Abstract
Aim
One condition associated with severe end-of-life pain that can lead to a poor quality of death is cancer. Cancer pain in people with dementia is of particular concern because of communication problems that occur with worsening disease. The aim of the current pilot study was to examine the association between hospice enrolment, dementia severity and pain among nursing home residents who died from advanced cancer.
Methods
Between-groups cross-sectional chart audits of 55 nursing home residents with dementia who died from cancer were carried out.
Results
A total of 45% of residents were in hospice at the end-of-life. Residents in hospice were more likely to receive an opioid (80% vs 43%, P = 0.005); but less likely to show severe cognitive impairment (20% vs 50%, P = 0.050). Enrolment in hospice was associated with an increased likelihood of receiving an opioid after controlling for level of cognitive impairment (OR = 3.9, 95% CI = 1.1–14.0, P = 0.037). Lower levels of cognitive functioning were associated with a decreased likelihood of receiving an opioid after controlling for enrolment in hospice (OR = 0.3, 95% CI = 0.1–0.8, P = 0.030). Notably, 40% of nursing home residents with dementia who died from cancer did not receive any opioid during this time.
Conclusions
Preliminary results suggest that hospice enrolment might be influenced by the facility or region of this particular country. Hospice enrolment predicts more opioid pain treatment in residents with dementia and terminal cancer; however, no resident with very severe dementia and terminal cancer was placed in hospice care. Severely cognitively impaired nursing home residents requiring opioids are at great risk of suffering from untreated advanced cancer pain. New methods are urgently required to improve end-of-life palliative care for nursing home residents with terminal cancer and severe dementia.
Keywords: cancer, dementia, discomfort, end-of-life, hospice, pain
Introduction
Untreated terminal cancer pain in people with dementia is of critical public health importance. Estimates are that from 50%1 to 88%2 of people with cancer experience pain during the last year of life. Evidence suggests that cancer pain seems to vary little in severity over the final year3 and months4 of life. Among people in whom treatment is no longer an option (life expectancy less than 6 months), 70% experience moderate to severe pain,1 and 50% of people with terminal cancer experience pain at a level that requires sedation during the final week of life.5
Between 2010 and 2050, the population of older adults worldwide over the age of 65 years is expected to increase from 34 million to 88 million.6 Nearly half of those over the age of 85 years will develop dementia, and a majority (75%) of these will live in nursing homes.7,8 The prevalence of pain and painful conditions in nursing homes has been estimated to be between 45% and 83%.9–12 Thus, many older adults residing in nursing homes with dementia have pain. One condition commonly associated with severe pain in the nursing home is cancer.10,13 Furthermore, older adults with cancer experience more comorbid medical conditions leading to increased pain.14 Unfortunately, cognitively impaired residents might receive little or no pain medication, even when they have medical conditions known to be painful in people who are cognitively intact.15,16 Thus, people with dementia and cancer might be at increased risk for untreated pain. One approach to improve pain management in older adults with dementia and cancer is to place them in hospice care.17 People in hospice are more likely to receive pain treatment than those who are not in hospice,18,19 yet nursing home residents with cancer receive fewer hospice services than other older adults with cancer (19%20 vs 65%21). Understanding the relationships among hospice enrolment, dementia severity and pain treatment is important in designing effective pain management programs for nursing home residents with cancer.
Two previous papers have been published from the current data. The first paper reported that African American nursing home residents with cancer and dementia experienced significantly higher discomfort pain scores when compared with Caucasians, suggesting that pain and distress might be experienced differently across different racial groups.22 The second paper found that when compared to those with moderate dementia, people with very severe dementia had significantly fewer behavioral indicators of pain.23 The aim of the current study was to examine the associations between hospice enrolment, dementia severity and pain among nursing home residents who died from advanced cancer. We tested three hypotheses: (i) behavioral indicators of pain and dementia severity will differ between nursing home residents in hospice and those who are not in hospice; (ii) levels of opioid administration during the last 2 weeks of life will differ between nursing home residents in hospice and those who are not in hospice; and (iii) dementia severity during the last 90 days of life and hospice enrolment will be associated with opioid administration during the last 2 weeks of life.
Methods
After receiving Institutional Review Board approval from The University of Tennessee Health Science Center, we carried out a between-groups retrospective chart audit study. The independent variable for the analysis of the first two hypothesis was hospice enrolment, and the dependent variables were: (i) dementia severity (Cognitive Performance Scale; [CPS] score); (ii) pain/discomfort (Discomfort Behavior Scale [DBS]) score; and (iii) opioid administration. Dementia severity and hospice enrolment were both independent variables for the third hypothesis, with opioid administration being the dependent variable. The primary author recruited nursing homes from those in a regional telephone directory, from local Internet listings and from word-of-mouth referrals. The nursing homes were stratified to include for-profit (3) and not-for-profit (8), ranging in size from 68–180 beds located in and up to 150 miles from a large southern USA metropolitan area (population >1.5 million). Of these, nine (2 for-profit and 7 not-for-profit) agreed to participate. To be included in the study, decedents had a diagnosis of dementia and must have died from cancer in the nursing home. The cancer diagnoses included in the present study were determined from the Centers for Disease Control (CDC) top 10 cancers of the overall population for all races and genders in 2004.24 These common cancers were chosen to enhance sample homogeneity and generalizability of findings. Each medical record with a cancer diagnosis was then cross-referenced to determine a diagnosis of Alzheimer’s disease (International Classification of Diseases, Ninth Revision [ICD9]-331.0), Lewy body dementia (ICD9-290.4), vascular dementia (ICD9-331.82) or any combination of the three. All medical charts were physically retrieved to verify the findings from the electronic medical record database. Exclusion criteria were inability to determine the cause of death, incomplete records, dementia with co-occurring psychosis or dying outside the nursing home. A total of 76 records meeting initial inclusion criteria were located. Because of the inability to definitively determine cause of death or not dying in the nursing home, 21 records were excluded leaving a final sample of 55.
Measures
Hospice enrolment
Each medical record was physically retrieved and the medical order for hospice services – and verification of enrolment in hospice – was reviewed before collecting all medication-related measures.
Minimum Data Set (2.0)
Nursing staff in several countries, including Japan and the USA, use the Minimum Data Set (MDS) to assess residents on admission, quarterly and with a significant change in health status.25 The MDS includes over 250 assessment items on demographic, diagnostic, clinical, functional, psychosocial and cognitive status.26 The MDS has strong interrater item reliability (>0.75),27 and the MDS is reliable for data collection in nursing home residents.28 Cognitive ability and pain assessment instruments have been derived from the MDS. They include the CPS26 and the DBS.29 Each of these measures was calculated from the same MDS assessment within the last 90 days of life.
Cognitive ability
The CPS26 was developed from five MDS 2.0 items relevant to cognitive ability. The CPS is scored from 0 to 6, indicating no impairment (score = 0) to very severe impairment (score = 6).26 Individuals with scores from 1 to 6 were included in this analysis. Based on previous work,19 in the current study, CPS scores were collapsed into the following categories: CPS score of 1 and 2 = mild dementia, CPS score of 3 and 4 = moderate dementia, and CPS score of 5 and 6 = severe dementia. The CPS tool developers reported interrater reliability at 0.85,26 and the CPS has been reported to be both reliable and valid.30 As the CPS is calculated quarterly, cognitive status related to dementia was assumed to remain fairly stable for at most 90 days, and could be meaningfully used for associations with opioid administration in the last 2 weeks of life.
Pain
The DBS was developed as an alternative for pain assessment when self-report might be limited. The 17 DBS tool items were taken from specific MDS 2.0 items relevant to behavioral indicators of discomfort and pain, such as repetitive verbalizations, insomnia/change in sleep or being sad/worried. The DBS tool developers reported a composite reliability of 0.98.29
Opioid administration
The Equianalgesic Opioid Conversion Ratios for Patients Previously Receiving Other Opioids online calculator,31 was used to convert opioid medications to an equivalent dose unit (EDU) continuous outcome measure. Opioids identified in the current analysis were: fentanyl, hydrocodone, hydromorphone, meperidine, morphine, oxycodone and oxymorphone. No resident received partial opioid-agonists during the period of the chart review. All opioid analgesics were converted to a continuous measure standardized to levorphanol (1 mg = 1 EDU), where 10 mg of codeine = 0.05 EDU; 10 mg of hydrocodone = 0.33 EDU; 10 mg of hydromorphone = 1.33 EDU; 10 mg of meperidine = 0.03 EDU; 10 mg of methadone = 2.5 EDU; 10 mg morphine = 0.33 EDU; 10 mg of oxycodone = 0.50 EDU; 10 mg of oxymorphone = 1 EDU; and 25mcg of fentanyl = 3 EDU.31
Non-narcotic analgesics
Total numbers of doses of non-narcotic analgesics (i.e. acetaminophen, acetylsalicylic acid, ibuprofen and naproxen sodium) from those recorded in the medical record.
General chart abstraction techniques
Using a retrospective chart review, residents with a diagnosis of at least one of the 10 cancers were located using the Vista KEANE MDS software (Keane Care, Redmond, WA, USA) in each nursing home. Vista KEANE is used by nurses to complete the MDS assessment forms and to interrogate the data. All medical records were then physically retrieved and reviewed to verify findings of cancer and dementia diagnoses. Total amount of opioid administered over the last 2 weeks of life was converted to EDU as described earlier. Total numbers of doses of non-narcotic analgesics were counted over the last 2 weeks of life.
Analyses
Data were analyzed using SPSS 18.0 (IBM Corporation, Somers, NY, USA). Means and standard deviations were used to summarize the distributions of age and cognitive performance (CPS) in this sample. Because of the skewed discomfort (DBS) and opioid (EDU) data distributions, median and 25–75th interquartile range values representing the middle 50% of the cases described were used to summarize that demographic characteristic. Other demographic and clinical characteristics were nominal in nature and were summarized using frequency distributions. Comparisons between the hospice and non-hospice groups were carried out using independent t-tests (age, CPS), Mann–Whitney tests (opioid EDU, non-narcotic doses, DBS) and likelihood χ2-tests (nominal variables). Comparisons among the cognitive status groups (mild impairment, moderate impairment, severe impairment) were carried out using Kruskal–Wallis tests (opioid EDU, non-narcotic doses, DBS) and likelihood χ2-tests (nominal variables). Multiple logistic regression was used to test for the adjusted associations of hospice enrolment and cognitive status on the receipt of opioid. A maximum alpha of 0.05 was used for determining statistical significance.
Results
Demographic (age, sex, race) and clinical characteristics (type of dementia, level of cognitive impairment) for the subjects enrolled in hospice (n = 25) and those who were not (n = 30) are summarized in Table 1. The median age of the sample was 87.5 years; approximately 55% (n = 28) were female, and 71% (n = 39) where white. Nearly 82% (n = 45) of the sample had Alzheimer’s disease, and 15% (n = 8) had vascular dementia. Approximately 53% (n = 29) of the sample had either colon/rectal or breast cancer. The average CPS score for the entire sample was 3.75 (SD 1.69). However, the CPS scores for the nursing home residents enrolled in hospice were statistically significantly lower (less cognitive impairment) on average than those for the residents not enrolled (hospice: mean 3.00, SD 1.35; not in hospice: mean 4.37, SD 1.71; t(d.f.=53) = 3.24; P = 0.002). A total of 50% (15/30) of those not enrolled in hospice showed severe cognitive impairment (CPS = 5–6), whereas just 20% (5/25) of those in hospice showed that level of impairment (likelihood χ2 (d.f.=2) = 5.78, P = 0.050). None of the nursing home residents with very severe cognitive impairment (CPS = 6, n = 12) were enrolled in hospice.
Table 1.
Study sample characteristics
| Characteristic | Total sample (n = 55) n (%) |
Hospice (n = 25) n (%) |
Non-hospice (n = 30) n (%) |
P-value |
|---|---|---|---|---|
| Sex | 0.843 | |||
| Female | 30 (54.5) | 14 (56.0) | 16 (53.3) | |
| Male | 25 (45.5) | 11 (44.0) | 14 (46.7) | |
| Race/ethnicity | 0.446 | |||
| White | 39 (70.9) | 19 (76.0) | 20 (66.7) | |
| Black | 16 (29.1) | 6 (24.0) | 10 (33.3) | |
| Dementia type | 0.217 | |||
| Alzheimer’s disease | 45 (81.8) | 19 (76.0) | 26 (86.7) | |
| Vascular dementia | 8 (14.5) | 5 (20.0) | 3 (10.0) | |
| Lewy body dementia | 1 (1.8) | 1 (4.0) | 0 (0.0) | |
| Mixed dementia | 1 (1.8) | 0 (0.0) | 1 (3.3) | |
| Age | a Mean (SD) | a Mean (SD) | a Mean (SD) | 0.175 |
| Years | 86.4 (7.8) | 85.2 (8.9) | 87.3 (6.9) | |
| Cognitive Performance Scale | 3.8 (1.7) | 3.0 (1.4) | 4.4 (1.7) | 0.002 |
| Levels of cognitive impairment | n (%) | n (%) | n (%) | 0.050 |
| Mild (CPS = 1–2) | 14 (14.4) | 9 (36.0) | 5 (16.7) | |
| Moderate (CPS = 3–4) | 21 (38.2) | 11 (44.0) | 10 (33.3) | |
| Severe (CPS = 5–6) | 20 (36.4) | 5 (20.0) | 15 (50.0) |
IQR: 25th and 75th interquartile range representing the middle 50% of the values. CPS, Cognitive Performance Scale.
A total of 20 of 25 (80.0%) of those in hospice received some opioid analgesic during the last 2 weeks of life compared with just 13 of 30 (43.3%) of those subjects not in hospice (likelihood X2(d.f.=1) = 7.96, P = 0.005; Table 2). However, if any opioid was administered, there was no statistically significant difference in the amount received regardless of whether the participant was in hospice or not (Mann–Whitney U = 119.00, Z = −0.40, P = 0.703). A total of 64% (16/25) of the participants in hospice reported at least some level of discomfort on the DBS measure compared with just 33.3% (10/30) of those not in hospice (likelihood χ2 (d.f.=1) = 5.22, P = 0.022). Residents in hospice with some level of discomfort (DBS > 0) tended to report less discomfort (median = 6.0) than did the respective residents not in hospice (median = 11.5); however. given the size of the sample this difference was not statistically significant, (Mann–Whitney U = 44.00, Z = 1.92, P = 0.055; Table 3).
Table 2.
Pain medication by hospice status
| Measure | Total sample (n = 55) | Hospice (n = 25) | Non-hospice (n = 30) | P-value |
|---|---|---|---|---|
| Received some opioid, n (%) | 33 (60.0) | 20 (80.0) | 13 (43.3) | 0.005 |
| a Median (Min, Max) (n = 33) | a Median (Min, Max) (n = 20) | a Median (Min, Max) (n = 13) | ||
| Opioid received (EDU) | 12.8 (0.1, 81.9) | 12.2 (0.1, 45.4) | 12.8 (0.1, 81.9) | 0.685 |
| Received some non-narcotic, n (%) | 18 (32.7) | 9 (36.0) | 9 (30.0) | 0.637 |
| a Median (Min, Max) (n = 18) | a Median (Min, Max) (n = 9) | a Median (Min, Max) (n = 9) | ||
| Total doses of non-narcotic | 2.0 (1, 56) | 1.0 (1, 43) | 4.0 (1, 56) | 0.579 |
IQR: 25th and 75th interquartile range representing the middle 50% of the values. Medication variables collected over the final 2 weeks of life. EDU, equivalent dose unit.
Table 3.
Discomfort level by hospice status
| Measure | Total sample (n = 55) | Hospice (n = 25) | Non-hospice (n = 30) | P-value |
|---|---|---|---|---|
| Any level of discomfort (DBS >0), n (%) | 26 (47.3) | 16 (64.0) | 10 (33.3) | 0.022 |
| Median (Min, Max) (n = 26) | Median (Min, Max) (n = 16) | Median (Min, Max) (n = 10) | ||
| If experienced discomfort, (DBS) score | 7.0 (3, 48) | 6.0 (3, 48) | 11.5 (5–32) | 0.055 |
Discomfort level determined during the last 90 days of life. DBS, Discomfort Behavior Scale.
A total of 10 of 14 residents with mild dementia (71%) and 16 of 21 residents with moderate dementia (76%) on their last CPS assessment before death received some amount of opioid during the last 2 weeks of life compared with just seven of 20 (35%) of those subjects with severe dementia on their last CPS assessment before death (likelihood χ2 (d.f.=2) = 8.33, P = 0.016; Table 4). However, if any opioid was administered, there was no statistically significant difference in the amount received regardless of whether the resident had mild, moderate or severe dementia. No statistically significant differences in non-narcotic analgesic intake (Table 4) were identified among those in the mild, moderate or severe dementia groups.
Table 4.
Pain medication by cognitive status
| Measure | Cognitive Impairment | P-value | ||
|---|---|---|---|---|
| Mild (n = 14) Mean (SD) |
Moderate (n = 21) Mean (SD) |
Severe (n = 20) Mean (SD) |
||
| Received some opioid, n (%) | 10 (71.4) | 16 (76.2) | 7 (35.0) | 0.016 |
| a Median (Min, Max) (n = 10) | a Median (Min, Max) (n = 16) | a Median (Min, Max) (n = 7) | ||
| Total opioid (EDU) | 14.2 (2.9, 81.9) | 10.8 (0.2, 45.4) | 12.8 (0.1, 21.0) | 0.418 |
| Received some non-narcotic, n (%) | 5 (35.7) | 7 (33.3) | 6 (30.0) | 0.938 |
| a Median (Min, Max) (n = 5) | a Median (Min, Max) (n = 7) | a Median (Min, Max) (n = 6) | ||
| Total doses of non-narcotic | 1.0 (1, 2) | 14.0 (1, 40) | 6.5 (1, 56) | 0.097 |
IQR: 25th and 75th interquartile range representing the middle 50% of the values. Medication variables collected over the final 2 weeks of life. Cognitive status determined during the last 90 days of life. We assumed that dementia severity would either remain the same or likely worsen until death. EDU, equivalent dose unit.
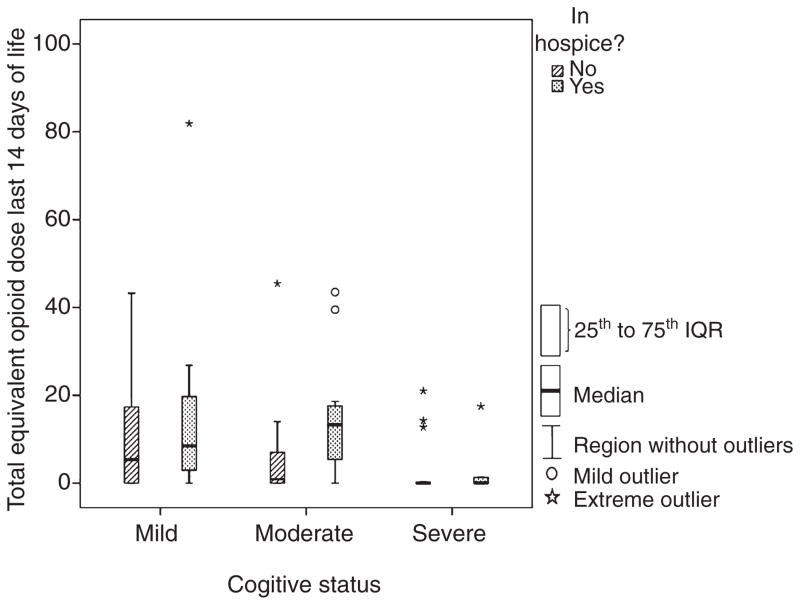
Because both enrolment in hospice and cognitive impairment showed statistically significant associations with the receipt of opioids during the last 2 weeks of life, both variables were included in a multiple logistic regression analysis to assess whether after controlling for the observed association between cognitive status and hospice enrolment both would remain statistically significant. These multivariate analyses showed that the patterns of the associations appeared to be similar as those found from the simple, unadjusted analyses (see Fig. 1). That is, being in hospice was associated with an increased likelihood of receiving an opioid after controlling for the level of cognitive impairment (OR = 3.9, 95% CI = 1.1–14.0, P = 0.037), and increasing cognitive impairment was associated with a decreased likelihood of receiving an opioid regardless of or even after controlling for being in hospice (OR = 0.3, 95% CI = 0.1–0.8, P = 0.030). None of the demographic or clinical variables were associated with the likelihood of receiving an opioid (P > 0.05).
Figure 1.
Box and whisker plot. The y-axis = total equivalent doses of opioid analgesic standardized to 1 mg of levorphanol = 1 equivalent dose unit (range 0–82). The x-axis = cognitive status reported in Cognitive Performance Scale (CPS) scores (range 1–6). Mild dementia = CPS of 1 and 2, moderate dementia = CPS of 3 and 4, severe dementia = CPS of 5 and 6. Note: No resident with a CPS score of 6 (n = 12) was enrolled in hospice.
Discussion
The purpose of the present study was to determine the association of hospice status, dementia severity and pain treatment in nursing home residents who died from cancer. Our first hypothesis that behavioral indicators of pain and dementia severity would differ between nursing home residents with and without hospice care was accepted. We found that residents enrolled in hospice were more cognitively intact and more likely to report some level of discomfort. Our second hypothesis that opioid administration during the last 2 weeks of life would differ between nursing home residents with and without hospice care was accepted. Nursing home residents in hospice were more likely to receive an opioid during the last 2 weeks of life. The third hypothesis that dementia severity during the last 90 days of life and hospice enrolment would be associated with opioid administration during the last 2 weeks of life was accepted. Nursing home residents enrolled in hospice were more cognitively intact and were more likely to receive an opioid.
Despite the fact that severe dementia should lead to greater hospice enrolment,32,33 we found that subjects enrolled in hospice were significantly more cognitively intact than those who were not. This cognitive status difference for enrolment is not explained by the data. Possible explanations are a selection bias by clinicians who perceive that patients who are more cognitively intact benefit more from hospice enrolment or that clinicians are biased because they have little understanding about end-of-life dementia care and hospice eligibility. Another possibility is that people who are more cognitively intact are better able to advocate for their placement in hospice care. For example, in the USA, hospice use is influenced by facility culture, religious beliefs, region of the country and medical benefits33
Preliminary findings show that low opioid administration might be a serious problem in nursing home residents with dementia and terminal cancer. A total of 40% (22/55) of subjects with dementia and cancer did not receive any opioid during the last 2 weeks of life. We also found that residents in hospice were more likely to have some level of discomfort (DBS scores >0), and that these residents were also more likely to receive an opioid. However, on average, residents not in hospice had higher DBS scores (more pain) and these residents were less likely to receive an opioid – indicating that being in hospice might be associated with better terminal cancer pain management. One reason for low opioid administration could be that clinicians who are using the World Health Organization’s guidelines for cancer pain treatment34 might believe that they are appropriately following the guidelines because people with severe dementia may appear to not be in pain. Thus, no opioid would be administered. Other reasons for low opioid administration could be attributed to family concerns over decreased quality of life issues, such as constipation, increased risk of falls or to difficultly in determining when to use stronger pain medications.
A total of 45% (n = 25) of subjects with dementia who died from cancer were enrolled in hospice at the end-of-life. Previous investigators reported that from 11.8%35 to 18.6%36 of people with cancer and dementia were enrolled in hospice. Some possible reasons for the higher enrolment in hospice in the current study are that nursing home staff have improved attitudes toward the use of hospice, nursing home staff perceive an increased benefit to patient care in hospice, or a cultural bias favoring hospice exists in the region of the country in which the sample was taken.33
Future studies are required to explore the reasons clinicians decide not to administer medications to residents with dementia. Does the severity of dementia produce a perception that no pain exists? Currently, clinicians rely on verbal reports and behavioral cues in deciding to treat pain, and without these indicators they might not administer pain medication.37 Are persons with dementia suffering needlessly or do they experience pain differently in the advanced stages of the disease? An important approach to consider is screening all residents with high-risk conditions for pain, with special attention given to developing an individualized pain assessment and management plan.
This study has some limitations. Because of the rarity of nursing home residents with dementia who die from cancer, random assignment of group participants was not feasible. Thus, an experimentally accessible38 convenience sample was selected over 6.5 years. To help overcome the threat to internal validity regarding the selection of groups, we used strict guidelines for inclusion in the current study. Subjects had a diagnosis of dementia without co-occurring psychosis and must have died from advanced cancer. Further, residents were included only if the medical chart and/or death certificate listed the cause of death as cancer and place of death was the nursing home. We could not locate an estimate of the number of people with dementia who die from cancer; however, the rarity of nursing home residents with terminal cancer and advanced dementia has been documented. Mitchell et al. found that approximately 2% of nursing home residents die from advanced dementia and cancer.19 The MDS is carried out quarterly, and with a change in resident status and is completed by multiple providers39,40 who are responsible for charting diagnoses and medication administration. An assumption is that the data were coded and entered correctly in the nursing home databases, MDS records and the medication administration records. Furthermore, cognitive and discomfort variables in the current study had to be collected from the last MDS preceding death. Nonetheless, the MDS has been found to be a reliable and valid instrument for data collection.27,28
In summary, the preliminary findings from the present study indicate that: (i) nursing home residents with dementia and terminal cancer receiving hospice care had lower behavioral scores indicating pain (ii) nursing home residents with dementia who were dying from cancer were more likely to receive an opioid if they were in hospice; and (iii) nursing home residents with severe dementia are seldom enrolled in hospice. Notably, if opioid administration is an indicator of pain relief and decreased suffering at the end of life, 40% of nursing home residents with dementia who died from cancer did not receive any opioid during this time.
Future studies addressing the neurobiology of pain in people with dementia are required.41 A recent Japanese case study highlighted that older adults with painful cancers and dementia received no narcotic or antipsychotic medications for palliative cancer care.42 Findings from the current study confirm these observations, and suggest that highly vulnerable nursing home residents with dementia and terminal cancer are at risk of experiencing a decreased quality of death. We join our colleagues in Japan43 with an urgent call to reform end-of-life care in long-term care facilities worldwide and to increase pain research in people with dementia.44
Acknowledgments
The authors gratefully acknowledge Dr Keela Herr for her assistance in article refinement.
Footnotes
Disclosure statement
The authors have no conflicts of interest to disclose
References
- 1.van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. 2007;132:312–320. doi: 10.1016/j.pain.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Addington-Hall J, McCarthy M. Dying from cancer: results of a national population-based investigation. Palliat Med. 1995;9:295–305. doi: 10.1177/026921639500900404. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel D, Bloom JR. Pain in metastatic breast cancer. Cancer. 1983;52:341–345. doi: 10.1002/1097-0142(19830715)52:2<341::aid-cncr2820520227>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Morris JN, Suissa S, Sherwood S, Wright SM, Greer D. Last days: a study of the quality of life of terminally ill cancer patients. J Chronic Dis. 1986;39:47–62. doi: 10.1016/0021-9681(86)90106-2. [DOI] [PubMed] [Google Scholar]
- 5.Fainsinger R, Bruera E, Miller M, Hanson J, MacEachern T. Symptom control during the last week of life on a palliative care unit. J Palliat Care. 1991;7:5–11. [PubMed] [Google Scholar]
- 6.Administration on Aging. Projected future growth of older population. 2010 [Cited 23 Jun 2011.] Available from URL: http://www.aoa.gov/aoaroot/aging_statistics/future_growth/future_growth.aspx-age.
- 7.Alzheimer’s Association. Alzheimer’s disease facts and figures. 2011 doi: 10.1016/j.jalz.2011.02.004. [Cited 17 Oct 2009.] Available from URL: http://www.alz.org/national/documents/report_alzfactsfigures2011.pdf. [DOI] [PubMed]
- 8.Sawyer P, Lillis JP, Bodner EV, Allman RM. Substantial daily pain among nursing home residents. J Am Med Dir Assoc. 2007;8:158–165. doi: 10.1016/j.jamda.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro RS. Liability issues in the management of pain. J Pain Symptom Manage. 1994;9:146–152. doi: 10.1016/0885-3924(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 10.Ferrell BA, Ferrell BR, Rivera L. Pain in cognitively impaired nursing home patients. J Pain Symptom Manage. 1995;10:591–598. doi: 10.1016/0885-3924(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 11.Roy R, Thomas M. A survey of chronic pain in an elderly population. Can Fam Physician. 1986;32:513–516. [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner D. Pain in nursing home residents: an exploration of prevalence, staff perspectives, and practical aspects of measurement. Clin J Pain. 1999;15:92. doi: 10.1097/00002508-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc. 1990;38:409–414. doi: 10.1111/j.1532-5415.1990.tb03538.x. [DOI] [PubMed] [Google Scholar]
- 14.Thome B, Hallberg I. Quality of life in older people with cancer – a gender perspective. Eur J Cancer Care (Engl) 2004;13:454–463. doi: 10.1111/j.1365-2354.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 15.Herr KA, Decker S. Assessment of pain in older adults with severe cognitive impairment. Ann Long Term Care. 2004;12:46–52. [Google Scholar]
- 16.Husebo BS, Strand LI, Moe-Nilssen R, BorgeHusebo S, Aarsland D, Ljunggren AE. Who suffers most? Dementia and pain in nursing home patients: a cross-sectional study. J Am Med Dir Assoc. 2008;9:427–433. doi: 10.1016/j.jamda.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Tarzian AJ, Hoffmann DE. Barriers to managing pain in the nursing home: findings from a statewide survey. J Am Med Dir Assoc. 2005;6:S13–S19. doi: 10.1016/j.jamda.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Munn JC, Hanson LC, Zimmerman S, Sloane PD, Mitchell CM. Is hospice associated with improved end-of-life care in nursing homes and assisted living facilities? J Am Geriatr Soc. 2006;54:490–495. doi: 10.1111/j.1532-5415.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell S, Kiely D, Hamel M. Dying with advanced dementia in the nursing home. Arch Intern Med. 2004;164:321–326. doi: 10.1001/archinte.164.3.321. [DOI] [PubMed] [Google Scholar]
- 20.Buchanan RJ, Barkley J, Wang S, Kim M. Analyses of nursing home residents with cancer at admission. Cancer Nurs. 2005;28:406–414. doi: 10.1097/00002820-200509000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Keating NL, Herrinton LJ, Zaslavsky AM, Liu L, Ayanian JZ. Variations in hospice use among cancer patients. J Natl Cancer Inst. 2006;98(15):1053–1059. doi: 10.1093/jnci/djj298. [DOI] [PubMed] [Google Scholar]
- 22.Monroe T, Carter M. A retrospective pilot study of African American and Caucasian nurisng home residents with cancer and dementia. J Pain Symptom Manage. 2010;40(4):e1–e3. doi: 10.1016/j.jpainsymman.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monroe T, Carter M, Feldt K, Tolley B, Cowan R. Assessing advanced cancer pain in older adults with dementia at the end-of-life. J Adv Nurs. 2012;68(9):2070–2078. doi: 10.1111/j.1365-2648.2011.05929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control. United States cancer statistics: 1999–2004 incidence and mortality web-based report. 2007;2008(August 5) [Google Scholar]
- 25.U. S. Department of Health Human Services. Minimum Data Set (MDS) Version 2.0. 2000;2008(July 12) [Google Scholar]
- 26.Morris J, Brant E, Fries D, et al. MDS cognitive performance scale. J Gerontol. 1994;49(4):m174–m182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 27.Mor V, Angelelli J, Jones R, Roy J, Moore T, Morris J. Inter-rater reliability of nursing home quality indicators in the U.S. BMC Health Serv Res. 2003;3:1–13. doi: 10.1186/1472-6963-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hawes C, Morris J, Phillips C, Mor V. Reliability estimates for the minimum data set for nursing home resident assessment and care screening (MDS) Gerontologist. 1995;35:172–179. doi: 10.1093/geront/35.2.172. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson KM, Brown RL, Dahl JL, Ward SE, Brown MS. The discomfort behavior scale: a measure of discomfort in the cognitively impaired based on the minimum data set 2.0. Res Nurs Health. 2006;29:576–587. doi: 10.1002/nur.20168. [DOI] [PubMed] [Google Scholar]
- 30.Paquay L, De Lepeleire J, Schoenmakers B, Ylieff M, Fontaine O, Buntinx F. Comparison of the diagnostic accuracy of the Cognitive Performance Scale (Minimum Data Set) and the Mini-Mental State Exam for the detection of cognitive impairment in nursing home residents. Int J Geriatr Psychiatry. 2007;22:286–293. doi: 10.1002/gps.1671. [DOI] [PubMed] [Google Scholar]
- 31.Arkansas Medicaid. Opioid dosing conversion calculator.xls. 2007 [Cited 11 Nov 2007.] Available from URL: https://www.medicaid.state.ar.us/Download/provider/pharm/OpioidDosingConversionCalculator.xls.
- 32.Mitchell SL. A 93-year-old man with advanced dementia and eating problems. JAMA. 2007;298:2527–2536. doi: 10.1001/jama.298.17.jrr70001. [DOI] [PubMed] [Google Scholar]
- 33.Monroe T, Carter M. Hospice care in US nursing homes: benefits and barriers. Eur J Palliat Care. 2010;17:144–149. [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. WHO’s pain ladder. 2011 [Cited 24 Jun 2011.] Available from URL: http://www.who.int/cancer/palliative/painladder/en/
- 35.Wu N, Miller SC, Lapane K, Gozalo P. The problem of assessment bias when measuring the hospice effect on nursing home residents’ pain. J Pain Symptom Manage. 2003;26:998–1009. doi: 10.1016/s0885-3924(03)00328-2. [DOI] [PubMed] [Google Scholar]
- 36.Gozalo P, Miller S. Predictors of mortality: hospice enrollment and evaluation of its causal effect on hospitalization of dying nursing home patients. Health Serv Res. 2007;42:587–610. doi: 10.1111/j.1475-6773.2006.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafheutle EI, Cantrill JA, Noyce PR. Why is pain management suboptimal on surgical wards? J Adv Nurs. 2001;33:728–737. doi: 10.1046/j.1365-2648.2001.01714.x. [DOI] [PubMed] [Google Scholar]
- 38.Bracht GH, Glass GV. The external validity of experiments. Am Educ Res J. 1968;5:437–474. [Google Scholar]
- 39.Mentes J, Culp K, Maas M, Rantz M. Acute confusion indicators: risk factors and prevalence using MDS data. Res Nurs Health. 1999;22:95–105. doi: 10.1002/(sici)1098-240x(199904)22:2<95::aid-nur2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 40.Ryan J, Stone RI, Raynor CR. Using large data sets in long-term care to measure and improve quality. Nurs Outlook. 2004;52:38–44. doi: 10.1016/j.outlook.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Monroe T, Gore G, Chen L, Mion L, Cowan R. Pain in people with Alzheimer’s disease: potential applications for psychophysical and neurophysiological research. J Geriatr Psychiatry Neurol. 2012;25:240–255. doi: 10.1177/0891988712466457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kosaka Y, Fujii M, Ishizuka S, Azumi M, Yamamoto T, Sasaki H. Dementia of patients with cancer. Geriatr Gerontol Int. 2010;10:269–271. doi: 10.1111/j.1447-0594.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- 43.Hirakawa Y, Masuda Y, Kuzuya M, Iguchi A, Uemura K. Director perceptions of end-of-life care at geriatric health services facilities in Japan. Geriatr Gerontol Int. 2007;7:184–188. [Google Scholar]
- 44.Monroe TB, Herr KA, Mion LC, Cowan RL. Ethical and legal issues in pain research in cognitively impaired older adults. Int J Nurs Stud. 2012 doi: 10.1016/j/ijnurstu.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]