gait variability and smoothness of walking than the standard group.
METHODS
Overview
The study protocol was approved by the University of Pittsburgh Institutional Review Board, and all subjects provided informed consent. The study was registered at ClinicalTrials.gov (PRO09080228). The Program to Improve Mobility in the Elderly (PRIME study) was a 12-week single-blind randomized clinical trial that compared two exercise interventions in older adults with subclinical gait dysfunction. Details of the methods and the main study outcomes have been published elsewhere.7
Participants and Inclusion Criteria
Briefly, the eligible participants consisted of men and women 65 years of age and older who had subclinical gait dysfunction defined as near normal gait speed (i.e. gait speed ≥ 1.0 m/s) and impaired motor skill in walking.7 Gait speed was assessed using an instrumented walkway. Motor skill in walking was assessed using the Figure of 8 Walk Test12 with impaired motor skill defined as ≥8 seconds to complete the test. The Figure of 8 Walk Test assesses walking around curves in both clockwise and counter-clockwise directions. This complex task, which is associated with measures of motor control and planning,12–15 requires smooth transitions from timing and coordination patterns of straight path walking to the different timing and coordination patterns of curved-path walking.16–19 Individuals with impaired walking skill slow down and take several small steps when walking the curves of the Figure of 8 Walk Test. Additional exclusion criteria included 1) reported dyspnea at rest or during activities, 2) hospitalization in the past 6 months for acute illness or injury, 3) progressive neuromuscular disorder such as Parkinson’s disease, 4) persistent lower extremity or back pain, 5) fixed or fused lower extremity joints, 6) resting systolic blood pressure greater than or equal to 200, diastolic blood pressure greater than or equal to 100, or resting heart rate greater than 100 beats per minute or less than 40 beats per minute, and 7) Mini Mental State Examination20 score less than 24. All participants had physician clearance to participate in a moderate intensity exercise program.
Sample Size and Randomization
As a pilot intervention trial, sample size (n=40) was based on feasibility within available resources rather than a given level of statistical power. The randomization sequence was generated by the study biostatistician using the high quality pseudo-random deviate generator in SAS® (SAS Institute, Inc., Cary, North Carolina) in 1:1 ratio and a blocked randomized scheme, and placed in sequentially numbered sealed envelopes. Participants were assigned to motor learning or standard interventions by the study coordinator at the time of randomization.
Interventions
Overview
Both interventions were physical therapist led, protocol driven interventions that lasted 60 minutes twice a week for 12 weeks. The programs included a brief warm-up period and strength training that was conducted on Magnum (Magnum Fitness Systems, South Milwaukee, WI) stacked weight equipment and included the following exercises: knee extension, knee flexion, leg press, hip abduction, and hip extension. When subjects were able to complete 2 sets of 15 repetitions with minimal effort (i.e. rating of perceived exertion < 10), resistance was increased for progression of the exercises.
Motor Learning Exercise
Subjects in the motor learning group also received 20–30 minutes of motor learning exercises in addition to the warm-up and strengthening exercises. The previously described motor learning program7, 9, 10 was based on the principles that enhance “skill” or smooth and automatic movement control.21–26 The motor learning exercise program included both stepping and walking patterns. The goal oriented, progressively more difficult stepping patterns were designed to shift the center of pressure posteriolaterally then forward, encouraging hip extension prior to stepping, loading the trailing limb, coordinating activation of the abductors of the soon-to-be-swung leg with adductors of the stance limb, and shifting the center of pressure in medial stance to unload the stepping limb.27–29 Walking patterns incorporated patterns of muscle coordination and interlimb timing into walking and were progressed by altering speed, amplitude (e.g. narrowing oval width), or accuracy of performance (e.g. without straying from the desired path). More complex walking patterns involved walking past others and with upper extremity object manipulation tasks, such as carrying or bouncing a ball.26 Treadmill walking reinforced the rhythmic stepping and was completed at preferred walking speed with brief intervals of increased speed.
Standard Exercise
Subjects in the standard group also received endurance training in addition to the warm-up and strengthening exercises. The endurance training consisted of treadmill walking at a submaximal workload with a self-reported rating of perceived exertion (RPE) of 10–13, somewhat hard. Once subjects tolerated a 10–13 RPE level for 15 minutes, the workload was increased by first increasing the duration of walking up to 30 minutes and then by increasing walking speed. The goal was to achieve 30 minutes of continuous treadmill walking at a somewhat hard level of exertion (i.e. RPE 10–13).
Measures of Motor Control of Gait
Aging and disease alter the motor control and the timing and coordination of gait as reflected by slowed neuromotor performance, increased gait variability30 and reduced smoothness of movement.31–34 In order to capture the motor control of walking we measured gait variability and smoothness of walking. All outcomes were assessed before and after the 12 week intervention by individuals masked to the intervention group.
Gait Variability
Gait variability, defined as fluctuations in gait characteristics from one step to the next, is an important indicator of impaired mobility in older adults. We were primarily interested in gait speed and variability of step length, step width, step time, stance time, and double support time. These were selected because they represent both spatial and temporal characteristics and because they have been studied by other investigators,35–38 and shown to be predictive of future falls35, 37 and mobility disability.39
The GaitMat II™ system was used for the analysis of speed and spatiotemporal variability.40 The GaitMat II™ is an instrumented 4-meter long walkway that records and analyzes footfall data. In addition to the 4-meter instrumented walkway, there are initial and final one meter inactive sections that allow for acceleration and deceleration of the participant. After two practice passes on the GaitMat II™, each participant completed four passes on the GaitMat II™ at their self-selected walking speed for data collection. The within-subject standard deviations of the gait characteristics determined from all of the right and left steps recorded over 4 passes were included in the calculation for each person and used as measures of gait variability. Approximately 25–30 steps were collected from 4 passes on the GaitMat which is more than adequate to achieve a stable measure of gait variability. Our prior work has shown that 20 steps are sufficient to achieve a reliability of ICC=0.75 and 30 are sufficient for ICC=0.80.41
Smoothness of Walking
Smoothness of walking, quantified by harmonic ratios (HRs), measures the symmetry and repeatability of the timing and magnitude of trunk accelerations in antero-posterior (AP), vertical (V) and mediolateral (ML) directions.42 Harmonic ratio theory and calculation has been previously described.43, 44 Briefly, HRs have been used to characterize the complex acceleration trajectories for each direction of motion as a single value for a given stride by quantifying the deviation from an ideally symmetrical acceleration pattern. Higher HRs are interpreted as greater walking smoothness.43 Older adults, who likely have impaired gait biomechanics and neural control,31, 45–47 individuals with Parkinson’s disease,48, 49 and individuals with sensory impairment,50 are less smooth in walking than young adults or individuals without disease. In addition, smoothness of walking is related to physical function, independent of gait speed, thus suggesting smoothness represents aspects of the motor control of walking important for physical function.51
A triaxial accelerometer (BIOPAC Systems, Inc., Santa Barbara, CA, USA) secured over the third lumbar spine vertebra and wireless transmission technology were used to record 3D trunk accelerations.45 Trunk accelerations were sampled at 100 Hz. A footswitch system was used to determine heel contact events for stride segmentation. HRs were determined for each stride and averaged across strides resulting in a mean HR for each direction of motion. Acceleration data were recorded during three over ground walking conditions, usual pace, narrow, and dual task which are described below:
Usual walk: Subjects walked 12.2 m over ground at their usual, preferred speed.
Narrow path walk: Brightly colored tape was used to mark a 15 cm wide, 4 meter long rectangular path in the center of the 12 meter long walkway. Subjects walked 4 meters on the usual width walkway, 4 meters in the narrow path, and then 4 meters on the usual width walkway at their preferred speed.
Dual task walk: While standing, subjects listened to a string of digits (presented at the rate of one digit per second), with the number of digits defined by subject’s memory span, as determined prior to gait testing. The digit list was provided to the subjects prior to the walk, the subject then walked for 12.2 m over ground at their preferred speed, and then upon stopping was asked to recall the digits in reverse order.
Trunk triaxial accelerometric gait analysis has shown high test-retest reliability, with ICC3,1 values ranging from .77 to .9652, 53 and high stride-to-stride reliability for trunk acceleration signals across a range of walking speeds (coefficient of multiple determination, a waveform repeatability statistic, ranged from .60 to .95).54
Data Analysis
All statistical analyses were performed using SAS® version 9.3 (SAS Institute, Inc., Cary, NC). Participant characteristics and baseline measurements were compared between arms using t-tests for continuous variables and chi-square tests for categorical variables. To obtain adjusted differences of outcomes between treatment arms, we fitted a series of analysis of covariance models using baseline to follow-up change in each outcome as the response variable; treatment arm as the main factor of interest; and baseline value of the outcome and baseline gait speed as covariates.
RESULTS
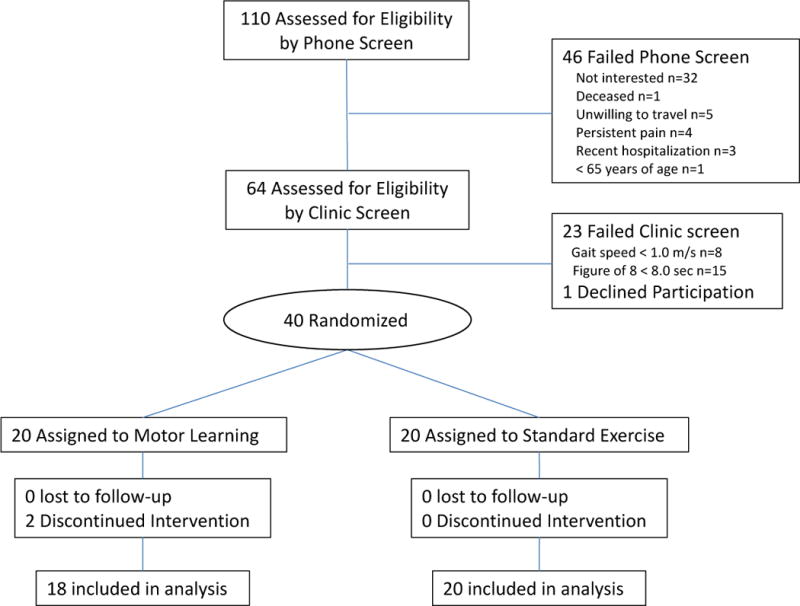
Of 110 people initially screened by telephone, 64 underwent on site screening. Forty-one participants met all criteria and 40 were randomized (1 subject deferred) and 38 completed the study.7 All 38 completers participated in at least 22 exercise sessions with 37 participants (97%) completing all 24 sessions. The two drop-outs developed unrelated medical conditions and walked more slowly than those who completed the study. Participants had a mean age of 77.1 years, near normal gait speed (mean gait speed 1.18 m/s) and impaired motor skill in walking (mean Figure 8 time 9.2 seconds); Table 1. Participants in the two treatment arms were similar on all health and demographic characteristics (Table 1).
Table 1.
Baseline characteristics by treatment group
| Motor Learning n=18 |
Standard n=20 |
P | |
|---|---|---|---|
| Age, years | 75.7 (5.5) | 78.5 (6.2) | 0.16 |
| female, n (%) | 10 (55.6) | 13 (68.4) | 0.42 |
| white, n (%) | 18 (100) | 19 (95) | 0.32 |
| married, n (%) | 12 (67) | 9 (47) | 0.36 |
| Graduate education, n (%) | 10 (56) | 11 (58) | 0.99 |
| Chronic conditions | |||
| Cardiac, n (%) | 2 (11) | 1 (5) | 0.49 |
| Musculoskeletal, n (%) | 12 (67) | 18 (90) | 0.08 |
| Visual, n (%) | 13 (72) | 18 (90) | 0.16 |
| Diabetes, n (%) | 4 (22) | 2 (10) | 0.30 |
| Cancer, n (%) | 8 (44) | 5 (25) | 0.21 |
| Lung, n (%) | 4 (22) | 2 (10) | 0.30 |
| Gait speed, m/s | 1.22 (0.16) | 1.14 (0.15) | 0.79 |
| Figure 8, s | 9.1 (0.93) | 9.3 (0.92) | 0.97 |
Baseline Motor Control of Walking
Participants in the two treatment arms were similar on all baseline gait measures. Though not statistically significant, baseline gait speed was 0.08 m/s faster in the motor learning than the standard group which is considered to be a small but meaningful difference.55 The group mean baseline values of gait variability and smoothness of walking were indicative of impaired motor control in walking (Tables 2 and 3). Baseline stance time variability was 0.39 s which is greater than the 0.034 s cut-off value for future mobility disability,56 and baseline smoothness of walking during the usual walking condition ranged from 2.00 to 3.03 which is much less than the values for young adults (i.e. 4.46).45
Table 2.
Baseline and post-intervention gait variability by treatment group
| Treatment Group | ||||||
|---|---|---|---|---|---|---|
| Motor Learning n=18 | Standard n=20 | Adjusted group differences | ||||
| Gait Variability | Baseline Mean (SD) |
Post-intervention Mean (SD) |
Baseline Mean (SD) |
Post-intervention Mean (SD) |
Adjusted group Difference (SE) |
p |
| Step length, m | 0.031 (0.012) | 0.030 (0.010) | 0.033 (0.013) | 0.036 (0.011) | −0.005 (0.003) | 0.09 |
| Step width, m | 0.034 (0.010) | 0.033 (0.008) | 0.032 (0.009) | 0.031 (0.005) | 0.002 (0.002) | 0.48 |
| Step time, s | 0.032 (0.024) | 0.021 (0.010) | 0.036 (0.022) | 0.024 (0.010) | −0.003 (0.003) | 0.45 |
| Stance time, s | 0.039 (0.023) | 0.026 (0.010) | 0.039 (0.020) | 0.031 (0.011) | −0.005 (0.004) | 0.14 |
| Double support time, s | 0.019 (0.006) | 0.015 (0.003) | 0.022 (0.012) | 0.021 (0.012) | −0.006 (0.003) | 0.03 |
Table 3.
Baseline and post-intervention smoothness of walking during usual, narrow path and dual task conditions by treatment group
| Treatment Group | ||||||
|---|---|---|---|---|---|---|
| Motor Learning n=18 | Standard n=20 | * Adjusted group differences | ||||
| Harmonic Ratio | Baseline Mean (SD) |
Post-intervention Mean (SD) |
Baseline Mean (SD) |
Post-intervention Mean (SD) |
Adjusted group Difference (SE) |
p |
| Vertical HRV | ||||||
| Usual | 3.03 (1.10) | 3.38 (0.92) | 2.94 (1.17) | 2.79 (1.07) | 0.58 (0.35) | 0.10 |
| Narrow path | 2.77 (1.00) | 2.95 (0.78) | 2.39 (0.86) | 2.26 (0.78) | 0.71 (0.27) | 0.01 |
| Dual task | 3.10 (1.27) | 3.50 (0.91) | 2.71 (1.13) | 2.55 (1.04) | 0.89 (0.35) | 0.01 |
| Antero-Posterior HRAP | ||||||
| Usual | 2.45 (0.99) | 2.67 (0.67) | 2.27 (0.78) | 2.12 (0.85) | 0.53 (0.26) | 0.05 |
| Narrow path | 2.18 (0.73) | 2.37 (0.67) | 1.91 (0.60) | 1.76 (0.54) | 0.56 (0.21) | 0.01 |
| Dual task | 2.46 (0.93) | 2.65 (0.76) | 2.31 (0.96) | 2.03 (0.93) | 0.57 (0.27) | 0.04 |
| Mediolateral HRML | ||||||
| Usual | 2.01 (0.65) | 2.24 (0.61) | 2.00 (0.81) | 1.96 (0.79) | 0.31 (0.21) | 0.16 |
| Narrow path | 1.81 (0.53) | 1.83 (0.53) | 1.72 (0.49) | 1.68 (0.62) | 0.13 (0.18) | 0.48 |
| Dual task | 2.00 (0.77) | 2.10 (0.53) | 1.94 (0.75) | 1.91 (0.81) | 0.17 (0.23) | 0.46 |
Adjusted for baseline value of each outcome and baseline gait speed.
Baseline to Post-Intervention Change in the Motor Control of Walking
The motor learning group had greater improvements than the standard group in double support time variability (Table 2). Improvements in step length, step width, step time, and stance time variability did not differ between the two groups. The motor learning group had greater improvements in smoothness of walking than the standard group (Table 3). The motor learning group had a greater increase than the standard group in AP and V smoothness measured specifically during the narrow and dual task walks. Changes in ML smoothness during all walking conditions were not different between the groups (p>0.10).
DISCUSSION
Older adults with near normal gait speed and subclinical gait deficits who participated in a task-oriented motor learning exercise program demonstrated greater improvements in the motor control of walking than those who participated in a standard impairment-based exercise program. Individuals in the motor learning program had greater improvements in gait variability (double support time variability) and smoothness of walking (HRAP and HRV during narrow and dual task conditions) two indicators of the motor control of walking.
The standard impairment-based exercise program and the task-oriented motor learning exercise program have very different proposed underlying mechanisms for improving walking.8 The standard impairment-based exercise program aims to increase the physiologic capacity in body systems that contribute to walking, but does not include the task specific exercise necessary to make use of the capacities for the walking. The result is a bigger engine for walking – the ability to use the excess capacity to tolerate the age-related gait abnormalities and high energy cost of poor walking. Whereas, the task-oriented motor learning program is intended to improve the older adult’s appropriate motor plan selection from an enhanced repertoire of motor plans for walking. More specifically, the task-specific motor learning intervention aims to improve the motor skill of walking by re-aligning biomechanical and neuromotor control, strengthening motor programs, and improving feedback for adjusting movements. In a sense it is comparable to a “tune-up” of an engine. The result is a more efficient system for walking which requires less energy and can last longer. The improvements in motor control of walking in the task-oriented motor learning group and not the standard impairment-based group support the proposed underlying mechanisms of the interventions.
Differences in gait variability between the groups after the intervention were smaller than expected. This may be partially explained by the fact that both groups trained on a treadmill, which acts as an external pacer and step generator and has been shown to reduce gait variability.57 Though the rationale and the protocol for training on the treadmill were different for the groups, both groups did walk on the treadmill as part of their intervention. Therefore, it is not surprising that both groups improved or reduced their step time and stance time variability after training.
The motor learning group demonstrated consistent improvements in smoothness of walking (HR values increasing after intervention) across all walking conditions whereas the standard group demonstrated consistent worsening (HR values decreasing after intervention). Group differences in smoothness of walking were more apparent during the challenging conditions of narrow and dual task walking. In higher functioning older adults, a challenge may be necessary to bring out deficits and demonstrate improvements with intervention.58 Also, neither group practiced these specific challenging tasks as part of their exercise intervention. The task-oriented motor learning approach prepares the older adult to select the appropriate motor plan for walking. The goal is not to practice every possible walking condition (which is virtually impossible) but to train the older adult to make the appropriate motor plan selection for the walking task. To make appropriate changes in the selection of motor plans, the brain needs to be updated about the body capacities through continued recent and relevant movement experiences in walking.25 The stepping and walking patterns included in the task-oriented motor learning program provide the older adult with such movement experiences relevant to walking.
Smoothness of walking is related to gait speed,43 so the improvements in smoothness of walking may be a result of the increased gait speed after the intervention. Though the motor learning group had greater improvements in gait speed than the standard group [adjusted group difference (SE) = 0.11 m/s (0.04), p=0.008] both groups did increase gait speed with the intervention.7 Despite this increase in gait speed, the standard group’s smoothness of walking worsened. Therefore, the improvements in the smoothness of walking in the motor control group cannot be attributed to the improvements in gait speed.
Study Limitations
The current work has several limitations that must be considered when interpreting the findings. First, this was a pilot study that was not powered to detect differences between groups; the sample size was based on feasibility and availability of resources. However, we were able to find a few important differences with relatively smaller p-values despite low statistical power. Secondly, both groups trained on a treadmill which has been shown to impact gait variability.57 Future studies examining the impact of standard versus task-oriented motor learning on the motor control of walking should utilize alternative forms of endurance training for the standard exercise group such as over ground walking. Lastly, given the number of comparisons the potential for Type I error needs to be considered. The current work also has strengths that should be recognized. The study’s design, a single-blind randomized clinical trial minimizes unwanted bias. Secondly, motor control of walking was objectively quantified using measures of gait variability and smoothness of walking under usual and challenging walking conditions.
CONCLUSION
Among older adults with subclinical walking difficulty, there is initial evidence that task-oriented motor learning exercise results in gains in the motor control of walking, while standard exercise does not. Task-oriented motor learning exercise is a promising intervention for improving timing and coordination deficits related to mobility difficulties in the older adults, and needs to be evaluated in a definitive larger trial.
Acknowledgments
This work was supported by the Pittsburgh Older Americans Independence Center (NIA P30 AG024827) and Beeson Career Development Award (NIA K23 AG026766). A portion of this work was presented at the APTA Combined Sections Meeting, January 2013, San Diego, CA.
Clinical Trials registration number: PRO09080228
Abbreviations
- AD
adjusted difference
- AP
antero-posterior
- HR
harmonic ratio
- ICC
intraclass correlation coefficient
- ML
mediolateral
- m/s
meters per second
- RPE
rating of perceived exertion
- V
vertical
References
- 1.LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: results of the Lifestyle Interventions and independence for elders pilot (LIFE-P) study. J Gerontol Med Sci. 2006;61A:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 2.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. New Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 3.Fiatarone MA, Marks MA, Ryan EC, Meredith ND, Lipsitz CN, Evans WJ. High intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. [PubMed] [Google Scholar]
- 4.Brown M, Holloszy JO. Effects of a low intensity exercise program on selected physical performance characteristics of 60- to 71-year olds. Aging (Milano) 1991;3:129–139. doi: 10.1007/BF03323989. [DOI] [PubMed] [Google Scholar]
- 5.Judge JO, Underwood M, Gennosa T. Exercise to improve gait velocity in older persons. Archives of Physical Medicine and Rehabilitation. 1993;74(4):400–406. [PubMed] [Google Scholar]
- 6.Manini T, Marko M, VanArnam T, et al. Efficacy of resistance and task-specific exercise in older adults who modify tasks of everyday life. J Gerontol Med Sci. 2007;62A:616–623. doi: 10.1093/gerona/62.6.616. [DOI] [PubMed] [Google Scholar]
- 7.Brach JS, VanSwearingen JM, Perera S, Wert DM, Studenski SA. Motor learning versus standard walking exercise in older adults with subclinical gait dysfunction: A randomized clinical trial. J Am Geriatr Soc. 2013;61:1879–1886. doi: 10.1111/jgs.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brach JS, VanSwearingen JM. Interventions to improve walking in older adults. Curr Transl Geriatr and Exp Gerontol Rep. 2013;2(4) doi: 10.1007/s13670-013-0059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanSwearingen JM, Perera S, Brach JS, Cham R, Rosano C, Studenski SA. A randomized trial of two forms of therapeutic activity to improve walking: effect on the energy cost of walking. J Gerontol A Biol Sci Med Sc. 2009;64A:1190–1198. doi: 10.1093/gerona/glp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanSwearingen JM, Perera S, Brach JS, Wert DM, Studenski S. Exercise to Improve Gait Efficiency: Impact on Activity and Participation in Older Adults with Mobility Limitations. Phys Ther. 2011;91:1740–1751. doi: 10.2522/ptj.20100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.VanSwearingen JM, Perera S, Brach JS, Cham R, Rosano C, Studenski SA. Exercise to reduce the energy cost of walking: a randomized trial. J Gerontol Med Sci. 2009;64(1):1190–1198. doi: 10.1093/gerona/glp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess RJ, Brach JS, Piva SR, VanSwearingen JM. Walking skill can be assessed in older adults: Validity of figure-of-8 walk test. Phys Ther. 2010;90(1):89–99. doi: 10.2522/ptj.20080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry KA, Brach JS, Nebes RD, Studenski SA, VanSwearingen JM. Contributions of cognitive function to straight- and curved-path walking in older adults. Arch Phys Med Rehabil. 2012;93:802–807. doi: 10.1016/j.apmr.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odonkor CA, Thomas JC, Holt N, et al. A comparison of straight- and curved-path walking tests among mobility-limited older adults. J Gerontol Med Sci. 2013 doi: 10.1093/gerona/glt060. EPub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbs RM, VanSwearingen JM, Studenski SA, Brach JS. Curved path walking predicts 12-month physical function in older adults without mobility limited physical function. J Am Geriatr Soc. 2012;60:S70. [Google Scholar]
- 16.Courtine G, Schieppati M. Human walking along a curved path. I. Body trajectory, segment orientation and the effect of vision. Euro J Neuro. 2003;18:177–190. doi: 10.1046/j.1460-9568.2003.02736.x. [DOI] [PubMed] [Google Scholar]
- 17.Patla AE, Adkin A, Ballard T. Online steering: coordination and control of body center of mass, head and body reorientation. Exp Brain Res. 1999;129:629–634. doi: 10.1007/s002210050932. [DOI] [PubMed] [Google Scholar]
- 18.Hollands MA, Sorensen KL, Patla AE. Effects of head immobilization on the coordination and control of head and body reorientation and translation during steering. Exp Brain Res. 2001;140:223–233. doi: 10.1007/s002210100811. [DOI] [PubMed] [Google Scholar]
- 19.Imai T, Moore ST, Raphan T, Cohen B. Interaction of the body, head, and eyes during walking and turning. Exp Brain Res. 2001;136:1–18. doi: 10.1007/s002210000533. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folsetein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Nelson WL. Physical principles for economies of skilled movements. Biol Cybernetics. 1983;46:135–147. doi: 10.1007/BF00339982. [DOI] [PubMed] [Google Scholar]
- 22.Daly JJ, Ruff RL. Construction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model-based measures for stroke patients. The Scientific World Journal. 2007;7:2031–2045. doi: 10.1100/tsw.2007.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lay BS, Sparrow WA, Hughes KM, O’Dwyer NJ. Practice effects on coordination and control, metabolic energy expenditure, and muscle activation. Human Movement Science. 2002;21:807–830. doi: 10.1016/s0167-9457(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 24.Newman MA, Dawes H, van den Berg M, Wade DT, Burridge J, Izadi H. Can aerobic treadmill training reduce the effort of walking and fatigue in people with multiple sclerosis: a pilot study. Multiple Sclerosis. 2007;13:113–119. doi: 10.1177/1352458506071169. [DOI] [PubMed] [Google Scholar]
- 25.Brooks VB. The Neural Basis of Motor Control. New York: Oxford University Press; 1986. [Google Scholar]
- 26.Gentile A. Skill acquisition: action, movement, and neuromotor processes. In: Carr JH, Shepherd RB, Gordon J, Gentile AM, Held JM, editors. Movement Sciences. 1. Rockville: Aspen Publishers; 1987. pp. 93–154. [Google Scholar]
- 27.Polcyn AF, Lipsitz LA, Kerrigan CD, Collins JJ. Age-related changes in the initiation of gait: degradation of central mechanisms for momentum generation. Arch Phys Med Rehabil. 1998;79:1582–1589. doi: 10.1016/s0003-9993(98)90425-7. [DOI] [PubMed] [Google Scholar]
- 28.Capaday C. The special nature of human walking and its neural control. Trends in Neurosciences. 2002;25(7):370–376. doi: 10.1016/s0166-2236(02)02173-2. [DOI] [PubMed] [Google Scholar]
- 29.Alexander RM. Walking made simple. Science. 2005;308:58–59. doi: 10.1126/science.1111110. [DOI] [PubMed] [Google Scholar]
- 30.Gabell A, Nayak USL. The effect of age and variability in gait. Journal of Gerontology. 1984;39(6):662–666. doi: 10.1093/geronj/39.6.662. [DOI] [PubMed] [Google Scholar]
- 31.Menz HB, Lord SR, Fitzpatrick RC. Age-related differences in walking stability. Age Aging. 2003;32:137–142. doi: 10.1093/ageing/32.2.137. [DOI] [PubMed] [Google Scholar]
- 32.Morgan M, Phillips J, Bradshaw J, Mattingley J, Iansek R, Bradshaw J. Age-related motor slowness: simply strategic? Journal of Gerontology. 1994;49(3):M133–M139. doi: 10.1093/geronj/49.3.m133. [DOI] [PubMed] [Google Scholar]
- 33.Welford AT. Motor Skills and Aging. In: Mortimer JA, Pirozzolo FJ, Maletta GJ, editors. The Aging motor System. New York: Praeger Publishers; 1982. pp. 152–187. [Google Scholar]
- 34.Welford AT. Between bodily performance and slowing with age. Exp Aging Res. 1984;(10):73–88. doi: 10.1080/03610738408258548. [DOI] [PubMed] [Google Scholar]
- 35.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45(3):313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 36.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78(3):278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 37.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82(8):1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 38.Owings TM, Grabiner MD. Step width variability, but not step length variability or step time variability, discriminates gait of healthy young and older adults during treadmill locomotion. Journal of Biomechanics. 2004;37(6):935–938. doi: 10.1016/j.jbiomech.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Brach JS, Studenski S, Perera S, VanSwearingen JM, Newman AB. Gait variability and the risk of incident mobility disability. J Gerontol Med Sci. 2007;62A:983–988. doi: 10.1093/gerona/62.9.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh JP. Foot fall measurement Technology. In: Craik RL, Oatis CA, editors. GaitAnalysis: Theory and Application. St. Louis: Mosby-Year Book, Inc; 1995. pp. 125–142. [Google Scholar]
- 41.Perera S, Brach JS, Talkowski JB, Wert d, Studenski SA. Measuring stride time variability: estimating test-retest reliability and required walk length using bootstrapping. Program & Abstracts of the ISPGR 18th International Conference. 2007:55–56. [Google Scholar]
- 42.Bellenca JL, Lowry KA, VanSwearingen JM, Brach JS, Redfern MS. Harmonic ratios: A quantification of step to step symmetry. J Biomech. 2013;46:828–831. doi: 10.1016/j.jbiomech.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture. 2003;18:35–46. doi: 10.1016/s0966-6362(02)00159-5. [DOI] [PubMed] [Google Scholar]
- 44.Smidt GL, Arora JS, Johnston RC. Accelerographic analysis of several types of walking. Am J Phys Med. 1971;50(6):285–300. [PubMed] [Google Scholar]
- 45.Brach JS, McGurl D, Wert d, et al. Validation of a measure of smoothness of walking. J Gerontol A Biol Sci Med Sc. 2011;66(1):136–141. doi: 10.1093/gerona/glq170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Menz HB, Lord SR, Fitzpatrick RC. Acceleration patterns of the head and pelvis when walking are associated with risk of falling in community-dwelling older people. J Gerontol A Biol Sci Med Sc. 2003;58:m446–m452. doi: 10.1093/gerona/58.5.m446. [DOI] [PubMed] [Google Scholar]
- 47.Lowry KA, Smiley-Oyen AL, Carrel AJ, Kerr JP. Age- and speed-related differences in harmonic ratios during walking. Gait Posture. 2012;35:272–276. doi: 10.1016/j.gaitpost.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 48.Latt MD, Menz HB, Fung VS, Lord SR. Acceleration patterns of the head and pelvis during gait in older people with Parkinson’s disease: a comparison of fallers and nonfallers. J Gerontol A Biol Sci Med Sc. 2009;64:700–706. doi: 10.1093/gerona/glp009. [DOI] [PubMed] [Google Scholar]
- 49.Lowry KA, Smiley-Oyen AL, Carrel AJ, Kerr JP. Walking stability using harmonic ratios in Parkinson’s disease. Movement Disorders. 2009;24(2):261–267. doi: 10.1002/mds.22352. [DOI] [PubMed] [Google Scholar]
- 50.Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85(2):245–252. doi: 10.1016/j.apmr.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Lowry KA, VanSwearingen JM, Perera S, Studenski SA, Brach JS. Walking smoothness is associated with self-reported function after accounting for gait speed. J Gerontol Med Sci. 2013;68(10):1286–1290. doi: 10.1093/gerona/glt034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moe-Nilssen R. Test-retest relaibility of trunk accelerometery during standing and walking. Arch Phys Med Rehabil. 1998;79:1377–1385. doi: 10.1016/s0003-9993(98)90231-3. [DOI] [PubMed] [Google Scholar]
- 53.Henriksen M, Lund H, Moe-Nilssen R, Bliddal H, Danneskiodsamsoe B. Test-retest reliability of trunk accelerometric gait analysis. Gait Posture. 2004;19:288–297. doi: 10.1016/S0966-6362(03)00069-9. [DOI] [PubMed] [Google Scholar]
- 54.Kavanagh JJ, Morrison S, James DA, Barrett R. Reliability of segmental accelerations measured using a new wireless gait analysis system. J Biomech. 2006;39:2863–2872. doi: 10.1016/j.jbiomech.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 55.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 56.Brach JS, Wert DM, VanSwearingen JM, Newman AB, Studenski SA. Use of stance time variability for predicting mobility disability in community-dwelling older persons: A prospective study. J Geriatric Phys Ther. 2012;35:112–117. doi: 10.1519/JPT.0b013e318243e5f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warabi T, Kato M, Kiriyama K, Yoshida T, Kobayashi N. Treadmill walking and overground walking of human subjects compared by recording sole-floor reaction forces. Neuroscience Research. 2005;53:343–348. doi: 10.1016/j.neures.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Brach JS, Perera S, VanSwearingen JM, Hile ES, Wert DM, Studenski SA. Challenging gait conditions predict 1-year decline in gait speed in older adults with apparently normal gait. Phys Ther. 2011;91:1857–1864. doi: 10.2522/ptj.20100387. [DOI] [PMC free article] [PubMed] [Google Scholar]


