Abstract
Aims
To characterize the effect of dapagliflozin on albuminuria and estimated glomerular filtration rate (eGFR) and to determine whether effects on albuminuria were mediated through changes in glycated haemoblogin (HbA1c), systolic blood pressure (SBP), body weight or eGFR.
Methods
We conducted a post hoc analysis of data pooled from two phase III clinical trials in hypertensive patients with type 2 diabetes (T2DM) on stable angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy, randomly assigned to dapagliflozin 10 mg/day or matched placebo. This analysis included only patients with microalbuminuria or macroalbuminuria at baseline.
Results
Patients were randomized to receive dapagliflozin 10 mg (n = 167) or placebo (n = 189). Dapagliflozin resulted in greater 12‐week reductions in albuminuria compared with placebo: −33.2% [95% confidence interval (CI) −45.4, −18.2]. The reduction in albuminuria was also present after adjusting for age, sex and changes in HbA1c, SBP, body weight and eGFR: −23.5% (95% CI −37.6, −6.3). There was a decrease in eGFR with dapagliflozin versus placebo that was readily reversed 1 week after last dose. No serious renal‐related adverse events were observed in any group.
Conclusions
Dapagliflozin was effective in lowering albuminuria in patients with T2DM and hypertension using renin‐angiotensin system blockade therapy. Reductions in albuminuria were still present after adjusting for changes in HbA1c, SBP, body weight and eGFR. Dapagliflozin‐induced improvements in glycaemic control and reductions in SBP, coupled with other potentially beneficial renal effects, may lead to a reduced long‐term renal and cardiovascular risk.
Keywords: albuminuria, dapagliflozin, diabetes, hypertension, sodium glucose cotransporter‐2
Introduction
People with type 2 diabetes mellitus (T2DM) are at a high risk of both cardiovascular and renal disease 1. Known risk factors for cardiovascular and renal disease include hyperglycaemia, hypertension and albuminuria, and controlling these factors is critical in order to reduce this risk 1. Dapagliflozin, a highly selective sodium‐glucose co‐transporter 2 (SGLT2) inhibitor, has been shown to improve glycaemic control by decreasing renal glucose reabsorption in the kidneys and increasing urinary glucose excretion 2, 3, 4. In addition to improving glycaemic control, dapagliflozin has been shown to have beneficial effects on body weight, blood pressure and other cardiovascular risk factors 5, 6, 7.
Evidence suggests that SGLT2 inhibition may also confer renoprotective effects 8, 9. The underlying potential renoprotective mechanism of SGLT2 inhibition may be explained by enhanced tubulo‐glomerular feedback, leading to a reduction in intraglomerular pressure and an accompanying acute fall in estimated glomerular filtration rate (eGFR) 10, 11, 12. Additionally, experimental studies have suggested that treatment with SGLT2 inhibitors results in a reduction of intrarenal inflammation 13, similar to that seen with angiotensin receptor blockers (ARBs) 14, and may also exert additive anti‐inflammatory effects when combined with angiotensin‐converting enzyme (ACE) inhibitors 15. Conversely, a recent animal model study did not show any renal‐specific effect apart from glucose‐lowering with SGLT2 inhibitor treatment 16.
Given the divergent preclinical findings, we first characterized in a clinical setting the effect of dapagliflozin on albuminuria and eGFR in patients with T2DM, hypertension and microalbuminuria or macroalbuminuria, and second, investigated whether any observed changes in albuminuria were dependent or independent of changes in glycated haemoglobin (HbA1c), systolic blood pressure (SBP), body weight or eGFR.
Methods
Study Design
This was a post hoc analysis of data pooled from two multicentre, randomized, double‐blind, parallel, placebo‐controlled phase III trials, conducted during 2010–2013, that evaluated the efficacy and safety of dapagliflozin in patients with T2DM with inadequately controlled hypertension despite receiving ACE inhibitor or ARB therapy (NCT01137474, NCT01195662). The trial designs comprised a qualification period (≤14 days following enrolment), a lead‐in period (4 weeks), a double‐blind treatment period (12 weeks) and a follow‐up period (1 week). The study protocols were approved by the institutional review board/independent ethics committee at each site and all patients provided written informed consent. The trials were conducted according to the principles of the Declaration of Helsinki. Detailed descriptions of the methods and primary results of the two trials have been published 17, 18.
Eligibility Criteria
Patients included in this analysis met the following criteria: 18–89 years of age with inadequately controlled T2DM [defined as HbA1c 7.0–10.5% (53–91 mmol/mol)]; inadequately controlled hypertension (defined as seated SBP 140–165 mmHg and seated diastolic blood pressure 85–105 mm Hg); and albuminuria [defined as urine albumin/creatinine ratio (UACR) ≥30 mg/g]. All patients were required to be taking a stable dose of an oral antidiabetic drug (OAD) for ≥6 weeks (12 weeks for thiazolidinediones) or insulin (monotherapy or in combination with an OAD) for 8 weeks, and a stable dose of an ACE inhibitor or an ARB for ≥4 weeks. Patients with a C‐peptide level ≥0.8 ng/ml (0.3 nmol/l), a body mass index ≤45.0 kg/m2, and serum creatinine <1.50 mg/dl (114.4 µmol/l) for men or <1.40 mg/dl (106.8 µmol/l) for women were included. Patients with an estimated creatinine clearance <60 ml/min were excluded.
Randomization and Treatment
After a 4‐week placebo lead‐in, patients were randomized using an interactive voice response system in a 1 : 1 ratio to receive dapagliflozin 10 mg or placebo once daily for 12 weeks. Randomization was stratified by additional antihypertensive medication use and/or insulin use at baseline.
Outcome Measures
The main endpoints in the present analysis were changes from baseline to week 12 in UACR and changes from baseline to 1 week after treatment cessation for eGFR. UACR was derived from a single spot urine sample. Urinary albumin and urinary creatinine concentration were measured in a central laboratory (Quintiles Laboratories, Marietta, GA, USA; Livingston, UK; Mumbai, India; or Mexico City, Mexico). eGFR was calculated using the modification of diet in renal disease formula 19. Changes from baseline in UACR to week 12 were also examined after controlling for changes in HbA1c, SBP, body weight and eGFR.
Safety assessments were performed during the double‐blind treatment period plus 4 days post‐dose for non‐serious adverse events (AEs) and 30 days post‐dose for serious AEs. Safety outcomes also included discontinuations attributable to AEs, AEs of special medical interest and laboratory abnormalities.
Statistical Analysis
Descriptive statistics were used to describe the baseline characteristics and safety of patients in the pooled analysis of these trials.
The UACR values were log‐transformed and analysed with a longitudinal repeated‐measures mixed model using direct likelihood, with fixed categorical effects of treatment, week, treatment‐by‐week interaction and study, and continuous covariates of baseline and baseline‐by‐week interaction in the model. The influence of other covariates on changes in UACR was explored by adding continuous fixed covariates of change from baseline to week 12 in HbA1c, SBP, body weight and eGFR to the model. In addition, age and gender were added to the model. Changes in HbA1c, eGFR, body weight and SBP were analysed with a longitudinal repeated‐measures mixed model using direct likelihood, with fixed categorical effects of treatment, week, treatment‐by‐week interaction, study and continuous covariates of baseline and baseline‐by‐week interaction in the model.
To further characterize the impact of the individual variables on the overall change in UACR, an additional post hoc multiple regression analysis was performed on only patients completing the 12‐week study period. By imputing representative mean changes versus placebo for HbA1c, SBP, body weight and eGFR in addition to treatment assignment into this model, estimates for the contribution of the individual components to the overall change in UACR were received.
Finally, data from the dapagliflozin‐treated patients were explored in an analysis of HbA1c and SBP responders/non‐responders to further examine the effect of these variables on UACR. An HbA1c or SBP responder was defined as a patient in the dapagliflozin treatment arm with a 12‐week HbA1c or SBP change lower than the median; a non‐responder was defined as a patient in the dapagliflozin treatment arm with a 12‐week HbA1c or SBP change on or above the median. The model included study, baseline, responder (yes/no) and study‐by‐baseline interaction.
All analyses were performed with SAS/STAT v.8.2 or higher (SAS Institute Inc., Cary, NC, USA).
Results
Disposition and Baseline Characteristics
Of 1062 patients randomized to receive dapagliflozin 10 mg or placebo in the two original studies, a total of 356 patients with a UACR ≥30 mg/g at baseline and at least one post‐baseline measurement in the double‐blind treatment period were included in this analysis (placebo, n = 189; dapagliflozin, n = 167). The baseline demographics, clinical and biochemical characteristics were generally similar between the treatment groups (Table 1). The mean age of patients was 55 (20–74) years. The durations of hypertension and of T2DM were ∼8 years, and the majority of patients (∼75%) had microalbuminuria.
Table 1.
Baseline characteristics.
| Placebo + ACE inhibitor/ARB n = 189 | Dapagliflozin 10 mg + ACE inhibitor/ARB n = 167 | |
|---|---|---|
| Male, % | 69.8 | 57.5 |
| Age, years | 55.1 ± 8.9 | 54.8 ± 8.6 |
| Race, % | ||
| White | 73.5 | 70.7 |
| Black | 4.2 | 8.4 |
| Asian | 20.1 | 18.6 |
| Other | 2.1 | 2.4 |
| BMI, kg/m2 | 31.3 ± 5.2 | 31.6 ± 5.6 |
| HbA1c, mmol/mol (%) | 65 ± 9.8 (8.1 ± 0.9) | 65 ± 10.9 (8.1 ± 1.0) |
| Diabetes duration, years | 8.3 ± 5.9 | 8.6 ± 6.5 |
| Microalbuminuria, n (%)* | 140 (74.1) | 128 (76.6) |
| Macroalbuminuria, n (%)† | 49 (25.9) | 39 (23.4) |
| UACR, mg/g (median, 25th, 75th percentile) | 78.0 (44.0, 267.0) | 75.0 (44.0, 267.0) |
| eGFR, ml/min/1.73 m2 | 85.8 ± 21.0 | 82.1 ± 19.7 |
| SBP, mmHg | 151.4 ± 8.0 | 151.9 ± 9.0 |
| DBP, mmHg | 91.5 ± 5.2 | 91.3 ± 5.1 |
| Hypertension duration, years | 7.4 ± 6.7 | 8.1 ± 7.2 |
All data are mean ± standard deviation unless otherwise stated.
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; SBP, systolic blood pressure; UACR, urine albumin/creatinine ratio.
Microalbuminuria was defined as UACR ≥30 to <300 mg/g.
Macroalbuminuria was defined as UACR ≥300 mg/g.
Effect of Dapagliflozin on Albuminuria and Estimated Glomerular Filtration Rate
Patients receiving dapagliflozin 10 mg had a median (25th, 75th percentile) UACR of 75.0 mg/g (44.0, 267.0) at baseline, which was reduced to 47.5 mg/g (23.5, 177.0) after 4 weeks of treatment and remained stable ending at 50.0 mg/g (23.0, 148.0) at 12 weeks. In patients treated with placebo, UACR decreased from 78.0 mg/g (44.0, 267.0) at baseline to 66.0 mg/g (32.0, 203.0) at 4 weeks and 74.0 mg/g (38.0, 181.0) at week 12. Relative to placebo, dapagliflozin significantly decreased albuminuria by −33.2% (95% CI −45.4, −18.2) after the 12‐week follow‐up (Figure 1A). Changes in UACR of a similar magnitude were observed in patients with baseline microalbuminuria −35.4% (95% CI −48.8, −18.7) or macroalbuminuria [−28.3% (95% CI −52.9, 9.2)]. The proportions of patients with at least a 30% reduction in albuminuria at week 12 were 49.7 and 37.4% in the dapagliflozin and placebo groups, respectively. The corresponding proportions of patients who improved from macroalbuminuria or microalbuminuria categories to a lower category (microalbuminuria or normoalbuminuria) were 40.5 and 23.9%, respectively.
Figure 1.
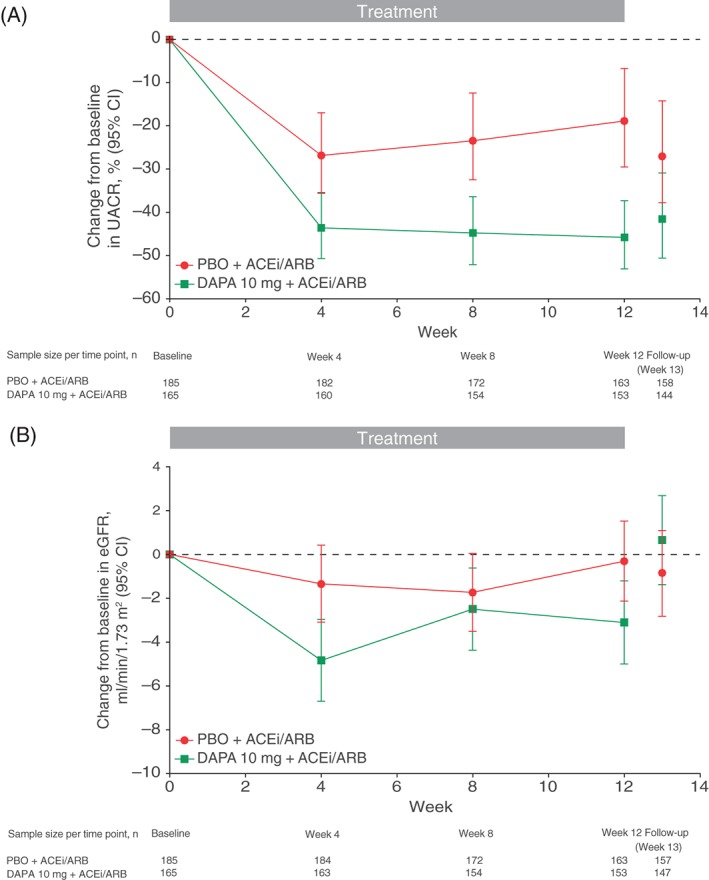
Change in (A) urine albumin/creatinine ratio (UACR) and (B) estimated glomerular filtration rate (eGFR) over time. Error bars represent 95% confidence interval (CI). ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; DAPA, dapagliflozin; PBO, placebo.
Baseline eGFR was 82.1 and 85.8 ml/min/1.73 m2 in the dapagliflozin and placebo groups, respectively. An initial decrease in eGFR was observed in the dapagliflozin group at the start of treatment (Figure 1B). At week 4, reductions in eGFR (ml/min/1.73 m2) were −4.8 ml/min/1.73 m2 (95% CI −6.7, −3.0) in the dapagliflozin group and −1.3 ml/min/1.73 m2 (95% CI −3.1, 0.4) in the placebo group. eGFR remained lower in the dapagliflozin group during the 12‐week follow‐up, with a difference from placebo at week 12 of −2.80 ml/min/1.73 m2 (95% CI −5.43, −0.16; Figure 1B). The initial reduction in eGFR in the dapagliflozin group was completely reversible after treatment discontinuation. At the 1‐week follow‐up visit after treatment discontinuation, eGFR was 0.7 ml/min/1.73 m2 (95% CI −1.4, 2.7) higher relative to baseline in the dapagliflozin group and −0.9 ml/min/1.73 m2 (95% CI −2.8, 1.1) lower relative to baseline in the placebo group.
Effect of Dapagliflozin on Glycated Haemoglobin, Blood Pressure and Body Weight
At week 12, patients receiving dapagliflozin had placebo‐corrected changes in HbA1c and SBP of −0.5% (95% CI −0.7, −0.3) and −3.5 mmHg (95% CI −5.9, −1.0), respectively (Figure 2). The corresponding change in body weight was −0.76 kg (95% CI −1.27, −0.26).
Figure 2.
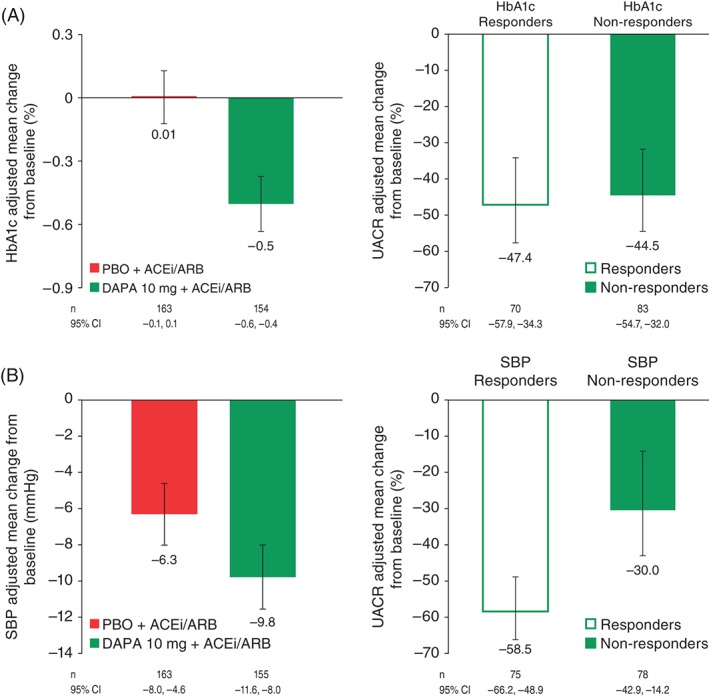
(A) Effect of dapagliflozin versus placebo on glycated haemoglobin (HbA1c) and effect of dapagliflozin on urine albumin/creatinine ratio (UACR) stratified by HbA1c response. (B) Effect of dapagliflozin versus placebo on systolic blood pressure (SBP) and effect of dapagliflozin on UACR stratified by SBP response.
Albuminuria‐lowering Effect Independent of Changes in Glycated Haemoglobin, Blood Pressure, Body Weight and Estimated Glomerular Filtration Rate
To assess whether the effect of dapagliflozin on albuminuria was mediated by changes in HbA1c, SBP or eGFR, the main analysis was repeated with adjustments for age, gender and changes in HbA1c, SBP, body weight and eGFR. The placebo‐corrected change in UACR at week 12 was −23.5% (95% CI −37.6, −6.3) for dapagliflozin, suggesting that the albuminuria‐lowering effect was still present after accounting for changes in HbA1c, SBP, body weight and eGFR and was to a large extent independent of changes in these covariates.
In two different post hoc analyses the impact of the different variables on change in UACR was further explored. First, a regression analysis in patients completing the 12‐week study was conducted in which the proportion of the effect explained by HbA1c, SBP, body weight and eGFR changes was quantified. When imputing mean changes versus placebo of −0.5% (HbA1c), −3.5 mmHg (SBP), −0.8 kg (body weight) and −2.8 ml/min/1.73 m2 (eGFR), this analysis (overall regression: p < 0.0001, r2 = 11.5%) indicated that the effect of dapagliflozin on albuminuria after 12 weeks of treatment was to a large extent independent of changes in HbA1c, SBP, body weight or eGFR (Figure 3). Further adjustment for baseline age or gender did not alter this finding. Furthermore, a second post hoc analysis in which dapagliflozin‐treated patients were stratified by the median value for 12‐week changes in HbA1c and SBP showed that the albuminuria‐lowering effect of dapagliflozin was present in both subgroups (Figure 2).
Figure 3.
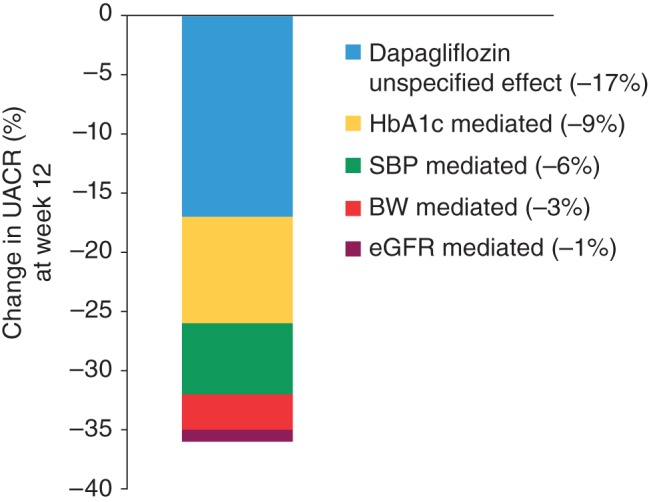
Effect of dapagliflozin versus placebo on albuminuria and proportion of effect mediated by changes in glycated haemoglobin (HbA1c), systolic blood pressure (SBP), body weight (BW) and estimated glomerular filtration rate (eGFR).
Safety
Overall, dapagliflozin was well tolerated with no increase in AEs leading to study drug discontinuation (Table 2). The frequency of AEs potentially related to volume depletion, urinary tract or genital infections and renal function was low; however, there were small numerical increases in these AEs in dapagliflozin‐treated patients as compared with placebo. None of these events was classified as serious. Similar proportions of patients between the two treatment groups experienced abnormal laboratory values (defined as increases in serum creatinine ≥1.5× baseline or potassium ≥6 mEQ/l).
Table 2.
Summary of adverse events.
| Number of patients, % | Placebo + ACE inhibitor/ARB n = 189 | Dapagliflozin 10 mg + ACE inhibitor/ARB n = 167 |
|---|---|---|
| Overall safety summary | ||
| ≥1 AE | 87 (46.0) | 81 (48.5) |
| AE leading to study drug discontinuation* | 2 (1.1) | 2 (1.2) |
| ≥1 serious AE | 0 | 6 (3.6) |
| Serious AE leading to study drug discontinuation* | 0 | 0 |
| ≥1 episode of hypoglycaemia | 5 (2.6) | 10 (6.0) |
| Hypoglycaemia leading to study drug discontinuation* | 0 | 0 |
| AEs of special interest | ||
| Renal events | 3 (1.6) | 5 (3.0) |
| Blood creatinine increased | 1 (0.5) | 4 (2.4) |
| GFR decreased | 1 (0.5) | 1 (0.6) |
| Renal impairment | 1 (0.5) | 0 |
| Volume reduction events | 0 | 2 (1.2) |
| Hypovolaemia | 0 | 1 (0.6) |
| Orthostatic hypotension | 0 | 1 (0.6) |
| Infections | ||
| Urinary tract infection | 3 (1.6) | 6 (3.6) |
| Genital infection | 5 (2.6) | 5 (3.0) |
| Marked laboratory abnormalities | ||
| Serum potassium ≥6 mmol/l | 4 (2.2) | 4 (2.4) |
| Serum creatinine ≥1.5× baseline value | 2 (1.1) | 2 (1.2) |
ACE, angiotensin‐converting enzyme; AE, adverse event; ARB, angiotensin receptor blocker.
Only hypoglycaemia reported as a serious AE was included as an AE, serious AE, or AE leading to discontinuation.
Discussion
This post hoc analysis studied the effects of dapagliflozin as an adjunct to ACE inhibitors and ARBs on renal variables in patients with T2DM and microalbuminuria or macroalbuminuria. Treatment with dapagliflozin reduced albuminuria compared with placebo and caused an initial fall in eGFR that was completely reversible 1 week after study drug discontinuation. The albuminuria‐lowering effect of dapagliflozin was to a large extent independent of the HbA1c‐, SBP‐, body weight‐ and eGFR‐lowering effects, suggesting that other mechanisms are also involved in the albuminuria‐lowering effect of dapagliflozin. These results also suggest that SGLT2 inhibition with dapagliflozin may confer renoprotective effects.
An initial fall in glomerular filtration rate (GFR) has been previously shown after administration of dapagliflozin 20. In the present study, however, we showed that the initial fall in eGFR was completely reversible only 1 week after dapagliflozin discontinuation. This reversibility indicates that the initial fall in eGFR did not reflect a reduction in the number of functioning nephrons but a haemodynamic‐induced reduction in single nephron GFR. Indeed, SGLT2 inhibition increases sodium delivery to the macula densa. The increased sodium delivery is sensed as an increase in circulating volume at the level of the juxtaglomerular apparatus, leading to a constriction of afferent renal arterioles, a reduction in intraglomerular pressure and a reversible reduction in single nephron GFR 10, 21. This reversible fall in eGFR is similar to that observed with ACE inhibitors and ARBs; however, the underlying mechanism is different from that of SGLT2 inhibitors, as ACE inhibitors and ARBs cause a vasodilatory efferent response, thereby decreasing intraglomerular pressure. Various studies have shown that a reduction in intraglomerular pressure is associated with long‐term renal preservation 22, 23, 24, suggesting that the reversible fall in eGFR with dapagliflozin is an indicator of its potential long‐term renal protective potency.
Dapagliflozin also significantly decreased albuminuria. This finding is consistent with other studies showing that SGLT2 inhibitors decrease albuminuria 25, 26. In patients with T2DM and chronic kidney disease (CKD), canagliflozin 100 mg/day and empagliflozin 25 mg/day decreased albuminuria by ∼22 and 35% relative to placebo, respectively 25, 26. A principal finding of the present study is that the albuminuria‐lowering effect of dapagliflozin is to a large extent independent of changes in eGFR, SBP, body weight or HbA1c. Apparently, other effects of dapagliflozin account for the albuminuria‐lowering properties, although we cannot exclude residual confounding caused by measurement variability. Continuous glucose excretion and the resulting metabolic effects not measured in this study may be one explanation for the potential renoprotective effects. Additionally, experimental studies have suggested that SGLT2 inhibitors exert intrarenal anti‐inflammatory effects that may be mediated by inhibition of glucose entry into tubular cells 13, 27. Anti‐inflammatory interventions have indeed been linked with albuminuria reduction 28. Unfortunately, inflammatory biomarkers were not measured in this study, and it is therefore not possible to verify this possibility.
The finding that the albuminuria‐lowering effect of dapagliflozin was independent of changes in HbA1c or SBP suggests a dissociation of the albuminuria response from responses in HbA1c or SBP. This uncoupling of responses in multiple characteristics of a single drug has also been observed with other drugs including ACE inhibitors, ARBs, dipeptidyl peptidase‐4 inhibitors and endothelin receptor antagonists 29, 30, 31. The underlying mechanisms of this uncoupling in response are unknown and require further study.
In the placebo arm, albuminuria decreased by nearly 20%, which is a larger placebo effect compared with other clinical trials 28, 32, 33. The reduction in albuminuria in the placebo arm probably reflects a regression to the mean phenomenon, as we selected only patients with UACR ≥30 mg/g based on the value from a single visit; therefore, it is particularly important to interpret the albuminuria reduction with dapagliflozin in the context of the placebo effect. Nevertheless, this still resulted in a reduction of >30% after 12 weeks of treatment. The placebo‐corrected reduction in albuminuria in the dapagliflozin arm would translate into a 30% relative risk reduction in end‐stage renal disease, assuming no changes in other renal risk markers 34.
As SGLT2 inhibition has been shown to increase urinary glucose excretion and reduce eGFR, there have been concerns around renal safety. There have also been concerns of unfavourable volume loss attributable to the diuretic action. In the present analysis in patients with albuminuria and a renal function mainly within CKD stage 1 and 2 categories, there were numerically more laboratory‐associated AEs related to renal function with dapagliflozin than with placebo, mainly as a result of increases in blood creatinine. This was an expected finding, given dapagliflozin's mode of action. Importantly, none of these events was classified as serious by the investigators. There was also no increase in the proportion of patients with more pronounced creatinine increases (≥1.5× baseline values) as compared with placebo. The proportion of patients with a marked increase in serum potassium was also similar to placebo. Although there were more patients with potentially volume‐related AEs as compared with placebo, none of these AEs was classified as serious. As observed in previous studies, more patients receiving dapagliflozin experienced a urinary tract infection, although the total number was low. Overall, this post hoc analysis indicated a beneficial renal profile in the studied CKD categories.
The present study has some limitations that should be taken into consideration. First, this was a post hoc analysis of two randomized controlled trials, and the original studies were not designed to assess the effect of dapagliflozin on renal variables. The results can therefore only be interpreted as hypothesis‐generating. The study did not include an active comparator to prospectively determine whether the albuminuria‐lowering effects were independent of glucose or blood pressure control. A study design with two additional treatment arms including a sulphonylurea derivative to control HbA1c and hydrochlorothiazide to control blood pressure could add to the interpretation. UACR was measured in a single spot urine sample. It is known that the day‐to‐day variability in spot urine samples is larger than first morning void samples. The use of single spot urine samples in the present study may have resulted in a lower precision (larger standard error) of the treatment effect compared with when first morning void samples were used; however, despite the use of single spot urine samples, a clear and highly significant treatment effect could still be detected. Finally, renal blood flow and filtration fraction were not measured to determine with ‘gold‐standard’ techniques the effect of dapagliflozin on intraglomerular pressure. The lack of measured GFR and use of eGFR, which is less accurate, may explain why the effect of dapagliflozin on albuminuria was not mediated by changes in GFR.
In conclusion, reductions in albuminuria in patients with T2DM and hypertension using renin‐angiotensin system blockade therapy were larger in dapagliflozin‐treated patients compared with placebo‐treated patients. The reduction in albuminuria appeared to be in large part independent of changes in HbA1c, SBP, body weight or eGFR. The safety profile of dapagliflozin in the present study was consistent with other dapagliflozin studies and no increase in the occurrence of serious renal AEs was observed. Improved glycaemic control and reductions in SBP and body weight with dapagliflozin treatment, in addition to beneficial renal effects, may alter the course of progressive diabetic kidney disease and warrant further study.
Conflict of Interest
H. J. L. H. is a consultant to Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Janssen and ZS‐Pharma (honoraria were paid to his employer). E. J., I. G.‐N. and C. D. S. are employees/shareholders of AstraZeneca. V. A. C. was an employee of AstraZeneca at the time of the study.
H. J. L. H. and C. D. S. designed the post hoc analysis, interpreted the data and wrote the manuscript. E. J., I. G.‐N. and V. A. C. participated in the analysis and interpretation of data and provided a critical review of the manuscript. All authors approved the final version for submission.
Acknowledgements
This study was funded by AstraZeneca. The authors would like to thank Irina Baldycheva of AstraZeneca for statistical support. Editorial support was provided by Jean Turner and Robert Axford‐Gatley of PAREXEL and was funded by AstraZeneca.
References
- 1. Heerspink HJ, de Zeeuw D. The kidney in type 2 diabetes therapy. Rev Diabet Stud 2011; 8: 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet 2010; 375: 2223–2233. [DOI] [PubMed] [Google Scholar]
- 3. Komoroski B, Vachharajani N, Feng Y, Li L, Kornhauser D, Pfister M. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2009; 85: 513–519. [DOI] [PubMed] [Google Scholar]
- 4. Komoroski B, Vachharajani N, Boulton D et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose‐dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009; 85: 520–526. [DOI] [PubMed] [Google Scholar]
- 5. Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med 2013; 125: 181–189. [DOI] [PubMed] [Google Scholar]
- 6. Sjöstrom CD, Johansson P, Ptaszynska A, List J, Johnsson E. Dapagliflozin lowers blood pressure in hypertensive and non‐hypertensive patients with type 2 diabetes. Diab Vasc Dis Res 2015; 12: 352–358. [DOI] [PubMed] [Google Scholar]
- 7. Bolinder J, Ljunggren Ö, Johansson L et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014; 16: 159–169. [DOI] [PubMed] [Google Scholar]
- 8. Komala MG, Panchapakesan U, Pollock C, Mather A. Sodium glucose cotransporter 2 and the diabetic kidney. Curr Opin Nephrol Hypertens 2013; 22: 113–119. [DOI] [PubMed] [Google Scholar]
- 9. Panchapakesan U, Pegg K, Gross S et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells–renoprotection in diabetic nephropathy? PLoS One 2013; 8: e54442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cherney DZ, Perkins BA, Soleymanlou N et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014; 129: 587–597. [DOI] [PubMed] [Google Scholar]
- 11. De Nicola L, Gabbai FB, Liberti ME, Sagliocca A, Conte G, Minutolo R. Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am J Kidney Dis 2014; 64: 16–24. [DOI] [PubMed] [Google Scholar]
- 12. Gilbert RE. Sodium‐glucose linked transporter‐2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int 2014; 86: 693–700. [DOI] [PubMed] [Google Scholar]
- 13. Terami N, Ogawa D, Tachibana H et al. Long‐term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One 2014; 9: e100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato‐Horiguchi C, Ogawa D, Wada J et al. Telmisartan attenuates diabetic nephropathy by suppressing oxidative stress in db/db mice. Nephron Exp Nephrol 2012; 121: e97–e108. [DOI] [PubMed] [Google Scholar]
- 15. Kojima N, Williams JM, Takahashi T, Miyata N, Roman RJ. Effects of a new SGLT2 inhibitor, luseogliflozin, on diabetic nephropathy in T2DN rats. J Pharmacol Exp Ther 2013; 345: 464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gangadharan KM, Gross S, Mudaliar H et al. Inhibition of kidney proximal tubular glucose reabsorption does not prevent against diabetic nephropathy in type 1 diabetic eNOS knockout mice. PLoS One 2014; 9: e108994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol 2016; 4: 211–220. [DOI] [PubMed] [Google Scholar]
- 18. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin‐angiotensin system blockade. Blood Press 2016; 25: 93–103. [DOI] [PubMed] [Google Scholar]
- 19. Foundation NK. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39: S1–S266. [PubMed] [Google Scholar]
- 20. Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose‐regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013; 15: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vallon V, Blantz RC, Thomson S. Glomerular hyperfiltration and the salt paradox in early [corrected] type 1 diabetes mellitus: a tubulo‐centric view. J Am Soc Nephrol 2003; 14: 530–537. [DOI] [PubMed] [Google Scholar]
- 22. Anderson S, Meyer TW, Rennke HG, Brenner BM. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest 1985; 76: 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Apperloo AJ, de Zeeuw D, de Jong PE. A short‐term antihypertensive treatment‐induced fall in glomerular filtration rate predicts long‐term stability of renal function. Kidney Int 1997; 51: 793–797. [DOI] [PubMed] [Google Scholar]
- 24. Holtkamp FA, de Zeeuw D, Thomas MC et al. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long‐term renal function. Kidney Int 2011; 80: 282–287. [DOI] [PubMed] [Google Scholar]
- 25. Barnett AH, Mithal A, Manassie J et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369–384. [DOI] [PubMed] [Google Scholar]
- 26. Yale JF, Bakris G, Cariou B et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013; 15: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ishibashi Y, Matsui T, Yamagishi S. Tofogliflozin, a highly selective inhibitor of SGLT2, blocks proinflammatory and proapoptotic effects of glucose overload on proximal tubular cells partly by suppressing oxidative stress generation. Horm Metab Res 2015. [9 Jul Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28. de Zeeuw D, Bekker P, Henkel E et al. The effect of CCR2 inhibitor CCX140‐B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol 2015; 3: 687–696. [DOI] [PubMed] [Google Scholar]
- 29. Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 2013; 36: 3460–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schievink B, de Zeeuw D, Parving HH, Rossing P, Lambers Heerspink HJ. The renal protective effect of angiotensin receptor blockers depends on intra‐individual response variation in multiple risk markers. Br J Clin Pharmacol 2015; 80: 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schievink B, de Zeeuw D, Smink PA et al. Prediction of the effect of atrasentan on renal and heart failure outcomes based on short‐term changes in multiple risk markers. Eur J Prev Cardiol 2015; DOI: 10.1177/2047487315598709 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Zeeuw D, Coll B, Andress D et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J Am Soc Nephrol 2014; 25: 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358: 2433–2446. [DOI] [PubMed] [Google Scholar]
- 34. Heerspink HJ, Kropelin TF, Hoekman J, de Zeeuw D. Drug‐induced reduction in albuminuria is associated with subsequent renoprotection: a meta‐analysis. J Am Soc Nephrol 2015; 26: 2055–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]


