Abstract
Although pharmacotherapy with atypical antipsychotics is common in child psychiatry, there has been little research on this issue. To compare the efficacy and safety of risperidone and aripiprazole in the treatment of preschool children with disruptive behavior disorders comorbid with attention deficit-hyperactivity disorder (ADHD). Randomized clinical trial conducted in a university-affiliated child psychiatry clinic in southwest Iran. Forty 3-6-year-old children, diagnosed with oppositional defiant disorder comorbid with ADHD, were randomized to an 8-week trial of treatment with risperidone or aripiprazole (20 patients in each group). Assessment was performed by Conners’ rating scale-revised and clinical global impressions scale, before treatment, and at weeks 2, 4, and 8 of treatment. The data were analyzed by SPSS version 16. Mean scores between the two groups were compared by analysis of variance and independent and paired t-test. Mean scores of Conners rating scales were not different between two groups in any steps of evaluation. Both groups had significantly reduced scores in week 2 of treatment (P = 0.00), with no significant change in subsequent measurements. Rates of improvement, mean increase in weight (P = 0.894), and mean change in fasting blood sugar (P = 0.671) were not significantly different between two groups. Mean serum prolactin showed a significant increase in risperidone group (P = 0.00). Both risperidone and aripiprazole were equally effective in reducing symptoms of ADHD and oppositional defiant disorder, and relatively safe, but high rates of side effects suggest the cautious use of these drugs in children.
Keywords: Aripiprazole, attention deficit-hyperactivity disorder, oppositional defiant disorder, risperidone
INTRODUCTION
Attention deficit-hyperactivity disorder (ADHD) is a common developmental disorder in children which often continues into adulthood. It is the most common psychiatric disorder in children so that it constitutes about 50% of referrals to child psychiatry clinics.[1] The prevalence of ADHD in children is 5–12% worldwide.[2]
ADHD has a high rate of comorbid psychiatric disorders. Half of clinical samples have disruptive behavior disorders (DBDs), consisting of oppositional defiant disorder or conduct disorder.[2] Many longitudinal studies have followed children from early childhood to evaluate the impact of early DBDs. Such DBDs have been associated with adult smoking, alcohol and illicit drug use, physical aggression, and risky sexual behavior; repeated or serious crimes, and involvement in destructive offenses.[3] Therefore, evidence-based treatments to mitigate these problems in early childhood are clearly warranted.
Treatment of behavioral disorders at an early age may help to prevent long-term disability. The cornerstone of treatment for these disorders is behavioral therapy, but psychotherapy may not be enough. Furthermore, some patients do not respond to these interventions. Therefore, physicians in daily practice sometimes require pharmacological intervention in preschool children with chronic, disabling, or resistant behavioral problems.[2]
Most psychiatric drugs are effective in ADHD.[2] Pharmacotherapy with atypical antipsychotics is increasing quickly in child and adolescent psychiatry, and this is especially the case for children with DBDs. For example, a study examining the prescription trend of atypical antipsychotics among 11,700 Arkansas Medicaid-covered children under age 18 who were newly treated with atypical antipsychotics from 2001 through 2005 found that the number of children receiving the medications doubled during this period. The most common condition was ADHD, followed by depression, conduct disorder, oppositional defiant disorder, and adjustment reactions. Most new users were given an initial prescription for risperidone.[4]
Risperidone is a safe and effective treatment for DBDs and ADHD in children.[5] Aripiprazole has a unique pharmacological profile, as a partial agonist at the dopamine D2 and serotonin 5HT1A receptors and an antagonist at the serotonin 5HT2A receptor; this drug has few side effects (such as extrapyramidal syndrome, hyperprolactinemia, weight gain, metabolic disorders, and sedation) which are common problems with other antipsychotic drugs. Efficacy and tolerability of aripiprazole in children and adolescents have been well-demonstrated in many clinical studies, which supported approvals granted by the US Food and Drug Administration for schizophrenia, bipolar diseases, and irritability associated with autistic disorder in children and adolescents.[6] This study was conducted to compare the efficacy and safety of risperidone and aripiprazole in the treatment of preschool children with DBD comorbid with ADHD.
SUBJECTS AND METHODS
This study was a randomized clinical trial conducted in the child psychiatry clinic affiliated with a university in Southwest Iran. Children 3–6-year-old with oppositional defiant disorder comorbid with ADHD, according to the diagnostic criteria of Diagnostic and Statistical Manual of Mental Disorders, Revision IV-Text Revised, were enrolled into the study. Children with mental retardation or other developmental disorders such as autism, chronic physical, or neurological illness, or those receiving other psychotropic drugs were excluded from the study. After the aims of the study were fully explained to the parents, they were asked to complete a consent form to participate in the study, whereas patient anonymity was preserved.
Treatment was initiated with 0.25 mg risperidone once daily, increased by 0.25 mg weekly, to a maximum dose of 2 mg/day,[7] or 2.5 mg aripiprazole, increased by 2.5 mg weekly, to a maximum dose of 10 mg/day.[8] For blinding, the patients were rated by a clinician who was blind to group assignment of the patients. At the beginning of the treatment, shortened and revised version of Conners parent rating scale, clinical global impressions (CGI) scale, body weight, fasting blood glucose (FBS), and serum prolactin were measured. Then at weeks 2, 4, and 8, CGI and Conners scale were readministered. Furthermore, weight, FBS, and prolactin were measured again at week 8. The instrument of data gathering was shortened and revised Conners parent rating scale, which contains 27 items in four subscales: Oppositional defiant, inattention, hyperactivity, and ADHD subscales. Reliability and validity of the Persian version of this questionnaire have been already confirmed.[9]
The data were analyzed by SPSS statistics software-IBM, and the mean score (standard deviation) of variables was calculated. Then, mean scores between the two groups were compared by analysis of variance (ANOVA), and independent and paired t-test.
RESULTS
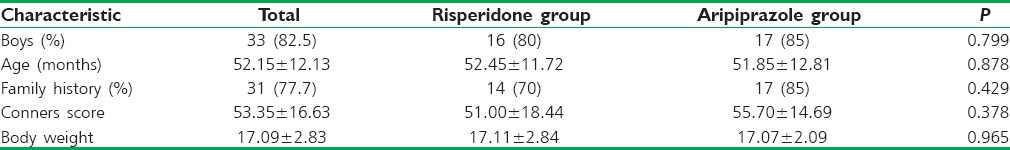
The two groups were not significantly different in age, gender, family history, and severity of illness [Table 1].
Table 1.
Demographic characteristics of participants
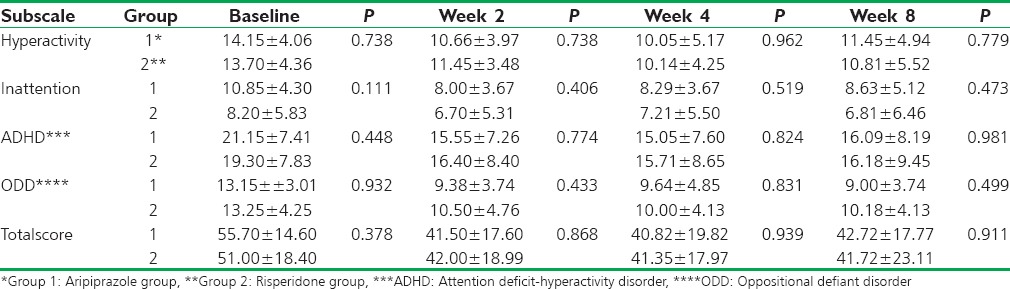
ANOVA showed that mean scores of Conners rating scale had statistically significant decrease in both groups, and t-test and paired comparison of different steps showed that there was significant difference between measurements at steps 1 and 2 (P = 0.00), but there was no significant difference between steps 2 and 3 (P = 1.00 and 0.431 for aripiprazole and risperidone groups, respectively) and steps 3 and 4 (P = 0.88 and 0.291 for aripiprazole and risperidone groups, respectively). All subscales of the Conners rating scale showed a significant decrease between steps 1 and 2 in both groups (P < 0.001). Mean scores of Conners rating scale are shown in Table 2.
Table 2.
Scores of Conners rating scale subscales
Results of CGI-improvement (CGI-I) showed that 7 (35%) patients of aripiprazole group had CGI-I scores of 1 or 2 (much or very much improvement) at week 8, which means significant improvement. This measure was 6 (30%) patients in week 8 for risperidone group. There was no significant difference between two groups at endpoint (P = 0.898). Similarly, no significant difference was observed between the two groups in CGI-severity (P = 0.380) at the endpoint.
The mean increase in body weight was 1.2 ± 1.13 kg in aripiprazole group and 1.25 ± 0.68 kg in risperidone group, with no significant difference (P = 0.894). The mean increase in FBS was − 0.25 ± 7.67 mg/dl in aripiprazole group and 3.5 ± 13.43 in risperidone group, with no significant difference (P = 0.671). The mean increase in serum prolactin level was 1.37 ± 0.87 in aripiprazole group and 22.38 ± 3.61 in risperidone group, with a significant difference (P = 0.00).
Six parents in risperidone group (30%), and 3 parents in aripiprazole group (15%) reported tolerance to drug effect, which was not a statistically significant difference (P = 0.289). The dose of aripiprazole was between 1.25 and 10 mg in week 8 of treatment; with a mean of 4.69 ± 1.25 mg. Dose of risperidone was between 0.25 and 2 mg in week 8; with a mean of 1.05 ± 0.51 mg.
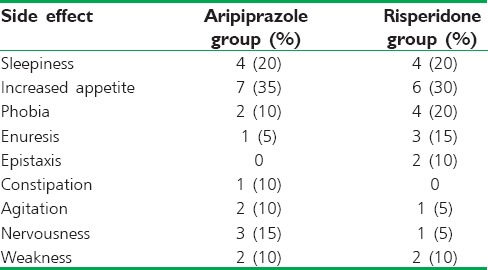
Most parents in both groups reported minor side effects. Sleepiness and increased appetite were the most frequently reported side effects in both groups. Frequency of side effects was not different between the two groups (P > 0.05). Most side effects were mild and transient. Four parents in aripiprazole group decided to discontinue the medication due to severely increased appetite (one patient), sleepiness (two patients), and agitation (one patient). Five parents in risperidone group discontinued medication due to tolerance to the drug effects (three patients), epistaxis (one patient), and severely increased appetite (one patient). None of the patients developed extrapyramidal side effects. Four parents in the risperidone group and three parents in aripiprazole group reported no side effects. Frequency of side effects is shown in Table 3.
Table 3.
Side effects reported by parents
DISCUSSION
Results of this study showed that clinical improvement was seen at the 2nd week of treatment with both drugs, and there was no further improvement with continuing medication or increasing the dosage. Some patients developed tolerance to the medication effects, which was more frequent in patients receiving risperidone. In a chart review study of 25 preschool-aged children treated with risperidone for DBDs, tolerance to risperidone was reported in 32% of the patients.[10]
Severity of illness at the beginning of treatment, and at weeks 2, 4, and 8 showed no statistically significant difference between the two groups, which means that both medications were equally effective in these patients. 35% of the patients in aripiprazole group and 30% in risperidone group experienced significant clinical improvement at week 8 of treatment based on CGI-I scores. In this study, some parents refused to increase the dose of medication as prescribed, which may have affected the results. In the chart review study of 25 preschool-aged children treated with risperidone for DBDs, the majority of subjects (72%) showed “much” to “very much” improvement in target disruptive behavior symptoms, and the duration of risperidone treatment ranged from 2 to 60 weeks (18.87 ± 15.19), which is longer than this study.[10]
In this study, there were no significant differences in weight gain and FBS level change between the two groups, but the increase in prolactin level in the group treated with risperidone was significantly higher than those treated with aripiprazole. No serious or life-threatening side effects were reported, and none of the patients experienced drug-induced extrapyramidal side effects.
Results of an 8-week study on bipolar children,[7] 16-week study on patients with pervasive developmental disorders,[11] and a 6-month study along with placebo showed that risperidone at low dose effectively controls prolonged behavioral disorders in preschool-aged children.[12] A retrospective study on children with aggressive behaviors associated with various diagnoses showed 36% decrease in symptoms after taking risperidone.[13] Results of these studies have shown obviously increased weight and increased prolactin levels in the patients taking risperidone.
In a 6-week, double-blinded study of 118 children with severely disruptive behaviors and subaverage intelligence, receiving 0.02–0.06 mg/kg/day of risperidone or placebo, the risperidone group showed significantly greater improvement on the conduct problem scale from week 1 through the endpoint. The most common adverse effects were headache and somnolence. The extrapyramidal symptom profile of risperidone was comparable to that of placebo. Mean weight increases of 2.2 kg and 0.9 kg occurred in the risperidone and placebo groups, respectively.[14]
In an open-label study of eight normally developing preschool children with conduct disorder, treated with risperidone for 8 weeks, all patients were classified as “responders” (very much or much improved). Tolerability was good, and serious adverse effects were not observed. They detected statistically significant increase in prolactin level (P < 0.05), but no clinical symptoms associated with prolactinemia.[15]
In another open-label study of risperidone monotherapy on 31 children and adolescents 4–15 years of age with pediatric bipolar disorder and ADHD, results showed that improvement in ADHD symptoms was contingent on improvement in manic symptoms. Although both hyperactive/impulsive and inattentive ADHD symptoms were significantly improved with risperidone, improvement was modest, and only 29% of the participants showed a 30% reduction in ADHD scores and had a CGI-I of 1 or 2.[16]
In a study assessing risperidone introduction into stimulant for severe aggression in 168 6–12-year-old children with ADHD, risperidone provided a moderate but variable improvement in aggressive and other seriously disruptive behaviors in children.[17]
Results of a 6-week open study on 23 hyperactive children aged 8–12 years old treated with aripiprazole, at a mean dose of 6.7 mg taken daily, showed significant improvement in symptoms and function. The most frequently reported adverse events were sedation (78.3%) and headache (47.8%).[18] In a study on patients with Tourette's disorder and ADHD, the drug was well-tolerated, and none of the patients discontinued medication due to adverse events. Furthermore, the drug led to significant improvement of Tourette's disorder and had a modest effect on the comorbid ADHD.[8]
CONCLUSIONS
Antipsychotic drugs, risperidone and aripiprazole, are relatively effective and safe for treatment of DBDs comorbid with ADHD in preschool-aged children. Side effects are generally mild to moderate but given the high rate of side effects of these drugs, they should be used with caution in this age group.
Recommendations
We recommend further studies with larger sample size and longer follow-up as well as to compare aripiprazole and risperidone with other atypical antipsychotics and Ritalin, or behavioral interventions in the treatment of behavioral disorders in preschool children.
Financial support and sponsorship
Shahrekord University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This research was conducted based on RCT No. 11983 and Project No. 92-6-18 funded by the Research and Technology Deputy of Shahrekord University of Medical Sciences. Hereby, we thank the Research and Technology Deputy of Shahrekord University of Medical Sciences.
REFERENCES
- 1.Sadock BJ, Kaplan HI, Sadock VA, Ruiz P. Kaplan & Sadock's Comprehensive Textbook of Psychiatry. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 2.Lacrimiora S, Arnold LE. Attention deficit hyperactivity disorders. In: Martin A, Wolkmar FR, editors. Lewis's Child and Adolescent Psychiatry: A Comprehensive Textbook. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 3.Stattin H, Magnusson D. The role of early aggressive behavior in the frequency, seriousness, and types of later crime. J Consult Clin Psychol. 1989;57:710–8. doi: 10.1037//0022-006x.57.6.710. [DOI] [PubMed] [Google Scholar]
- 4.Pathak P, West D, Martin BC, Helm ME, Henderson C. Evidence-based use of second-generation antipsychotics in a state Medicaid pediatric population, 2001-2005. Psychiatr Serv. 2010;61:123–9. doi: 10.1176/ps.2010.61.2.123. [DOI] [PubMed] [Google Scholar]
- 5.Turgay A, Binder C, Snyder R, Fisman S. Long-term safety and efficacy of risperidone for the treatment of disruptive behavior disorders in children with subaverage IQs. Pediatrics. 2002;110:e34. doi: 10.1542/peds.110.3.e34. [DOI] [PubMed] [Google Scholar]
- 6.Kirino E. Efficacy and safety of aripiprazole in child and adolescent patients. Eur Child Adolesc Psychiatry. 2012;21:361–8. doi: 10.1007/s00787-012-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biederman J, Mick E, Hammerness P, Harpold T, Aleardi M, Dougherty M, et al. Open-label, 8-week trial of olanzapine and risperidone for the treatment of bipolar disorder in preschool-age children. Biol Psychiatry. 2005;58:589–94. doi: 10.1016/j.biopsych.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Masi G, Gagliano A, Siracusano R, Berloffa S, Calarese T, Ilardo G, et al. Aripiprazole in children with Tourette's disorder and co-morbid attention-deficit/hyperactivity disorder: A 12-week, open-label, preliminary study. J Child Adolesc Psychopharmacol. 2012;22:120–5. doi: 10.1089/cap.2011.0081. [DOI] [PubMed] [Google Scholar]
- 9.Zargarinejad G, Yekkehyazdandoost R. Efficacy of parent's training on problem behaviors in ADHD children. Psychol Stud. 2007;3:29–48. [Google Scholar]
- 10.Coskun M, Zoroglu SS, Ozturk M. Risperidone treatment in preschool children with disruptive behavior disorders: A chart review study. Bull Clin Psychopharmacol. 2011;21:33–41. [Google Scholar]
- 11.Luby J, Mrakotsky C, Stalets MM, Belden A, Heffelfinger A, Williams M, et al. Risperidone in preschool children with autistic spectrum disorders: An investigation of safety and efficacy. J Child Adolesc Psychopharmacol. 2006;16:575–87. doi: 10.1089/cap.2006.16.575. [DOI] [PubMed] [Google Scholar]
- 12.Masi G, Cosenza A, Mucci M, Brovedani P. A 3-year naturalistic study of 53 preschool children with pervasive developmental disorders treated with risperidone. J Clin Psychiatry. 2003;64:1039–47. doi: 10.4088/jcp.v64n0909. [DOI] [PubMed] [Google Scholar]
- 13.Cesena M, Gonzalez-Heydrich J, Szigethy E, Kohlenberg TM, DeMaso DR. A case series of eight aggressive young children treated with risperidone. J Child Adolesc Psychopharmacol. 2002;12:337–45. doi: 10.1089/104454602762599880. [DOI] [PubMed] [Google Scholar]
- 14.Aman MG, De Smedt G, Derivan A, Lyons B, FindlingRL Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry. 2002;159:1337–46. doi: 10.1176/appi.ajp.159.8.1337. [DOI] [PubMed] [Google Scholar]
- 15.Ercan ES, Basay BK, Basay O, Durak S, Ozbaran B. Risperidone in the treatment of conduct disorder in preschool children without intellectual disability. Child Adolesc Psychiatry Ment Health. 2011;5:10. doi: 10.1186/1753-2000-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biederman J, Hammerness P, Doyle R, Joshi G, Aleardi M, Mick E. Risperidone treatment for ADHD in children and adolescents with bipolar disorder. Neuropsychiatr Dis Treat. 2008;4:203–7. doi: 10.2147/ndt.s1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aman MG, Bukstein OG, Gadow KD, Arnold LE, Molina BS, McNamara NK, et al. What does risperidone add to parent training and stimulant for severe aggression in child attention-deficit/hyperactivity disorder? J Am Acad Child Adolesc Psychiatry. 2014;53:47–60.e1. doi: 10.1016/j.jaac.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findling RL, Short EJ, Leskovec T, Townsend LD, Demeter CA, McNamara NK, et al. Aripiprazole in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2008;18:347–54. doi: 10.1089/cap.2007.0124. [DOI] [PubMed] [Google Scholar]


