Abstract
Objective:
To evaluate the diagnostic value of endoscopic ultrasonography (EUS) and contrast-enhanced harmonic (CEH) EUS in patients with gastrointestinal stromal tumors (GISTs).
Patients and Methods:
About 19 patients with suspected GISTs underwent EUS and CEH-EUS before tumor resection. The malignant potential was assessed according to the modified Fletcher classification system. Patients were divided into lower (Group I) and higher (Group II) malignant potential group. The clinical characteristics and EUS/CEH-EUS features were compared between two groups.
Results:
The tumor size in Group II was significantly larger than that in Group I (14.6 ± 5.8 mm vs. 32.1 ± 8.4 mm, P < 0.05). Heterogeneous echogenicity was observed in 4 (4/8) cases in Group II and none in Group I (P < 0.05). Irregular intratumoral vessels were detected in 6 cases in Group II and none in Group I (P < 0.05). The sensitivity and specificity of irregular vessel detection for discriminating higher from lower malignant potential GISTs were 75% and 100%, respectively. The positive predictive value and negative predictive value of detection of irregular vessels to high malignant potential GISTs were 33% and 100%, respectively.
Conclusion:
Detection of irregular intratumoral vessels can predict higher malignant potential before tumor resection. The tumor size and echogenicity are assistant factors for malignant potential assessment. Endoscopic resection is an efficacious treatment with good security for appropriate patients.
Keywords: Contrast enhanced harmonic endoscopic ultrasonography, gastrointestinal stromal tumor, irregular vessels, malignant potential
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract.[1] Approximately, two-thirds of all GISTs occur in the stomach, followed by the small intestine, rectum, and esophagus.[2] Moreover, the body and fundus are the most frequent site of GISTs in stomach. It is difficult to differentiate GISTs from other submucosal lesions with white light endoscopy. Endoscopy reveals a smooth mass with normal overlying mucosa or with mucosal ulceration in larger tumors. Tissue sampling with standard endoscopic mucosal biopsy is mostly negative in GISTs for lying underneath normal appearing mucosa. Endoscopic ultrasonography (EUS) has the ability to obtain differential diagnosis by the wall layer of origin and echogenicity. On EUS, GISTs are usually hypoechoic masses, which originate from the muscularis propria, also occasionally they can arise from the muscularis mucosa. In some larger tumors, the EUS features include irregular extra-luminal border, echogenic foci, and cystic spaces.[3,4]
Although only 10%-30% of GISTs are clinically malignant, all GISTs are known to have some degree of malignant potential.[5] Therefore, a simple classification of benign and malignant is not appropriate. At present, the modified Fletcher classification system[6] has been used most frequently to assess the malignant potential of GISTs [Table 1]. This risk classification system is composed of four factors: Tumor size, tumor site, mitotic count, and presence of tumor rupture and classifies the malignant potential as four grade: Very low, low, intermediate, and high grade. For patients with high risk of recurrence, preoperative neoadjuvant therapy with imatinib is recommended.[7] Among the four factors in Fletcher system, tumor size, tumor site, and presence of tumor rupture are easy to determine by endoscopy and EUS, whereas the mitotic count is generally obtained from resection specimens. Although EUS-guided fine needle aspiration (FNA) and EUS-guided trucut biopsy (TCB) can also obtain specimens before surgery, these techniques have risks of complications such as bleeding, localized abdominal pain, puncture site infection, and fever, and both have a limitation of inadequate specimen for immunohistochemical analysis.[8,9,10,11,12] For these reasons, EUS-FNA or EUS-TCB is only performed in patients with strong indications. Therefore, the malignant potential is hardly achieved before surgery. Contrast-enhanced ultrasound (CE-US) has been applied in clinical practice for more than 10 years, especially in diseases of digestive and cardiovascular system.[13,14] With the development of EUS techniques and the advent of second generation contrast agent, CE harmonic (CEH)-EUS has been used for diagnosis of lesions of pancreas, liver, and gallbladder.[15,16] In this prospective study, EUS and CEH-EUS were performed in patients with resectable GIST before resection. The relationship between features of EUS/CEH-EUS and the malignant potential in GISTs was evaluated.
Table 1.
Modified Flecther classification system of risk of malignant potential
PATIENTS AND METHODS
Patients
Between March 2015 and December 2015, a total of 19 patients suspected of having a GIST were enrolled in our study. The exclusion criteria include severe heart failure, severe chronic obstructive pulmonary disease, known allergic disposition to SonoVue (59 mg, Bracco societa per azioni, Milan, Italy), pregnancy or lactation, severe psychiatric disorders, and esophagogastric varices. This study was approved by the Ethics Committee of Beijing Friendship Hospital, Capital Medical University. All patients provided informed consent.
Endoscopy procedure
Before undergoing CEH-UES, endoscopy features including tumor location, size, mucosa appearance, and other lesions were obtained. Then standard B-mode EUS was performed in all patients to determine the tumor size, originating layer, echogenicity, and the growth patterns. Color Doppler mode was performed to detect intratumoral color Doppler flow signals. For CEH-EUS, the extended pure harmonic detection mode was used, with the mechanical index set at 0.25 and a transmitting frequency of 4.7 MHz. EUS and CEH-EUS were performed in the left lateral decubitus position under midazolam-induced conscious-sedation with heart rate and oxygen saturation monitoring. A bolus infusion of SonoVue (59 mg/5 ml) was administered via peripheral vein catheter, followed by a 10 ml saline flush. The agent arrival time (AAT) was recorded, and the vascular structures were assessed in real time. US video sequences were continuously recorded and stored in the hard disk for off-line analyses. All EUS and CEU-EUS were performed by two experienced endoscopists using an Olympus GF-UE260 (Olympus Medical Systems Co., Ltd., Tokyo, Japan) connected to a US system (ProSound SSD α-10; Hitachi-Aloka, Tokyo, Japan).
Endoscopic resection procedure
Routine blood count, liver and kidney function, and coagulation function were performed in all patients. CE-computed tomography (CT) and chest radiograph were obtained to exclude distant metastases. None of the 19 cases had distant or lymph node metastasis. The operation selection was based on the tumor size and growth patterns. Laparoscopic surgical procedures were performed in 3 cases with tumor size >50 mm and/or with obviously extraluminal growth. Endoscopic resections were performed in the other 16 patients under a complete general anesthesia. An Olympus GIF260J endoscope with an auxiliary water jet (Olympus Medical Systems Co., Ltd., Tokyo, Japan) was used. The steps of endoscopic resection were as follows: (1) Marking of the entire circumference using a Dual knife at intervals of a few millimeters, approximately 2–5 mm laterally from the lesion; (2) local injection of Glycerin-Fructose-Methylene mixture (consisted of 10% glycerin, 5% fructose, and methylene 4 mg in 250 ml normal saline solution); (3) circumferential mucosal incision using Dual knife; (4) dissection of the tumor using IT knife; (5) en bloc resection of the tumor using snare; (6) withdrawal of the resect specimen using the grasping forceps; (7) wound closure with clips and hemostasis using hemostatic forceps or by argon plasma coagulation anytime when hemorrhage is observed. After surgery or endoscopic resection, fasting, water-deprivation, and intravenous fluid continued for at least 48 h. Patients without complication started drinking water after 48 h and were discharged within 4–7 days.
Pathology and immunohistochemistry examination
The histologic diagnosis of GIST was defined as subepithelial tumors composed of spindle cells that stained positive for c-kit and CD34.[17] Mitotic count was obtained from histological analysis of hematoxylin-eosin stained resected specimens. Risk classification of malignancy was performed according to the modified Fletcher classification system. The immunohistochemistry examination included the following: CD34, c-kit, DOG-1, smooth muscle actin (SMA), desmin, and Ki67.
Statistical analyses
The independent sample t-test was used for the comparison of two groups regarding continuous variables. The Chi-square test was used to compare the frequency data of two groups including clinical characteristics, EUS, and CEH-EUS features. A difference was considered significant when P < 0.05. Statistical analyses were performed with SPSS software (version 17.0; SPSS, Chicago, IL, USA).
RESULTS
One patient was pathologically diagnosed as middle differentiation adenocarcinoma and was excluded from this study. The other 18 patients were confirmed by pathology and immunohistochemistry examination. According to the modified Fletcher classification system, 18 resected tumors consisted of 4 very low, 6 low, 6 intermediate, and 2 high malignant potential GISTs. We divided the 18 patients into 2 groups: Group I (lower malignant potential) included low and very low malignant potential and Group II (higher malignant potential) included high and intermediate malignant potential. Mean (mean ± standard deviation [SD]) age was 51.5 ± 10.0 years in Group I and 58.9 ± 14.1 years in Group II [Table 2]. One (1/10) patient of Group I and 3 (3/8) patients of Group II were symptomatic. The other 14 patients were asymptomatic and were discovered incidentally or by other image examinations. The majority of tumors (7/10 vs. 5/8) located in fundus and cardia of stomach. No significant difference of these 3 features was found between Group I and Group II (P = 0.21, 0.28 and 0.95).
Table 2.
Clinical characteristics of 18 patients with gastrointestinal stromal tumors
Mean (mean ± SD) diameter measured on EUS was 14.6 ± 5.8 mm and 32.1 ± 8.4 mm [Table 3] and tumor size in Group II was significantly larger than that in Group I (P < 0.05). Furthermore, the number of patient with tumor size ≥20 mm was 3 of 10 in Group I and 7 of 8 in Group II (P < 0.05). Intraluminal growth was the predominant growth pattern in the two groups except that extraluminal growth was found in one patient in Group II, who underwent surgical resection. Homogeneous hypoechogenicity was observed in all ten patients in Group I while heterogeneous hypoechogenicity with focal hyperechoic area or anechoic area was found in four of eight patients in Group II (P < 0.05). After a bolus infusion of SonoVue, the mean value of AAT was 10.9 ± 0.2 s in Group I and 10.7 ± 0.2 s in Group II (P = 0.10). No irregular intratumoral vessel was found in Group I, regular fine intratumoral vessels were found in six patients, and no vessel was detected in other four patients. On contrast, in Group II, irregular intratumoral vessels were found in six of eight patients [Figure 1] and regular fine intratumoral vessels were found in other two patients.
Table 3.
Endoscopic ultrasonography and contrast enhanced harmonic-endoscopic ultrasonography features of 18 patients with gastrointestinal stromal tumors
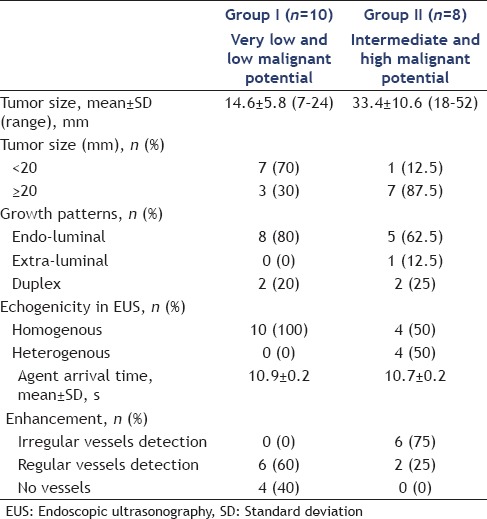
Figure 1.
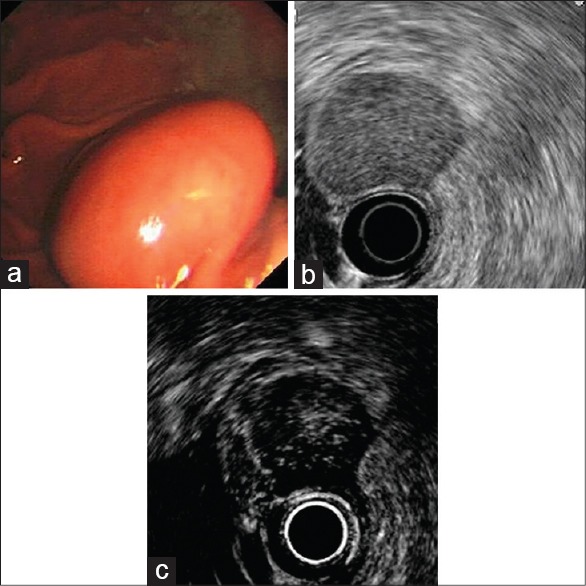
An intermediate malignant potential gastrointestinal stromal tumor of the stomach in a 76-year-old man. (a) White light endoscopy shows a ball-shaped mass with normal overlying mucosa; (b) endoscopic ultrasonography shows a hypoechoic mass originated from the muscularis propria. (c) Contrast enhanced harmonic -endoscopic ultrasonography shows irregular vessels
Except one patient with middle differentiation adenocarcinoma, the other 18 patients met GIST diagnostic criteria. According to the modified Fletcher classification system, tumor size and mitotic count were used for assessing the malignant potential, and no tumor rupture was found in all patients. In Group I, four and six patients were very low and low malignant potential, respectively; in Group II, six and two patients were intermediate and high malignant potential, respectively. DOG-1 was positive in all 18 patients. SMA was positive in one patient with very low malignant potential GIST and one with low malignant potential GIST. Desmin was only positive in one patient with low malignant potential GIST. Ki67 was positive in both two patients with high malignant potential GIST and one with intermediate malignant potential GIST [Figure 2].
Figure 2.
Pathology and immunohistochemistry examination (the same case as in Figure 1). (a) H and E; (b) CD34; (c) c-kit; (d) DOG-1; (e) smooth muscle actin; (f) desmin; (g) Ki67 (all for ◊200)
DISCUSSION
All GISTs are known to have some degree of malignant potential.[5] The modified Fletcher classification system is the most commonly used standard for assessing the malignant potential of GISTs, which comprises four factors: Tumor size, tumor site, mitotic count, presence of tumor rupture. The assessment of malignant potential is difficult to perform before tumor resection for the reason that the modified Fletcher classification system depends on postoperative pathology. In the present study, we observed features of EUS and CEH-EUS preoperatively and evaluated the correlation between the features and malignant potential of GISTs. The modified Fletcher classification system classifies the malignant potential as four grades: Very low, low, intermediate, and high malignant potential. We collapsed patients with very low and low malignant potential GISTs into Group I (the lower group) as well as intermediate and high malignant potential GISTs into Group II (the higher group). No significant difference was found in the mean age, gender and tumor location between Group I and Group II. The majority of patients in both groups were asymptomatic, of which subepithelial masses were detected incidentally during routine endoscopic screening or other image examinations. The tumor size is one of the four factors in the modified Fletcher classification system, and the mean tumor size in Group II was significantly larger than that in Group I (4.6 ± 5.8 mm vs. 33.4 ± 10.6 mm, P < 0.05). The tumor size was < 20 mm in most patients in Group I while only few in Group II (7/10 [70%] vs. 1/8 [12.5%], P < 0.05). This result seems to be inconsistent with that of some previous studies.[18,19] We interpret this result as that the percentage of larger size (≥20 mm) in higher malignant potential GISTs is higher than that in lower malignant potential GISTs. Furthermore, it does not mean that the malignant potential of a larger GIST must be higher than that of a smaller GIST. On the contrary, some smaller GISTs revealed higher malignant potential.
On EUS, GISTs are usually homogenous hypoechoic masses or heterogeneous hypoechoic masses with anechoic cystic spaces due to cystic degeneration and liquefaction necrosis. In our study, all of the ten GISTs in Group I were homogenous hypoechoic and originated from the muscularis propria. On contrast, only four GISTs in Group II were heterogenous hypoechoic with focal hyperechoic area (P < 0.05). Appearance of heterogenous echogenicity was only observed in higher malignant potential GISTs. Only two GISTs were extraluminal growth, which were resected by laparoscopic procedure. The other 16 GISTs were resect by endoscopic procedure. No severe complication such as bleeding or peritonitis was observed in all 18 patients. To date, no recurrence was found in all patients during endoscopy surveillance, and the follow-up is in progress.
In some previous studies,[20,21] researchers found that detection of intratumoral irregular vessels had important value in assessing the malignant potential of GISTs. In our study, we detected intratumoral irregular vessels by CEH-EUS. In Group II, irregular vessels were detected in both two high malignant potential GISTs and four intermediate malignant potential GISTs. In Group I, no irregular vessel was found in ten low and very low malignant potential GISTs. There was a significant difference for the detection of irregular vessels between the two groups. Detection of irregular vessels had good sensitivity and specificity in discriminating higher and lower malignant potential GISTs, which are 75% and 100%, respectively. The positive predictive value of detection of irregular vessels to high malignant potential was 33%, and the negative predictive value was 100%. CE-CT, color, and PD-EUS, CEH-EUS are used for detection of intratumoral vessels clinically,[22,23] and the efficacy of the former two methods is correlated to the diameter and flow of vessels. For CEH-EUS, the harmonic signal of microbubbles was directly depicted, particularly in the vessels with small diameter and slow flow, which were hard to be identified by CE-CT or PD-EUS.[24,25] Therefore, CEH-EUS can be used to diagnose disease in various organs with abundant as well as poor blood supply. Some researchers had reported the diagnostic value of CEH-EUS in lesions of pancreas and gallbladder. AAT is one of parameters in CE-US. Song et al.[26] found that AAT of endometrial carcinoma was shorter than that of normal myometrium, and contributed this to by numerous and complex neovascularization in malignant lesions. In a study on evaluation of severity of chronic hepatitis C, Ridolfi et al. also revealed that the mean hepatic vein arrival time decreased progressively with increasing severity of liver disease.[27] However, no significant difference of AAT was found between colorectal adenocarcinoma and adenoma.[28] In this study, we also found no significant difference of AAT between the two groups.
The diagnosis of GISTs must be confirmed by pathology and immunohistochemistry examinations. At present, the diagnostic criterion of GISTs was defined as subepithelial tumors composed of spindle cells c-kit(+) and CD34(+). Eighteen patients, who met the criterion in this study except one patient, were diagnosed middle differentiated adenocarcinoma. DOG-1 is a calcium dependent, receptor activated chloride channel protein expressed in GISTs,[6] and it is positive in all 18 patients in our study. The result is consistent with previous studies.[29] Leiomyoma is another common subepithelial tumor in gastrointestinal tract, which is difficult to discriminate from GISTs by endoscopy and EUS. However, CD34 and c-kit are rarely positive in leiomyoma, whereas SMA and Desmin are always positive. In this study, SMA and Desmin are positive only in few GISTs.[30] Ki67 is a nuclear proliferation associated antigen expressed in the growth and synthesis phases of the cell cycle (G1, S, G2, and M) but not in the resting phase (G0). This antigen provides information about the proportion of active cells in the cell cycle and is correlated with tumor recurrence and prognosis.[31] We observed that Ki67 was positive in two high malignant potential GISTs and in one intermediate malignant potential GISTs in Group I and was all negative in Group II. Hence, strict follow-up and endoscopy surveillance are needed in these three patients with Ki67(+).
CONCLUSION
Irregular vessel is an important factor for evaluating malignant potential of GISTS. Detection of irregular intratumoral vessels can predict higher malignant potential before tumor resection. Meantime, the tumor size and echogenicity are assistant factors for malignant potential assessment. The limitations of this study are the small number of patients enrolled and the relatively short period of follow-up. The follow-up is still in progress, and further study with larger number of cases is needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: A clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70–83. doi: 10.1053/j.semdp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Palazzo L, Landi B, Cellier C, et al. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000;46:88–92. doi: 10.1136/gut.46.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yasuda K, Cho E, Nakajima M, et al. Diagnosis of submucosal lesions of the upper gastrointestinal tract by endoscopic ultrasonography. Gastrointest Endosc. 1990;36(2 Suppl):S17–20. doi: 10.1016/s0016-5107(90)71010-3. [DOI] [PubMed] [Google Scholar]
- 5.Croom KF, Perry CM. Imatinib mesylate: In the treatment of gastrointestinal stromal tumours. Drugs. 2003;63:513–22. doi: 10.2165/00003495-200363050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008;39:1411–9. doi: 10.1016/j.humpath.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 7.Rammohan A, Sathyanesan J, Rajendran K, et al. A gist of gastrointestinal stromal tumors: A review. World J Gastrointest Oncol. 2013;5:102–12. doi: 10.4251/wjgo.v5.i6.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoshida S, Yamashita K, Yokozawa M, et al. Diagnostic findings of ultrasound-guided fine-needle aspiration cytology for gastrointestinal stromal tumors: Proposal of a combined cytology with newly defined features and histology diagnosis. Pathol Int. 2009;59:712–9. doi: 10.1111/j.1440-1827.2009.02433.x. [DOI] [PubMed] [Google Scholar]
- 9.Na HK, Lee JH, Park YS, et al. Yields and utility of endoscopic ultrasonography-guided 19-gauge trucut biopsy versus 22-gauge fine needle aspiration for diagnosing gastric subepithelial tumors. Clin Endosc. 2015;48:152–7. doi: 10.5946/ce.2015.48.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Esparrach G, Sendino O, Solé M, et al. Endoscopic ultrasound-guided fine-needle aspiration and trucut biopsy in the diagnosis of gastric stromal tumors: A randomized crossover study. Endoscopy. 2010;42:292–9. doi: 10.1055/s-0029-1244074. [DOI] [PubMed] [Google Scholar]
- 11.Kida M, Araki M, Miyazawa S, et al. Comparison of diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration with 22- and 25-gauge needles in the same patients. J Interv Gastroenterol. 2011;1:102–7. doi: 10.4161/jig.1.3.18508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekine M, Imaoka H, Mizuno N, et al. Clinical course of gastrointestinal stromal tumor diagnosed by endoscopic ultrasound-guided fine-needle aspiration. Dig Endosc. 2015;27:44–52. doi: 10.1111/den.12333. [DOI] [PubMed] [Google Scholar]
- 13.Rettenbacher T. Focal liver lesions: Role of contrast-enhanced ultrasound. Eur J Radiol. 2007;64:173–82. doi: 10.1016/j.ejrad.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Kuecherer HF, Will M, da Silva KG, et al. Contrast-enhanced Doppler ultrasound for noninvasive assessment of pulmonary artery pressure during exercise in patients with chronic congestive heart failure. Am J Cardiol. 1996;78:229–32. doi: 10.1016/s0002-9149(96)90404-x. [DOI] [PubMed] [Google Scholar]
- 15.Kamata K, Kitano M, Omoto S, et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy. 2016;48:35–41. doi: 10.1055/s-0034-1393564. [DOI] [PubMed] [Google Scholar]
- 16.Park CH, Chung MJ, Oh TG, et al. Differential diagnosis between gallbladder adenomas and cholesterol polyps on contrast-enhanced harmonic endoscopic ultrasonography. Surg Endosc. 2013;27:1414–21. doi: 10.1007/s00464-012-2620-x. [DOI] [PubMed] [Google Scholar]
- 17.Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): Definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol. 2003;54:3–24. [PubMed] [Google Scholar]
- 18.Park SS, Ryu JS, Oh SY, et al. Surgical outcomes and immunohistochemical features for gastrointestinal stromal tumors (GISTS) of the stomach: With special reference to prognostic factors. Hepatogastroenterology. 2007;54:1454–7. [PubMed] [Google Scholar]
- 19.Chen TH, Hsu CM, Chu YY, et al. Association of endoscopic ultrasonographic parameters and gastrointestinal stromal tumors (GISTs): Can endoscopic ultrasonography be used to screen gastric GISTs for potential malignancy? Scand J Gastroenterol. 2016;51:374–7. doi: 10.3109/00365521.2015.1095350. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto H, Kitano M, Matsui S, et al. Estimation of malignant potential of GI stromal tumors by contrast-enhanced harmonic EUS (with videos) Gastrointest Endosc. 2011;73:227–37. doi: 10.1016/j.gie.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Kannengiesser K, Mahlke R, Petersen F, et al. Contrast-enhanced harmonic endoscopic ultrasound is able to discriminate benign submucosal lesions from gastrointestinal stromal tumors. Scand J Gastroenterol. 2012;47:1515–20. doi: 10.3109/00365521.2012.729082. [DOI] [PubMed] [Google Scholar]
- 22.Liu GJ, Xu HX, Lu MD, et al. Enhancement pattern of hepatocellular carcinoma: Comparison of real-time contrast-enhanced ultrasound and contrast-enhanced computed tomography. Clin Imaging. 2006;30:315–21. doi: 10.1016/j.clinimag.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Saftoiu A, Vilmann P, Hassan H, et al. Utility of colour Doppler endoscopic ultrasound evaluation and guided therapy of submucosal tumours of the upper gastrointestinal tract. Ultraschall Med. 2005;26:487–95. doi: 10.1055/s-2005-858905. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto H, Kitano M, Suetomi Y, et al. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol. 2008;34:525–32. doi: 10.1016/j.ultrasmedbio.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Kudo M. Contrast Harmonic Imaging in the Diagnosis and Treatment of Hepatic Tumors. Tokyo: Springer; 2003. pp. 1–30. [Google Scholar]
- 26.Song Y, Yang J, Liu Z, et al. Preoperative evaluation of endometrial carcinoma by contrast-enhanced ultrasonography. BJOG. 2009;116:294–8. doi: 10.1111/j.1471-0528.2008.01981.x. [DOI] [PubMed] [Google Scholar]
- 27.Ridolfi F, Abbattista T, Marini F, et al. Contrast-enhanced ultrasound to evaluate the severity of chronic hepatitis C. Dig Liver Dis. 2007;39:929–35. doi: 10.1016/j.dld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang H, Yang ZG, Chen HJ, et al. Time-intensity curve parameters in colorectal tumours measured using double contrast-enhanced ultrasound: Correlations with tumour angiogenesis. Colorectal Dis. 2012;14:181–7. doi: 10.1111/j.1463-1318.2011.02546.x. [DOI] [PubMed] [Google Scholar]
- 29.Espinosa I, Lee CH, Kim MK, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32:210–8. doi: 10.1097/PAS.0b013e3181238cec. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Liu YH. Expression of CD117, CD34, SMA, S-100 protein, Vim and desmin in patients with gastrointestinal stromal tumors. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:438–40. [PubMed] [Google Scholar]
- 31.Liu M, Lawson G, Delos M, et al. Prognostic value of cell proliferation markers, tumour suppressor proteins and cell adhesion molecules in primary squamous cell carcinoma of the larynx and hypopharynx. Eur Arch Otorhinolaryngol. 2003;260:28–34. doi: 10.1007/s00405-002-0512-8. [DOI] [PubMed] [Google Scholar]