Abstract
Aim:
To reveal the impact of preoperative endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of pancreatic ductal adenocarcinoma (PDAC).
Materials and Methods:
We retrospectively reviewed 242 patients who underwent surgery for PDAC at our institution between January 1996 and July 2012. Among them, there were three patients with R2 resection and 30 patients with a follow-up period of less than 1 year, who were excluded because they did not meet the conditions for evaluating recurrence. Consequently, 209 patients were enrolled in the present study. The patients were divided into two groups: 126 patients who underwent preoperative EUS-FNA (FNA group) and 83 patients who did not (non-FNA group) undergo preoperative EUS-FNA.
Results:
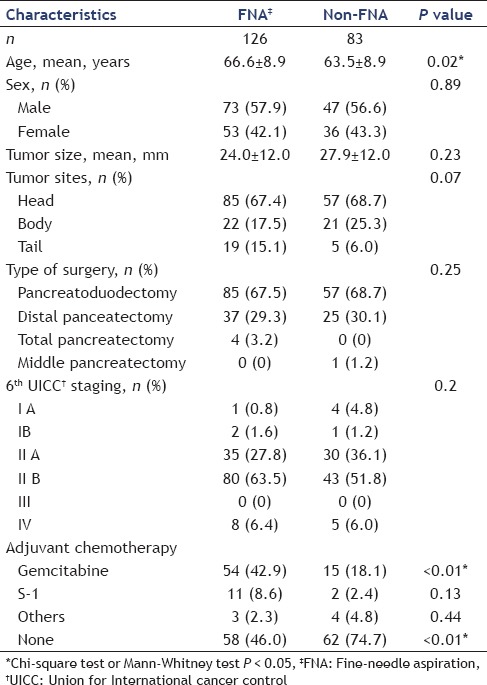
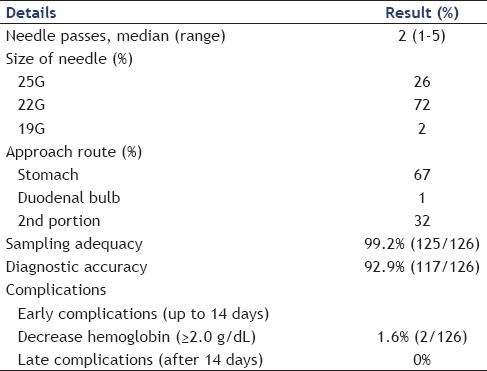
There were no significant differences in baseline characteristics between FNA and non-FNA groups except mean age (66.6 ± 8.9 years vs. 63.5 ± 8.9 years, respectively, P = 0.02) and the administration rate of gemcitabine as adjuvant chemotherapy (42.9% vs. 18.1%, P < 0.01). Sampling adequacy of preoperative EUS-FNA was 99.2% (125/126) and sensitivity for diagnosis was 92.9% (117/126). The rate of complications related to EUS-FNA was 1.6% (2/126); two patients experienced reduction in hemoglobin (≥2.0 g/dL). These two patients did not have any apparent bleeding and could be managed conservatively. No severe complications were seen. We evaluated long-term outcomes of preoperative EUS-FNA, especially disease-free survival, needle-track seeding and recurrence. Kaplan-Meier analysis indicated no significant difference in disease-free survival between the two groups (P = 0.12). The site of recurrence was not significantly different between groups. Needle-track seeding was not observed in this study. Multivariate analysis of recurrence factors showed that preoperative EUS-FNA did not affect postoperative recurrence.
Conclusion:
Preoperative EUS-FNA for PDAC was shown to be a safe procedure with high diagnostic ability, and not a risk factor for postoperative recurrence.
Keywords: Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA), pancreatic ductal adenocarcinoma, pancreatic tumor, tumor dissemination, tumor seeding
INTRODUCTION
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) is established as a procedure to confirm the cytopathological evidence of solid pancreatic tumors before therapy. EUS-FNA of pancreatic masses has been reported to have a sensitivity of 82%-95%, specificity of 92%-100%, positive predictive value of 98%-100%, negative predictive value of 58%-91%, and overall accuracy of 55%-96%.[1,2,3,4]
In preoperative cases, the indications for EUS-FNA have remained controversial because of concern about false negativity, needle-track seeding, and tumor dissemination related to EUS-FNA. Ngamruengphong et al.[5] reported that preoperative EUS-FNA was not associated with an increased rate of gastric or peritoneal cancer recurrence in patients with resected pancreatic cancer. Kudo et al.[6] suggested by their data that the use of EUS-FNA neither influenced recurrence free survival or overall survival nor did it increase the risk of peritoneal recurrence. However, a few cases have been reported in which needle-track seeding of the gastric wall following EUS-FNA was suspected as the cause of recurrence of solid pancreatic tumors.[7,8,9,10]
The purpose of the present study was to analyze patients who underwent preoperative EUS-FNA for pancreatic cancer and reveal the clinical impact of preoperative EUS-FNA.
MATERIALS AND METHODS
We retrospectively reviewed 242 patients who underwent surgery for pancreatic ductal adenocarcinoma (PDAC) at our institution between January 1996 and July 2012. Among them, there were three patients with R2 resection and 30 patients with a follow-up period of less than 1 year who were excluded because they did not meet the conditions for evaluating recurrence. Consequently, 209 patients were enrolled in the present study. All study participants provided informed consent for participation, and the study protocol was approved by the institutional review board of our institution (1-001). No funding was received for this work. The authors have no conflicts of interest to declare.
The patients were divided into two groups: 126 patients who underwent preoperative EUS-FNA (FNA group) and 83 patients who did not (non-FNA group) [Figure 1]. Disease-free survival time was defined from the day of surgery to the day of recurrence or from the day of surgery to July 2013 (in cases with no recurrence). EUS-FNA was performed under conscious sedation with the administration of intravenous midazolam 5-20 mg (Astellas, Tokyo, Japan) and intravenous pethidine hydrochloride 35 mg (Mitsubishi Tanabe Pharma, Osaka, Osaka Prefecture, Japan). It was performed with a convex array echoendoscope (GF-UC30P, GF-UC240P-AL5, GF-UCT240-AL5, or GF-UCT260-AL5, Olympus Medical Systems, Tokyo, Japan) connected to an ultrasound scanning system (SSD-5500, Prosound SSD α-10; Hitachi Aloka Medical, Tokyo, Japan). Various types of needles were employed (19G, 22G, and 25G, Expect, Boston Scientifics Corporation, Marlborough, MA, USA; EchoTip Ultra, Cook Medical, Bloomington, IN, USA; NA10J1, NA11JKB, NA-200H-8022; Olympus Medical Systems, Tokyo, Japan or Sono Tip Pro Control; Medi-Globe Corp., Rosenheim, Bavaria, Germany). Selection of the type and size of needle was at the discretion of the endosonographer. In our institution, only experienced specialists are allowed to perform preoperative EUS-FNA. Cytological diagnoses were interpreted as “insufficient,” “no atypia” (normal pancreatic tissue), “atypical” (including regenerative atypia by inflammatory changes), “suspicious,” or “malignant.” After obtaining tissue from a pancreatic lesion via EUS-FNA, the tissue was reviewed immediately (rapid on-site cytopathological evaluation: ROSE) by a cytopathologist or cytotechnician. Subsequent punctures in the same patient were not performed before confirming the results of ROSE so as to minimize the complications. In preoperative cases, no more EUS-FNA samples were obtained if the tissue was diagnosed as “suspicious” or ‘malignant.’ If it was difficult to obtain an adequate specimen, we finished the procedure with five needle passes. Both cytology and cell block techniques were usually used for diagnosis.
Figure 1.
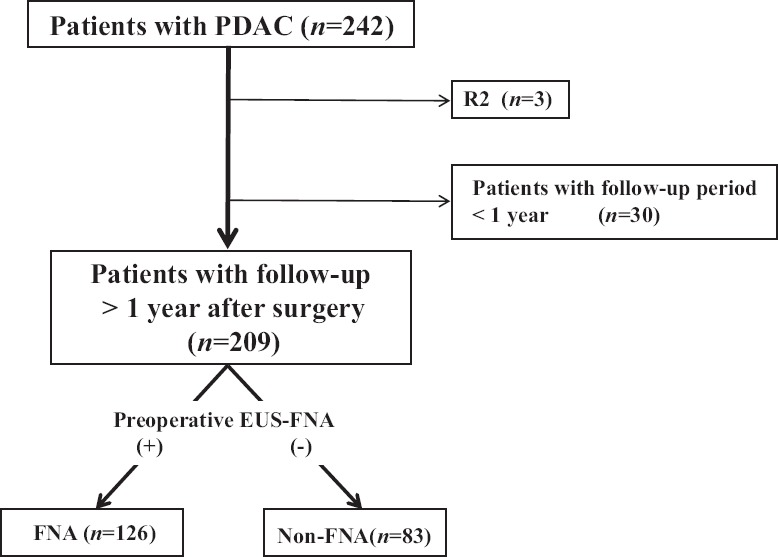
Flowchart showing the inclusion of patients and their follow-up in the current study. PDAC: Pancreatic ductal adenocarcinoma, EUS-FNA: Endoscopic ultrasound-guided fine-needle aspiration
The timing of complications was classified as postprocedure (up to 14 days) and late (any time after 14 days).[11]
Statistical analysis
The statistical methods of this study were reviewed by Kenichi Yoshimura Innovative Clinical Research Center, Kanazawa University, Kanazawa, Ishikawa Prefecture, Japan.
Statistical analysis was performed using the chi-square test, Fisher's exact test, or the Mann-Whitney test in univariate analysis. The Kaplan-Meier method was used to calculate survival curves and the log-rank test was used for comparing the two groups. Cox proportional hazards models were used in multivariate analysis of factors associated with disease-free survival. Values of P < 0.05 were regarded as statistically significant. All statistical analyses were performed using StatMate IV software (ATMS, Tokyo, Japan).
RESULTS
Patient characteristics
Patient characteristics in each group are shown in Table 1. We usually did not perform EUS-FNA before surgery from 1996 to 2008. Thereafter, from 2008 onward, we tried to perform EUS-FNA for all preoperative cases to best manage their treatment. In comparisons of the FNA group with the non-FNA group, significant differences were found in the mean age (66.6 ± 8.9 years vs. 63.5 ± 8.9 years, respectively, P = 0.02) and in the administration of gemcitabine as adjuvant chemotherapy (42.9% vs. 18.1%, P < 0.01). After the CONKO-001 study was reported,[12] most patients at our institution received adjuvant chemotherapy. There were no significant differences in other characteristics [sex, tumor size, tumor site, type of surgery, and 6th Union for International Cancer Control (UICC) staging].
Table 1.
Patient characteristics
EUS-FNA
The details of preoperative EUS-FNA and cytopathological diagnosis are shown in Table 2. The median number of needle passes was 2 (1-5) times. Overall sampling adequacy and diagnostic accuracy were 99.2% (125/126) and 92.9% (117/126). We were not able to cytopathologically confirm the presence of malignancy with EUS-FNA in nine cases, five of whom subsequently underwent endoscopic retrograde cholangiopancreatography (ERCP) for cytopathological diagnosis. Finally, we could confirm the cytopathological evidence of malignancy in four cases prior to surgery. The remaining five cases underwent surgery without cytopathological evidence of malignancy. The rate of complications related to EUS-FNA was 1.6% (2/126); two patients experienced a reduction in hemoglobin (≥2.0 g/dL). These two patients did not have any apparent bleeding and were successfully managed conservatively. There were no other early complications (within 14 days) of EUS-FNA.
Table 2.
Results of preoperative EUS-FNA (n = 126)
Long-term outcomes
We evaluated the long-term outcomes of preoperative EUS-FNA, specifically disease-free survival, needle-track seeding, and recurrence. There was no significant difference in disease-free survival between the two groups by Kaplan-Meier analysis (P = 0.12) [Figure 2]. We also evaluated preoperative EUS-FNA in only pancreatic body and tail cancers. In pancreatic body and tail cancers, significant differences were found in adjuvant chemotherapy between the FNA and non-FNA groups. There were no significant differences in other characteristics (mean age, sex, tumor size, tumor site, type of surgery, and 6th UICC staging) [Table 3]. No significant difference in disease-free survival was shown between body and tail cancers by Kaplan-Meier analysis (P = 0.82) [Figure 3].
Figure 2.
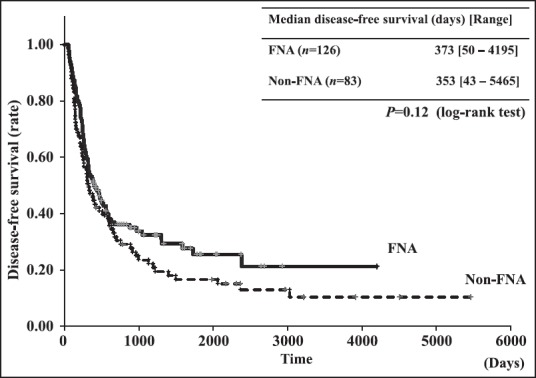
Kaplan-Meier analysis showing disease-free survival of patients with pancreatic cancers with and without the performance of FNA
Table 3.
Characteristics of patients with pancreatic body and tail cancers
Figure 3.
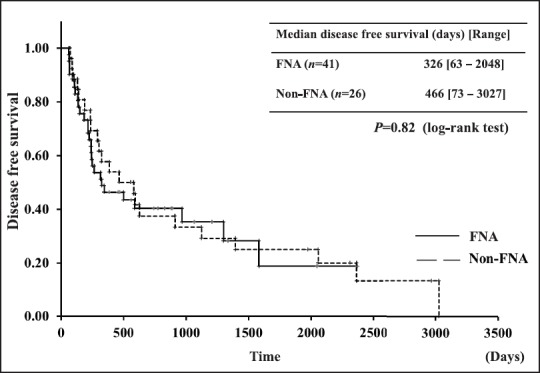
Kaplan-Meier analysis showing disease-free survival of pancreatic body and tail cancers with and without the performance of FNA
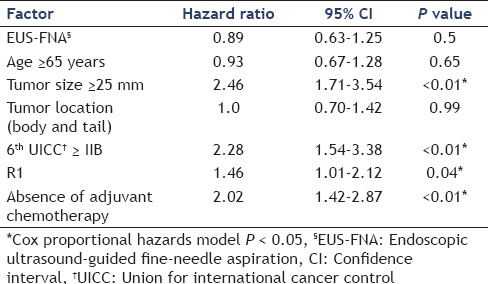
Table 4A compares the sites of recurrence between FNA and non-FNA groups and Table 4B shows this comparison in body and tail cancers only. No needle-track seeding was observed in this study. There was no significant difference in the site of recurrence between FNA and non-FNA groups, as seen in Table 4A (liver: 23.8% vs. 31.3%, respectively, P = 0.26; peritoneal: 17.5% vs. 19.3%, respectively, P = 0.85; local: 13.5% vs. 14.5%, respectively, P = 0.84; lung: 11.9% vs. 8.4%, respectively, P = 0.5; lymph nodes: 7.9% vs. 13.3%, respectively, P = 0.24; and bone: 0.7% vs. 1.2%, respectively, P = 1). There were no significant differences in the site of recurrence between body and tail cancers as well, even after adjustment [Table 4B]. In a multivariate analysis of factors related to disease-free survival by the use of a Cox proportional-hazards model, tumor size ≥25 mm [hazard ratio (HR): 2.46, P < 0.01] and 6th UICC ≥IIB (HR: 2.28, P < 0.01), R1 resection (HR: 1.46, P = 0.04), and absence of adjuvant chemotherapy (HR: 2.02, P < 0.01) were shown to be independent factors associated with disease-free survival [Table 5]. Performance of preoperative EUS-FNA and the tumor site were not related to disease-free survival.
Table 4A.
Comparison of sites of recurrence
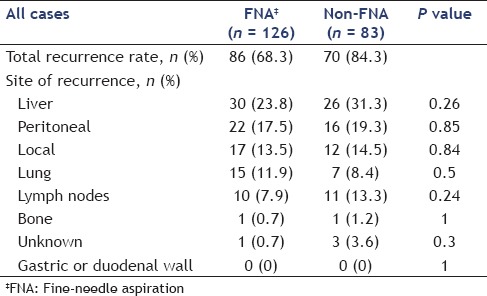
Table 4B.
Comparison of sites of recurrence in body and tail cancers only
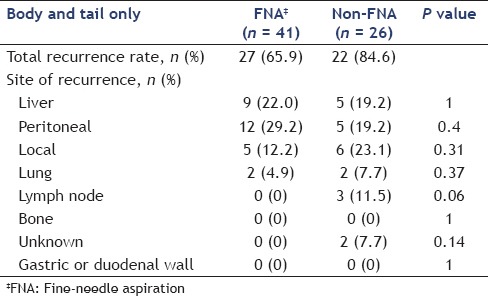
Table 5.
Multivariate analysis of the factors associated with disease-free survival
DISCUSSION
Pancreatic carcinoma remains an intractable disease, with a reported 5-year survival rate of only 6%[13] and a reported 5-year survival rate of patients who undergo surgery of only 7%-23%.[14] Although curative surgery is the only way to obtain long-term survival in patients with PDAC, surgery for pancreatic carcinoma generally has a high morbidity rate (9%-44%) and a high mortality rate (0.7%-21%).[15,16,17,18] Performing surgery before confirming the cytopathological diagnosis may result in an unnecessary surgery for patients. Although EUS-FNA is a very useful diagnostic procedure, tumor seeding is recognized as a very rare complication of this procedure. Previous reports of suspected tumor seeding related to preoperative EUS-FNA showed that all recurrences occurred in the gastric wall.[7,8,9,10] Gastric wall recurrence during follow-up has a possibility of additional curative surgery. Peritoneal dissemination, however, has not been reported. Therefore, it is important to confirm the cytopathological evidence of malignancy before surgery to avoid unnecessary surgery. Recently, neoadjuvant chemotherapy and chemoradiotherapy have been discussed as therapies offering a possibility of improving the prognosis of PDAC.[19,20,21,22] Appropriate neoadjuvant therapy requires evaluation of the pathological diagnosis to determine the best treatment. ERCP and EUS-FNA are the procedures that are generally used for confirming cytopathological evidence before beginning therapy for pancreatic carcinoma. On comparing EUS-FNA and ERCP, EUS-FNA is safer and more accurate for the cytopathological diagnosis of pancreatic tumors.[23] Preoperative EUS-FNA is therefore, considered to be an adequate and much-needed procedure in clinical practice for the preoperative diagnosis of patients with suspected PDAC. Since 2008, in our institution, we have been performing EUS-FNA preoperatively for all solid pancreatic tumors to confirm their cytopathological profiles. Hence, we conducted this study to evaluate the diagnostic ability, morbidity, and clinical impact of preoperative EUS-FNA and to discuss the safety and adequacy of this procedure. Analysis of the diagnostic ability and morbidity of EUS-FNA in the present study revealed a sampling adequacy of 99.2% (125/126) and sensitivity for diagnosis of 92.9% (117/126). The complication rate was 1.6% (2/126), with the only complication being a reduction of hemoglobin (≥2.0 g/dL). There were no severe complications and no late complications. The present study found preoperative EUS-FNA to be safe and to have excellent diagnostic capability. In the comparison between FNA and non-FNA group patients, disease-free survival was not significantly different between them [Figure 2].
In cases of recurrence, recurrence sites were not significantly different between the two groups and also between body and tail cancers [Table 4]. In multivariate analysis of factors related to disease-free survival by use of a Cox proportional hazards model, tumor size ≥25 mm (HR: 2.46, P < 0.01) and 6th UICC ≥IIB (HR: 2.28, P < 0.01), R1 resection (HR: 1.46, P = 0.04), and absence of adjuvant chemotherapy (HR: 2.02, P < 0.01) were found to be independent factors associated with disease-free survival. The performance of preoperative EUS-FNA and tumor location had no relationship with disease-free survival [Table 5]. In our study, there were no cases of tumor seeding unlike previous reports. This could be because of the number of punctures, three or five, in previous reports.[7,8,9,10] In our study, the median number of punctures was only two times [Table 2]. Our novel approach of using rapid on-site evaluation (ROSE) could have contributed to minimizing the number of punctures.[24] Ngamruengphong et al.[5] also reported that preoperative EUS-FNA was not associated with an increased rate of gastric or peritoneal cancer recurrence in patients with resected pancreatic cancer. Another study evaluating not only preoperative cases but also inoperable ones investigated the risk of peritoneal carcinomatosis by comparing a group of patients undergoing ERCP and a group of patients undergoing EUS-FNA to confirm the pathological evidence of pancreatic cancer, and found that EUS-FNA did not increase the risk of peritoneal carcinomatosis.[25] The results of the present study also suggest that preoperative EUS-FNA does not decrease disease-free survival, and has less correlation with peritoneal recurrence. Gastric wall recurrence related to preoperative EUS-FNA was not seen in our study although there are some previous reports[7,8,9,10] of recurrence related to preoperative EUS-FNA. Tumor seeding is a very rare but possible complication of EUS-FNA. The indications of preoperative EUS-FNA are probably different between different institutes, countries, cultures, and individuals. Hence, the indications and complications of this procedure should be discussed with the patient before its performance. More importantly, unnecessary EUS-FNA should be avoided.
Some limitations must be considered regarding the present study. Since this was a retrospective study, the results could have been influenced by patient characteristics.
CONCLUSION
The present study evaluated the diagnostic performance and adequacy of preoperative EUS-FNA. At the current moment, preoperative EUS-FNA is a safe and adequate procedure to confirm the presence of malignancy before curative surgery for PDAC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Eloubeidi MA, Varadarajulu S, Desai S, et al. A prospective evaluation of an algorithm incorporating routine preoperative endoscopic ultrasound-guided fine needle aspiration in suspected pancreatic cancer. J Gastrointest Surg. 2007;11:813–9. doi: 10.1007/s11605-007-0151-x. [DOI] [PubMed] [Google Scholar]
- 2.Uehara H, Ikezawa K, Kawada N, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic malignancy in relation to the size of lesions. J Gastroenterol Hepatol. 2011;26:1256–61. doi: 10.1111/j.1440-1746.2011.06747.x. [DOI] [PubMed] [Google Scholar]
- 3.Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973–81. doi: 10.1007/s00535-012-0695-8. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamao K, Okubo K, et al. Differential diagnosis of pancreatic cancer and focal pancreatitis by using EUS-guided FNA. Gastrointest Endosc. 2005;61:76–9. doi: 10.1016/s0016-5107(04)02224-2. [DOI] [PubMed] [Google Scholar]
- 5.Ngamruengphong S, Xu C, Woodward TA, et al. Risk of gastric or peritoneal recurrence, and long-term outcomes, following pancreatic cancer resection with preoperative endosonographically guided fine needle aspiration. Endoscopy. 2013;45:619–26. doi: 10.1055/s-0033-1344216. [DOI] [PubMed] [Google Scholar]
- 6.Kudo T, Kawakami H, Kuwatani M, et al. Influence of the safety and diagnostic accuracy of preoperative endoscopic ultrasound-guided fine-needle aspiration for resectable pancreatic cancer on clinical performance. World J Gastroenterol. 2014;20:3620–7. doi: 10.3748/wjg.v20.i13.3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paquin SC, Gariépy G, Lepanto L, et al. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610–1. doi: 10.1016/s0016-5107(05)00082-9. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed K, Sussman JJ, Wang J, et al. A case of EUS-guided FNA-related pancreatic cancer metastasis to the stomach. Gastrointest Endosc. 2011;74:231–3. doi: 10.1016/j.gie.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Chong A, Venugopal K, Segarajasingam D, et al. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011;74:933–5. doi: 10.1016/j.gie.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Katanuma A, Maguchi H, Hashigo S, et al. Tumor seeding after endoscopic ultrasound-guided fine-needle aspiration of cancer in the body of the pancreas. Endoscopy. 2012;44(Suppl 2 UCTN):E160–1. doi: 10.1055/s-0031-1291716. [DOI] [PubMed] [Google Scholar]
- 11.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 14.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas? Is it really improving. Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–33. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa O, Ohhigashi H, Sasaki Y, et al. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg. 1988;208:215–20. doi: 10.1097/00000658-198808000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalton RR, Sarr MG, van Heerden JA, et al. Carcinoma of the body and tail of the pancreas: Is curative resection justified? Surgery. 1992;111:489–94. [PubMed] [Google Scholar]
- 18.Wade TP, Virgo KS, Johnson FE. Distal pancreatectomy for cancer: Results in US Department of Veterans Affairs Hospitals, 1987-1991. Pancreas. 1995;11:341–4. [PubMed] [Google Scholar]
- 19.Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: Gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088–96. doi: 10.1245/s10434-007-9384-x. [DOI] [PubMed] [Google Scholar]
- 20.Snady H, Bruckner H, Cooperman A, et al. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer. 2000;89:314–27. doi: 10.1002/1097-0142(20000715)89:2<314::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 21.Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: A new treatment paradigm? Oncologist. 2014;19:266–74. doi: 10.1634/theoncologist.2013-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DE Felice F, Musio D, Raffetto N, et al. Neoadjuvant strategy as initial treatment in resectable pancreatic cancer: Concrete evidence of benefit. Anticancer Res. 2014;34:4673–76. [PubMed] [Google Scholar]
- 23.Wakatsuki T, Irisawa A, Bhutani MS, et al. Comparative study of diagnostic value of cytologic sampling by endoscopic ultrasonography-guided fine-needle aspiration and that by endoscopic retrograde pancreatography for the management of pancreatic mass without biliary stricture. J Gastroenterol Hepatol. 2005;20:1707–11. doi: 10.1111/j.1440-1746.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- 24.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikezawa K, Uehara H, Sakai A, et al. Risk of peritoneal carcinomatosis by endoscopic ultrasound-guided fine needle aspiration for pancreatic cancer. J Gastroenterol. 2013;48:966–72. doi: 10.1007/s00535-012-0693-x. [DOI] [PubMed] [Google Scholar]


