Abstract
The purpose was to evaluate the clinical outcome in multi-drug resistant Pseudomonas aeruginosa (MDR-PA) bacterial keratitis and report the successful use of an alternative antibiotic, topical colistimethate in some of them. The medical records of 12 culture-proven MDR-PA keratitis patients, all exhibiting in vitro resistance by Kirby–Bauer disc diffusion method to ≥ three classes of routinely used topical antibiotics were reviewed. Eight patients were treated with 0.3% ciprofloxacin or ofloxacin, 1 patient with 5% imipenem/cilastatin and 3 patients with 1.6% colistimethate. The outcomes in 8 eyes treated with only fluoroquinolones were evisceration in 4 eyes, therapeutic corneal graft in 1 eye, phthisis bulbi in 1 eye, and no improvement in 2 eyes. The eye treated with imipenem/cilastin required a therapeutic corneal graft. All the three eyes treated with 1.6% colistimethate healed. Colistimethate may prove to be an effective alternative antibiotic in the treatment of MDR-PA keratitis.
Keywords: Antibiotic, bacterial keratitis, colistimethate, colistin, multi-drug resistant, Pseudomonas
Worldwide bacterial resistance to antibiotics in both systemic and ocular infections is rising. Specifically, the increasing trend in Gram-negative multi-drug resistant (MDR) bacterial infections is being viewed with more serious concern as there is a lack of effective antibiotics against them.[1] In general, bacterial keratitis caused by Pseudomonas aeruginosa (PA) is difficult to treat, more so when the isolate is drug resistant.[2,3] Very recently studies reported treating MDR-PA bacterial keratitis with topical pipericillin,[4] colistin,[5,6] and meropenem,[7] antibiotics which are not ordinarily used in ophthalmology. In this study, we are reporting the outcome in MDR-PA and our experience in successfully treating a subset of these patients with topical colistimethate.
Materials and Methods
The medical records of 12 culture-proven MDR-PA keratitis who presented from 2005 to 2013 were retrospectively reviewed for clinical features, ulcer progression, microbiology findings, treatment details, and outcome. All patients had undergone antibiotic susceptibility testing with Kirby–Bauer disc diffusion method as per Clinical and Laboratory Standards Institute guidelines. Each isolate was labeled resistant (resistant and intermediate) or susceptible to a particular antibiotic based on the zone of inhibition around the antibiotic impregnated filter paper disc. Multi-drug resistance was considered if the bacterial isolate exhibited resistance pattern to ≥ three classes of antibiotics. Initially following Gram-stain report these patients had been treated with 0.3% ciprofloxacin or ofloxacin, 1% atropine sulfate and oral ibuprofen. Two nonconventional antibiotics were used in four patients - 1.6% colistimethate sodium and 5% imipenem/cilastatin. The former was prepared by reconstituting 1 million IU/80 mg of colistimethate sodium (Xylistin, Cipla Ltd., Mumbai, India) powder with 5 ml distilled water, giving a concentration of 16 mg (200,000 IU)/ml (1.6%) and the latter by reconstituting 50 mg of imipenem/cilastin (Cilaster, Alkem Ltd., Mumbai, India) with 10 ml of distilled water to obtain a 5% concentration. Both these were prepared fresh every day. Patients were admitted, and the antibiotics were instilled half-hourly for 48–72 h and then 1 hourly during daytime and 3 hourly during the night and thereafter the frequency was reduced according to the clinical response. Before the use of these antibiotics, the patients were explained about the empirical nature of the treatment and informed consent was obtained. The ulcer was considered to be healed once there were corneal epithelialization and resolution of the infiltrate and inflammation.
Results
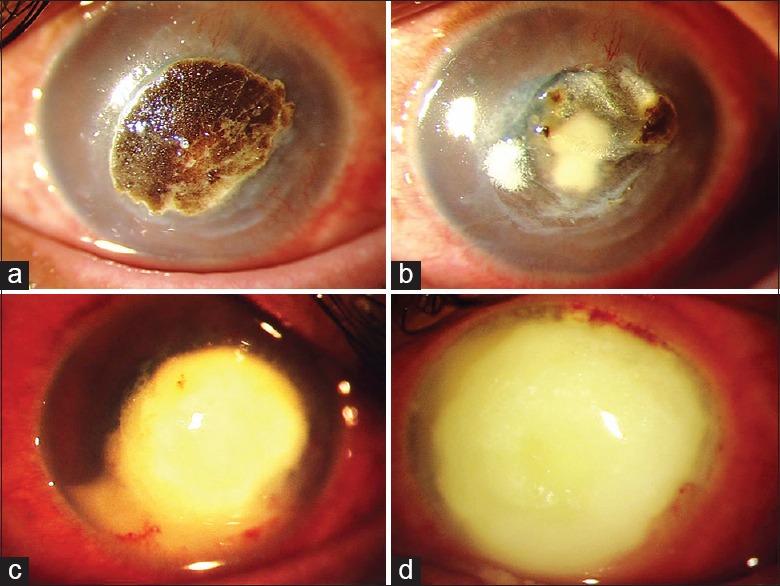
The demographic details, clinical features, treatment, and outcome of the 12 patients are given in Table 1. There were 8 (67%) males and 4 (33%) females with age ranging from 6 to 64 (mean: 44.33 ± 18.81) years. The mean time interval to the doubling of ulcer size or progression to perforation was 4.16 ± 2.41 (range: 2–10) days. Visual acuity was <20/200 in all cases. Seven(58.33%) patients progressed to corneal perforation while 4(33%) patients had endophthalmitis like Patient 3 [Fig. 1]. The antibiotic susceptibility findings are given in Table 2. The outcome in eight patients treated only with fluoroquinolones was evisceration in 4 (50%) patients, corneal graft in 1 (12.5%) patient, phthisis bulbi in 1 (12.5%) patient, and no clinical improvement in 2 (25%) patients. The only patient treated with imipenem/cilastatin did not improve clinically and underwent therapeutic corneal grafting. All the three patients treated with colistimethate healed with corneal scar formation [Figs. 2–4].
Table 1.
Clinical features, treatment details and outcome
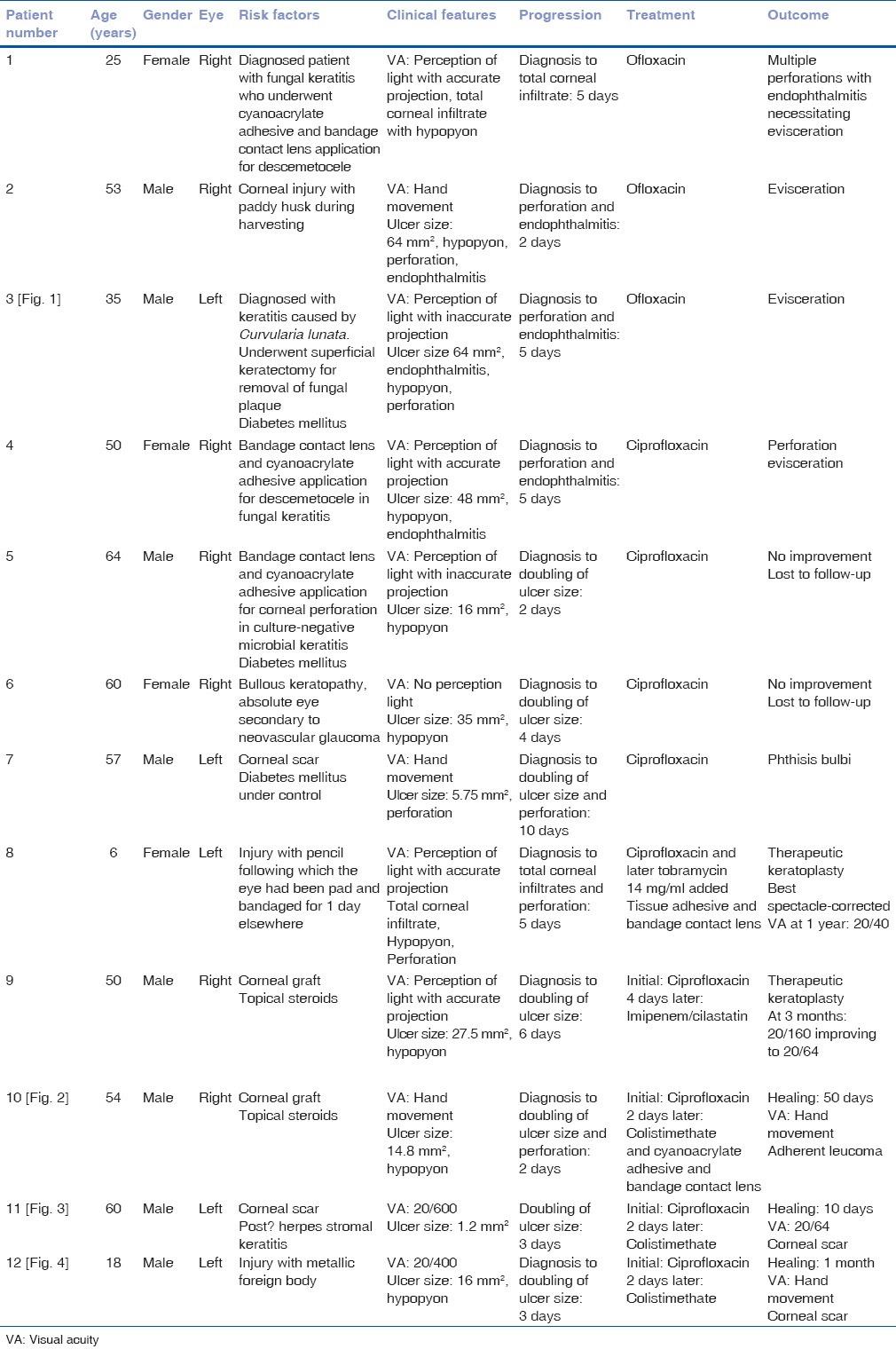
Figure 1.
Clinical slit-lamp photograph of the left eye of a 35-year-old farmer (patient no. 3) who had presented on August 07, 2006 with a pigmented fungal corneal ulcer (a) caused by Curvularia lunata and on the same day underwent superficial keratectomy to remove the pigmented plaque (b). Four days later he presented with worsening clinical features (c). Repeat scraping isolated multi-drug resistant Pseudomonas aeruginosa and 5 days later there was total corneal involvement with perforation and endophthalmitis (d)
Table 2.
Results of antimicrobial susceptibility test by Kirby–Bauer disc diffusion
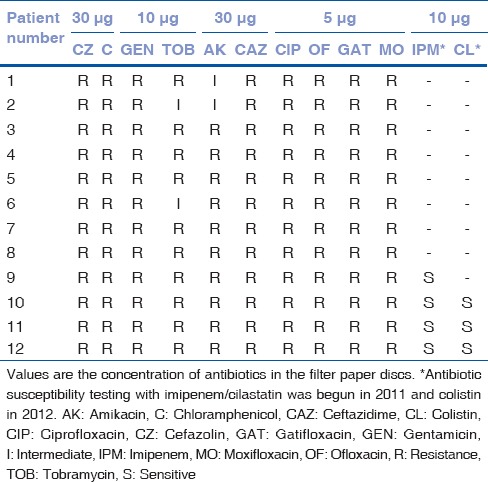
Figure 2.
Clinical slit-lamp photographs of the right eye of a 55-year-old farmer (patient no. 10) who presented on January 10, 2010 with a corneal graft infiltrate 2 months after undergoing penetrating keratoplasty (a). Two days later the infiltrate had doubled with corneal melting (b) necessitating the application of cyanoacrylate adhesive. Topical 1.6% colistimethate sodium was started 4 days from the presentation. Three weeks later the infiltrate had reduced significantly (c) and (d) complete healing occurred by 3 months
Figure 4.
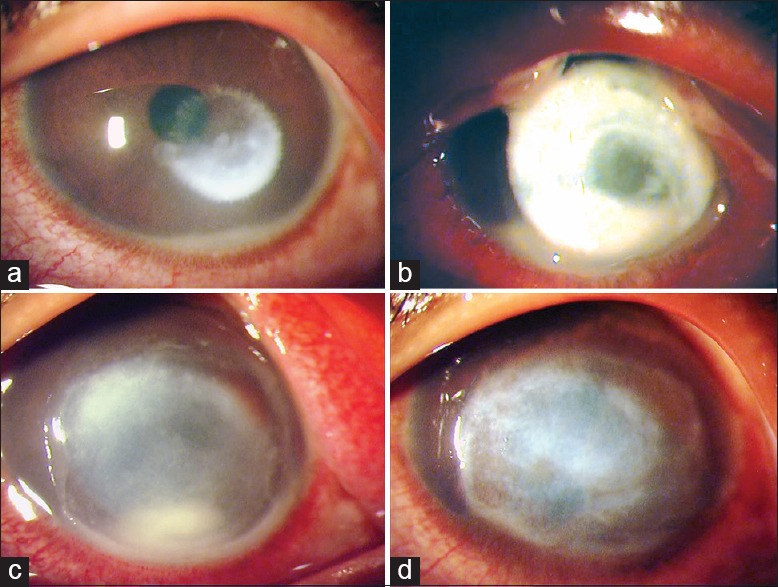
Clinical slit-lamp photographs of the left eye of an 18-year-old male (patient no. 12) who presented on May 06, 2013 with a corneal ulcer following trauma with a metallic foreign body (a). The ulcer had doubled 48-h later with corneal melting (b) when 1.6% colistimethate treatment was initiated. Four days later (c) there was reduction in infiltrate density and corneal melting appeared to have ceased and subsequently the ulcer healed (d) by 30 days
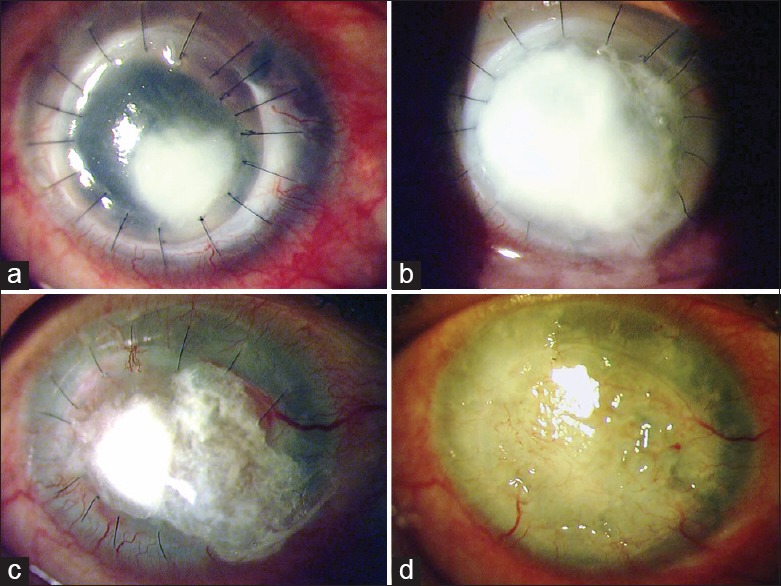
Figure 3.
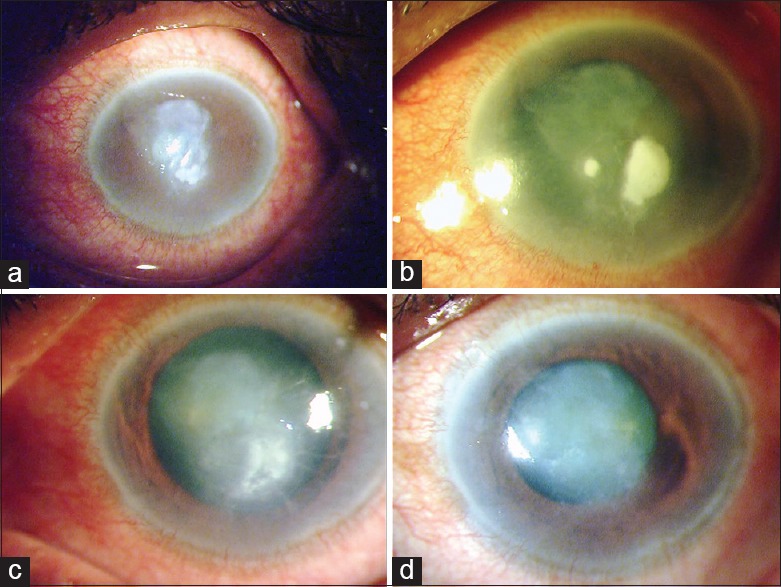
Clinical slit-lamp photographs of the left eye of a 60-year-old retired gentleman (patient no. 11), who had been previously treated for presumed herpes stromal keratitis, presented on March 08, 2012 with a corneal ulcer (a) which 48-h later had doubled in size (b). At this point, 1.6% colistimethate treatment was initiated. Four days later (c) the infection appeared to be under control and eventually healed (d) by 2 weeks
Discussion
Increasing resistance to topical ciprofloxacin, the drug of choice in PA bacterial keratitis has been reported since the late 1990s.[2] Alternative antibiotics suggested in different reports included amikacin,[2] or combinations of tobramycin and ticarcillin, or ceftazidime and a fluoroquinolone.[3] However, when the isolate is resistant to commonly available antibiotics the options are limited. Therefore, on the basis of reports from systemic infections[8,9] published in nonophthalmic literature, we selected two alternative antibiotics – imipenem/cilastatin and colistimethate. Our clinical experience with imipenem/cilastatin was not favorable, although the isolate was susceptible to the drug in vitro. We noticed that the topical preparation turned dark brown within few hours of reconstitution. This physical deterioration of the drug may have affected its potency and be the reason why treatment failed, and the patient eventually required therapeutic keratoplasty. However, in a report,[7] where meropenem, a newer carbapenem, was used, no such physical change was reported by the authors and all of their four cases had healed.
All the three patients in our series, of whom two had moderately severe ulcer [Table 2], who were treated with 1.6% colistimethate healed. Due to the limited treatment options in systemic infections with MDR Gram-negative bacteria, colistimethate (or polymyxin E) a polypeptide antibiotic introduced initially in the 1960s is seeing a resurgence in use.[9] After the submission of our paper for publication, a report by Jain et al. was published,[6] where the authors reported the use of 0.19% colistimethate in eight patients with keratitis due to MDR-PA. Similar to our series, the clinical presentation of their patients was characterized by rapid progression of the ulcers and nonresponsiveness to initial broad-spectrum antibiotics. The concentration of colistimethate used in this study, 16 mg/ml, was higher than the study reported by Jain et al. who used 0.19%.[5,6] The choice of 0.19% concentration was explained by the authors to be based on a previous paper published by Lund and Plains[10] however, none of these reports,[5,6,10] provide any scientific evidence or explanation based on which a 0.19% concentration was selected. Despite a higher concentration we did not observe any ocular features of toxicity. Lund et al. did not observe any ocular or systemic toxicity in experimental rats and dogs, by using oral doses of 6.67, 20 mg and 60 mg base/kg daily for a period of 90 days.[10] Colistimethate sodium is hydrolyzed to colistin sulfate, the active form of the drug and other sulfomethylated derivatives. The use of a higher concentration of colistimethate sodium would theoretically provide a higher concentration of colistin base as cited by Jain et al.[6] The higher concentration would be advantageous as it would besides increasing efficacy, also prevent the development of bacterial resistance. The preparation of 1.6% concentration of colistimethate involves a single step of dilution in contrast to 0.19% concentration, which requires double dilution.[5,6] Thus, the former process is simpler and would make compounding the solution less cumbersome in eye wards and pharmacies. Further studies are required to gain information on pharmacokinetics and pharmacodynamics of colistimethate in ocular use to come to an ideal concentration.
The clinical progression in MDR-PA keratitis is fulminant and so rapid that the eye is often beyond salvage even before the susceptibility report is available. Four of the eight patients in the series reported by Jain et al. required additional surgical intervention such as application of cyanoacrylate adhesive and corneal patch graft.[6] Of the three patients that we treated with colistimethate sodium, one (patient no. 10) [Fig. 2] required application of cyanoacrylate adhesive for impending perforation. In an earlier paper, the same authors had suggested performing early keratoplasty in larger ulcers as a globe-saving procedure.[5] A lower performance of therapeutic corneal grafts that resulted in a higher evisceration rate in our patients who were treated with conventional antibiotics is due to lack of donor corneal tissue, a limitation which is common in almost all developing countries. Furthermore, antibiotic susceptibility testing in an ophthalmic microbiology laboratory uses a limited number of antibiotics leading to delay in identifying alternative drugs. To avoid this delay we now routinely include colistimethate, piperacillin, and meropenem in antibiotic susceptibility test for Pseudomonas isolates.
Although this series is small, it underlines the severity of infection with MDR-PA and the efficacy of 1.6% colistimethate. There is a need for further studies, but as observed by others the low incidence rate of this particular infection is likely to make larger clinical trials a difficult proposition.[6] Pooling of data from multiple centers may give more information on antibiotic resistance trends and provide evidence-based solutions. Nevertheless, this study along with other reports provide an important evidence to support the use of these alternative antibiotics in an infection where treatment options are extremely limited.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: No ESKAPE! An update from the infectious diseases society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Garg P, Sharma S, Rao GN. Ciprofloxacin-resistant Pseudomonas keratitis. Ophthalmology. 1999;106:1319–23. doi: 10.1016/S0161-6420(99)00717-4. [DOI] [PubMed] [Google Scholar]
- 3.Rhee MK, Kowalski RP, Romanowski EG, Mah FS, Ritterband DC, Gordon YJ. A laboratory evaluation of antibiotic therapy for ciprofloxacin-resistant Pseudomonas aeruginosa. Am J Ophthalmol. 2004;138:226–30. doi: 10.1016/j.ajo.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Chew FL, Soong TK, Shin HC, Samsudin A, Visvaraja S. Topical piperacillin/tazobactam for recalcitrant Pseudomonas aeruginosa keratitis. J Ocul Pharmacol Ther. 2010;26:219–22. doi: 10.1089/jop.2009.0077. [DOI] [PubMed] [Google Scholar]
- 5.Jain R, Murthy SI, Motukupally SR. Clinical outcomes of corneal graft infections caused by multi-drug resistant Pseudomonas aeruginosa. Cornea. 2014;33:22–6. doi: 10.1097/ICO.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 6.Jain R, Murthy SI, Motukupally SR, Jain M. Use of topical colistin in multiple drug-resistant Pseudomonas aeruginosa bacterial keratitis. Cornea. 2014;33:923–7. doi: 10.1097/ICO.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 7.Pande R, Bhailume PV. Use of topical meropenem in management of hospital acquired Pseudomonas ocular infections. J Clin Ophthalmol Res. 2014;2:23–5. [Google Scholar]
- 8.Zhanel GG, Wiebe R, Dilay L, Thomson K, Rubinstein E, Hoban DJ, et al. Comparative review of the carbapenems. Drugs. 2007;67:1027–52. doi: 10.2165/00003495-200767070-00006. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: The re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 10.Lund MH, Plains M. Colistin sulfate ophthalmic in the treatment of ocular infections. Arch Ophthalmol. 1969;81:4–10. doi: 10.1001/archopht.1969.00990010006002. [DOI] [PubMed] [Google Scholar]


