Abstract
Purpose:
To compare the efficacy and tolerability of brinzolamide/timolol (BT) and dorzolamide/timolol (DT) fixed combinations on intraocular pressure (IOP) reduction.
Methods:
Patients with primary open angle glaucoma or normal tension glaucoma were randomized to receive either BT or DT. IOPs were measured at baseline, 2 weeks, and 1, 2, and 3 months. The primary outcome measures were the mean change in IOP from baseline at each visit. Secondary outcome measures included the tolerability of each fixed combination.
Results:
Seventy-three patients (73 eyes) were included; 37 eyes in BT group and 36 eyes in DT group. Baseline mean IOP were 24.14 ± 4.5 and 29.53 ± 6 mmHg for BT and DT, respectively (P < 0.001). Both BT and DT provided statistically significant mean IOP reductions from baseline values within each group at all study visits (P < 0.001). DT provided greater mean IOP reductions from baseline than BT at each visit which was statistically significant at 2 weeks (P = 0.037). Mean percentage of IOP reduction was 24.35% and 46.33% at 2 weeks (P < 0.001), and 24.65% and 47% at 3 months (P < 0.001) for BT and DT, respectively. Patients' tolerability appeared to be better for DT than for BT with complete ocular comfort without any ocular adverse effects in 31 patients (81.1%) in DT group and 11 patients (29.7%) in BT group (P < 0.001).
Conclusion:
Both drops provide effective IOP reduction which was greater, and patients were more likely to achieve lower target pressures with DT than with BT.
Keywords: Brinzolamide/timolol, dorzolamide/timolol, fixed combinations
The ocular hypertension treatment study[1] and the collaborative initial glaucoma treatment study[2] have reported that about 50–75% of patients will need combination therapy at any stage of the disease and may require two or more drugs to reach their target pressure. In such cases, a fixed combination rather than separate drugs has a number of potential advantages including no risk of drug washout,[3] reduced exposures to preservatives with reduced side effects, reduced costs of treatment, and ultimately better patient compliance and quality of life.[4] Few head to head studies[5,6] have found that brinzolamide/timolol (BT) and dorzolamide/timolol (DT) have almost similar efficacy at lowering intraocular pressure (IOP). This issue of efficacy needs to be carefully investigated as the early manifest glaucoma trial has suggested that every millimeter of IOP lowering corresponds to a reduction in the risk of glaucomatous progression by approximately 10%.[7]
DT is formulated at an acidic pH of 5.65 whereas BT has a near physiologic pH of 7.2. Any dissimilarity in tolerability is likely due to differences in pH between brinzolamide and dorzolamide which may affect the compliance of the patients to the treatment which may in turn affect the efficacy. All published studies demonstrated better patient tolerability with BT over DT.[8,9,10,11,12,13]
The purpose of this study was to compare the ocular hypotensive effect and tolerability of brinzolamide 1% plus timolol maleate 0.5% fixed combination (Alcon Lab, Cairo, Egypt) and dorzolamide 2% plus timolol maleate 0.5% fixed combination (Merk & Co Inc., Cairo, Egypt) in a hospital population with primary open-angle glaucoma (POAG) or normal tension glaucoma (NTG).
Patients and Methods
Approval for the study was obtained from the Hospital's Ethical Committee and followed the tenets of the declaration of Helsinki. All patients received a thorough explanation of the study design and aims. Study participants gave informed consent before initiation of any study-related procedures, and the study was conducted in compliance with informed consent regulations.
This is a prospective, randomized, controlled, clinical study. The study was carried out between January 2012 and December 2012 at University Hospitals. Enrolled patients were men or women of at least 18 years of age with a clinical diagnosis of POAG or NTG, who were uncontrolled on a single or a double medication other than the eye drops to be studied. Patients using ocular hypotensive drugs completed a washout of all drugs of appropriate duration before study entry (6 weeks for prostaglandin analogs, 4 weeks for topical beta-blockers, and 2 weeks for adrenergic agents or carbonic anhydrase inhibitors). Only the right eye of each patient was included. All participants confirmed their ability to follow study instructions and complete all required visits.
Exclusion criteria included
Active ocular disease other than POAG/NTG that would interfere with study outcomes; sensitivity or allergy to any component of either study drug; pregnancy, planning a pregnancy, or breastfeeding; functionally marked visual field loss; ocular surgery within the last 3 months; concomitant usage of ocular drugs (except intermittent use of artificial tears); and active systemic disease or plans to change ongoing systemic treatment that might affect IOP. Diabetic patients were included; however, those with diabetic retinopathy were excluded.
Intervention and outcome measures
After meeting all inclusion criteria and completing a washout of any ocular hypotensive agents, patients were randomized using a computer-generated randomization into two groups to receive either BT or DT twice daily. Patients were instructed to instill their study drugs between 8:00–9:00 and 20:00–21:00. Although the investigators (authors) were not blinded to the randomization, the multiple IOP measurements were done by different fellows and residents who were not aware of the study nature.
Patients were evaluated at baseline, 2 weeks, and 1, 2, and 3 months. IOPs were measured 3 times at 10:00 and another 3 times 18:00 at each study visit using Goldman applanation tonometry, and an average was taken for each eye (3 time points measurements were not feasible at our hospital). The recorded average IOPs were compared between the 2 groups and further analyzed in terms of age and gender. Central corneal thickness was not measured.
Baseline and follow-up visits' evaluations included medical and ophthalmic history and a complete ophthalmic examination (visual acuity, external exam, slit-lamp biomicroscopy, and measurement of IOP). Study drug was initiated after the examination and randomization. Patients were asked about adverse events and compliance, and their responses recorded at each follow-up study visit. Score 3 was given for the best tolerability with no ocular discomfort felt by the patients; Score 2 was given if the patients felt ocular discomfort in the form of burning or stinging sensation; Score 1 was given for ocular discomfort plus ocular pain; finally, Score 0 was given for ocular discomfort plus ocular pain plus blurred vision.
At the final study visit, the investigator completed a clinical success evaluation. A patient was considered clinically successful if the investigator, after considering IOP-lowering efficacy, tolerability, and any adverse events, continued the patient on his or her study drug at 3 months. The primary outcome measures were the mean change in IOP and the mean percentage of drop of IOP from baseline at each visit by applanation tonometer. Secondary outcome measures included the incidence of adverse events and tolerability of the used drug.
Statistical analysis
Data were statistically described in terms of mean ± standard deviation (±SD), frequencies (number of cases), and percentages when appropriate. Data distribution was tested using Kolmogorov–Smirnov test. Since numerical data were not violating normal assumption, comparison between the study groups was done using Student's t-test for independent samples. Within group, comparison was done using repeated measure ANOVA followed by paired t-test as a post hoc two group comparisons. For comparing categorical data, Chi-square test was performed. Exact test was used instead when the expected frequency is <5. P < 0.05 was considered statistically significant. All statistical calculations were done using Statistical Package for the Social Science (SPSS; SPSS Inc., Chicago, IL, USA) version 15 for Microsoft Windows.
Power analysis
Power analysis was done for the change in IOP over the study period as the primary outcome of this study. Student's t-test for independent samples was chosen to perform the analysis; the α-error level was fixed at 0.05, and the sample size was entered to be 36 participants for each group. To be most conservative, the intragroup SD was set at the highest recorded one. Accordingly, the power of our statistical results was 98.3%, 92.6%, 92.7%, and 89.8% at 2 weeks and 1, 2, and 3 months, respectively. Calculations were done using PS Power and Sample Size Calculations Software, version 2.1.30 for MS Windows (William D. Dupont and Walton D. Vanderbilt, USA).
Results
Seventy-three eyes of 73 patients were originally enrolled in this study; 37 patients (37 eyes) in BT group and 36 patients (36 eyes) in DT group. There were no statistically significant differences between the two treatment groups in patient demographics; mean patients' age were BT = 19.94 ± 50.73 (72–18) and DT = 18.41 ± 52.61 (72–18) (P = 0.677), and sex ratios were 1:1 for both groups. Baseline characteristics: Rate of hypertension showed no significant difference (BT = 10, DT = 14, P: 0.072); however, there was significant difference regarding the rate of diabetes (BT = 13, DT = 6, P = 0.042). In the BT, there were 13 (35.1%) diabetic patients; Type I diabetes mellitus (DM) was in 3 (23.1%) and Type II DM in 10 (76.9%). In the DT, there were 6 (16.7%) diabetic patients; Type I DM was in 2 (33.3%) and Type II DM in 4 (66.6%). However, subgroup analysis was not possible due to the small sample size. At the baseline visit, there were statistically significant between-group differences in mean IOP; 24.14 ± 4.49 mmHg (range: 14–35 mmHg, median = 25.00 mmHg) and 29.53 ± 5.99 mmHg (range: 18–44 mmHg, median = 30.00 mmHg) for BT and DT, respectively (P < 0.001).
Mean intraocular pressure reduction
The repeated measure of ANOVA for the mean IOP values within each group showed provided statistically significant reductions of baseline IOP at all study visits (P < 0.001). The mean IOP for DT was lower than BT at all visits and was significant only at 2 weeks (P = 0.037), with no significant difference at all other follow-up visits [Fig. 1]. This occurred despite a statistically higher mean IOP at baseline for DT.
Figure 1.
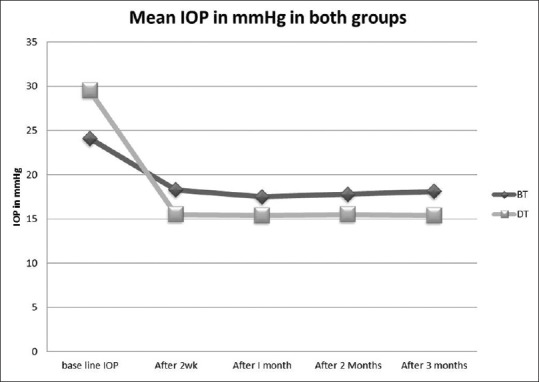
Mean intraocular pressure for brinzolamide/timolol and dorzolamide/timolol at baseline and follow-up visits
Percentage intraocular pressure reduction
There was a statistically significant higher percentage of IOP reduction for DT than BT at all study visits [Fig. 2].
Figure 2.
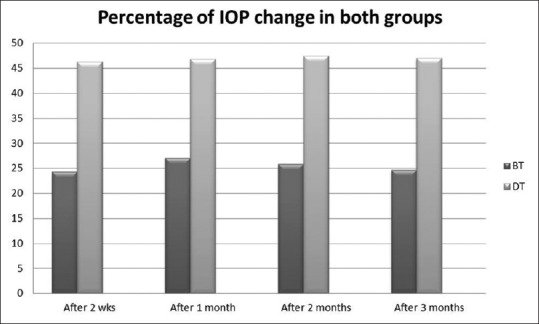
Comparison between the percentage intraocular pressure reduction in dorzolamide/timolol and brinzolamide/timolol group
Tolerability
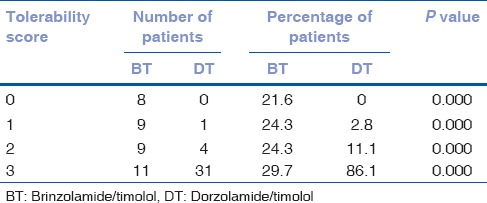
There was complete ocular comfort without any ocular adverse effects in 31 patients (81.1%) in the DT group and 11 patients (29.7%) in the BT group (P < 0.001). Ocular discomfort and ocular pain were experienced by patients from both groups, but it was unexpectedly higher in the BT group. Ocular discomfort was found in 9 patients (24.3%) in the BT group and only in 4 patients (11.1%) in the DT group (P < 0.001). Ocular pain was found in 9 patients (24.3%) from the BT group and in 1 patient (2.8%) from the DT group (P < 0.001). Blurred vision in 8 patients (21.6%) only in the BT and it was not found in the DT (P < 0.001) [Table 1].
Table 1.
Tolerability of brinzolamide/timolol and dorzolamide/timolol fixed combinations
Clinical success
The rate of clinical success was significantly different between each treatment group. In the BT group, 86.5% of patients (32/37) were considered clinically successful, compared with 94.4% of patients (34/36) in the DT group, which was statistically significant (P < 0.001).
Discussion
More than 50% of glaucoma patients will need more than one drug to reach their target IOP.[1,2] Reducing the amount of drops by including more than one medication in a fixed combination will reduce the side effects and increase compliance.[3,4] Although the tolerability is identified as a barrier to compliance, the issue of efficacy needs to be carefully investigated first before investigating patients' drug tolerability. There are limited published data[5,6] regarding the IOP-reducing power comparison between BT and DT as most of the studies[8,9,10,11,12,13] were more concerned with tolerability rather than the efficacy of the drug. The purpose of our study was to compare the ocular hypotensive effect of BT versus DT in a hospital-based population with POAG or NTG as the primary outcome with patients' tolerability as secondary outcome.
Regarding the efficacy of these drugs, only 2 head to head study reports[5,6] have found that the efficacy of BT is similar to DT. Other studies measuring BT efficacy alone showed similar efficacy to that of DT reported in the earlier literature.[14,15,16] However, in our study, we found that both BT and DT significantly reduced the IOP at all study visits, but there were lower mean IOPs for DT than BT at all study visits that was statistically significant at 2 weeks. There was also a statistically significant higher percentage of IOPs reduction with DT than BT at all study visits.
Manni et al.[5] compared the efficacy and tolerability of BT and DT; data were collected from 437 cases of open-angle glaucoma and ocular hypertension. They found that IOP reductions ranged from 7.2 to 9.2 mm Hg for BT and from 7.4 to 8.9 mm Hg for DT. Although a similar overall safety profile was observed between the 2 treatment groups, BT showed significantly less ocular irritation (2.7% vs. 10.6%; P = 0.0009) than BT. Akçay et al.[6] found the IOP reductions with BT ranged from 6.42 to 9.74 mmHg (26.09–37.46%), whereas DT produced mean IOP reductions ranging from 8.16 to 12.41 mmHg (31.19–41.44%) (P > 0.05). Cheng et al.[14] showed the IOP-reducing power of the BT (33%) and DT (30%) were to be similar. Holló et al.[16] reported that BT provided an approximately 30–33% IOP reduction from the untreated baseline IOP of 25–27 mmHg and concluded that BT is similarly effective but better tolerated than the DT.
Regarding the tolerability of the drug, because the beta-blocker component of the two carbonic anhydrase inhibitor containing fixed combination products is identical, any dissimilarity in tolerability is likely due to differences in pH between brinzolamide and dorzolamide. DT is formulated at an acidic pH of 5.65 whereas BT has a near physiologic pH of 7.2. The main difference between these two ocular hypotensive agents lies in their safety profile, with DT causing more ocular discomfort to appear while installing (burning and stinging).[8,9,10,11,12,13]
We used a scoring system to evaluate tolerability ranging from 0 (worst tolerability, with severe ocular discomfort) to 3 (best tolerability, with no ocular discomfort). Unexpectedly, we found that there was a significant difference in the tolerability of both fixed combinations with better tolerability in the DT group. In the BT group, we found that eight eyes (21.6%) suffered from ocular irritation in the form of stinging and burning sensation associated with ocular pain and blurred vision; these eyes was given Score 0. While eleven eyes (29.7%) did not suffer from any ocular discomfort and was given Score 3. While in the DT, no eyes suffered from severe ocular discomfort, and thirty-one eyes (86.1%) did not suffer from any ocular discomfort (P < 0.001).
The difference in the results could be related to the different sample size of each study which is considered one of the limitations of our study as we have a small sample size. The different scoring system to evaluate the tolerability could be another factor affecting the results. For example, Firat et al.[9] in 2012 used the ocular surface disorder index, as well as performing Schirmer test, tear breakup time and ocular impression cytology to test the tolerability of the drug. Lanz and Rabert[10] in 2011 judged the tolerability by being positive or negative tolerability; they found high satisfaction rating in 93.4% from a total of 14,025 eyes. Another scoring system was adopted by Vold et al.,[11] in 2008, where ocular discomfort score was judged by a score ranged from 0 (none) – 4 (very severe); they found very severe ocular irritation in <5% of the cases. Mundorf et al.[13] in 2008 performed their study on 127 patients; there was an ocular discomfort scale ranging from 0 (no discomfort) to 9 (significant discomfort). Ocular discomfort scores were significantly higher with DT than BT (2.9 vs. 1.4, respectively; P < 0.0001). They found significantly more patients reported ocular pain and discomfort after DT instillation and transient blurred vision than after BT instillation.
In our study, although the patients were randomly assigned to received DT and BT, the baseline mean IOPs were significantly higher in the DT group than the BT group. However, despite starting at significantly higher mean IOPs, the DT group had lower mean IOPs at each visit which was statistically significant at 2 weeks and a statistically significant higher percentage of IOP reduction by DT at 2 weeks and 3 months. The difference in structure between both brinzolamide and dorzolamide could lead to a different synergy of each drug with timolol. That is, the fixed combination does not usually have a power equals to the exact algebraic addition of each of its component drugs' powers when used alone. Thus, a fixed combination of each with timolol might have a different IOP-lowering power despite both (brinzolamide and dorzolamide) reported to have a similar efficacy when each was compared alone head to head.
Conclusion
The results of the present study suggest that BT and DT provide effective IOP-lowering in patients with POAG in this sample of a hospital population. Mean percentage of IOP reductions from baseline were clinically and statistically greater, and patients were more likely to achieve and maintain low target pressures with DT than with BT. Further, the tolerability of DT was better than BT. These findings suggest that DT is a more appropriate therapeutic choice for IOP lowering than BT for patients in need of more than eye drop. We do believe that future studies will confirm that DT is a stronger ocular hypotensive drop than BT. However, a large multicenter expansion of the present study is needed to further evaluate this wide gap we found between both medications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The ocular hypertension treatment study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 2.Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, et al. Interim clinical outcomes in the collaborative initial glaucoma treatment study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 3.Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63:333–8. doi: 10.1002/jps.2600630304. [DOI] [PubMed] [Google Scholar]
- 4.Dunker S, Schmucker A, Maier H Latanoprost/Timolol Fixed Combination Study Group. Tolerability, quality of life, and persistency of use in patients with glaucoma who are switched to the fixed combination of latanoprost and timolol. Adv Ther. 2007;24:376–86. doi: 10.1007/BF02849907. [DOI] [PubMed] [Google Scholar]
- 5.Manni G, Denis P, Chew P, Sharpe ED, Orengo-Nania S, Coote MA, et al. The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination versus dorzolamide 2%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension. J Glaucoma. 2009;18:293–300. doi: 10.1097/IJG.0b013e31818fb434. [DOI] [PubMed] [Google Scholar]
- 6.Sezgin Akçay BI, Güney E, Bozkurt KT, Unlü C, Akçali G. The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination versus dorzolamide 2%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2013;29:882–6. doi: 10.1089/jop.2013.0102. [DOI] [PubMed] [Google Scholar]
- 7.Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 8.Calugaru M, Calugaru D. Azarga, a new and useful fixed combination in glaucoma treatment. Oftalmologia. 2011;55:38–46. [PubMed] [Google Scholar]
- 9.Firat PG, Samdanci E, Doganay S, Cavdar M, Sahin N, Gunduz A. Short-term effect of topical brinzolamide-timolol fixed combination on ocular surface of glaucoma patients. Int J Ophthalmol. 2012;5:714–8. doi: 10.3980/j.issn.2222-3959.2012.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanzl I, Raber T. Efficacy and tolerability of the fixed combination of brinzolamide 1% and timolol 0.5% in daily practice. Clin Ophthalmol. 2011;5:291–8. doi: 10.2147/OPTH.S16355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vold SD, Evans RM, Stewart RH, Walters T, Mallick S. A one-week comfort study of BID-dosed brinzolamide 1%/timolol 0.5% ophthalmic suspension fixed combination compared to BID-dosed dorzolamide 2%/timolol 0.5% ophthalmic solution in patients with open-angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2008;24:601–5. doi: 10.1089/jop.2008.0030. [DOI] [PubMed] [Google Scholar]
- 12.Nebbioso M, Evangelista M, Librando A, Di Blasio D, Pescosolido N. Fixed topical combinations in glaucomatous patients and ocular discomfort. Expert Opin Pharmacother. 2012;13:1829–35. doi: 10.1517/14656566.2012.705830. [DOI] [PubMed] [Google Scholar]
- 13.Mundorf TK, Rauchman SH, Williams RD, Notivol R Brinzolamide/Timolol Preference Study Group. A patient preference comparison of Azarga (brinzolamide/timolol fixed combination) vs Cosopt (dorzolamide/timolol fixed combination) in patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2008;2:623–8. doi: 10.2147/opth.s4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng JW, Cheng SW, Gao LD, Lu GC, Wei RL. Intraocular pressure-lowering effects of commonly used fixed-combination drugs with timolol: A systematic review and meta-analysis. PLoS One. 2012;7:e45079. doi: 10.1371/journal.pone.0045079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckers HJ, Schouten JS, Webers CA. Role of fixed-combination brinzolamide 1%/timolol 0.5% in the treatment of elevated intraocular pressure in open-angle glaucoma and ocular hypertension. Clin Ophthalmol. 2009;3:593–9. doi: 10.2147/opth.s4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holló G, Bozkurt B, Irkec M. Brinzolamide/timolol fixed combination: A new ocular suspension for the treatment of open-angle glaucoma and ocular hypertension. Expert Opin Pharmacother. 2009;10:2015–24. doi: 10.1517/14656560903124388. [DOI] [PubMed] [Google Scholar]


