Abstract
Folk medicine (FM) is practiced by people without access to conventional medical services; it usually involves the use of natural remedies such as herbs or vegetable substances. Before the use of pharmaceutical drugs, and surgical procedures, these healing methods were used, and are still in use today. It is estimated that twenty five percent of all therapeutic drugs trace their origins to plants, and almost two-thirds of the people of the world rely on their healing powers. One hundred years ago, health care in the U.S. was provided by a highly competitive medical sect, and quite infrequently, folk medicine practitioners were patronized. However, FM usage in the U.S. has increased drastically during the past decade. National surveys of adults (18 years of age or older) show that one in three adults use unconventional therapies or Complementary and Alternative Medicine (CAM) in the U.S. The rate of CAM usage is more than eighty percent among cancer patients. Vernonia amygdalina (VA) is well known for its medicinal importance. Fractionation of the VA extracts with solvents of varying polarities, by silica gels analyses, UV Spectrophotometer, HPLC, TLC and NMR techniques have yielded some biologically-active fractions.
Keywords: Anti-cancer activity, Apoptosis, Sesquiterpene lactones, Vernonia amygdalina extracts
INTRODUCTION
Folk medicine is practiced by people without access to conventional medical services and that usually involves the use of natural remedies such as herbs or vegetable substances. Before the use of pharmaceutical drugs, and surgical procedures, these healing methods were used, and are still in use today. Herbs, roots, fruits, insects and food items are used for the treatment of diseases from arthritis to high blood pressure and cancer [1]. First the Egyptians, then the Chinese, and later the Greeks and Romans established the historical relationship between plant products and medicine, which goes back to the beginning of medicine itself. As a part of this history, alternative medicine, generally called “natural folk medicine”, “herbal medicine”, or “home remedies”, was derived from ancient traditions. These practices allowed for the survival of a vast number of individuals with no access to professional medical services. Through generations long past, folk remedies have been passed down and many such remedies have survived well into the twenty-first century, wherein twenty five percent of all drugs come from chemicals derived from natural products, and almost two-thirds of the people of the world rely on their healing powers. In America, the use of herbal medicine and remedies is deeply rooted in the folklore of native Indians, but health practices range from the use of herbs and dietary manipulations to acupuncture or acupressure to spiritual healing [2]. One hundred years ago, health care in America was provided by a highly competitive medical sect, and quite infrequently, folk medicine practitioners were patronized. However, today many Americans use medical doctors and folk practitioners simultaneously. The strategy of combining botanical products and conventional drugs is becoming increasingly popular in the U.S. For example, cross-sectional prevalence surveys on the use of Complementary and Alternative Medicine (CAM) amongst patients conducted by one of the nation’s largest cancer centers (University of Texas’s M.D. Anderson Cancer Center, Houston, Texas), show eighty-three percent of their patients, representing a spectrum of malignancies and disease stages acknowledged the use of CAM [3]. Cancer patients combine vitamins/botanical supplements with conventional therapies [4]. Patients turn to CAM products to relieve the side effects of cancer treatments, such as nausea/vomiting [5]. There are, at least, two combination therapy clinical trials (botanical product and conventional drug) recently funded by the National Center for Complementary and Alternative Medicine (NCCAM): 1) Gemcitabine combined with mistletoe extracts for treating patients with advanced solid tumors; and 2) Mistletoe for treating patients with advanced Non-Small Cell Lung Cancer (NSCLC) who are receiving palliative chemotherapy. It is estimated that more than 60 million Americans are now using herbal or botanical supplements either for the treatment of illnesses such as cancer or for overall health maintenance, and spending more than $600 million annually [6]. All cultures have some varieties of local healer specialists, whose influences have originated outside conventional medicine. It does not fade away with assimilation, as realized by the idea that the Chinese immigrants have administered acupuncture to countless Americans, just as the Indians have been administering Yoga to Americans for centuries, with great benefits [7].
People in many countries, particularly African nations still rely heavily on folk medicine as the main source of treatment for their ailment. Nigeria is one of such countries, and where Vernonia amygdalina (VA) is well known for its medicinal importance. VA, a member of the compositae family, is a small shrub that grows in the tropical Africa. VA is commonly called bitter leaf because of its bitter taste. The VA leaves are consumed either as a vegetable (macerated leaves in soup) or aqueous extracts as a tonic for the treatment of various illnesses. Many herbalists and local healers in Nigeria prescribe aqueous VA to their patients as treatment for emesis, nausea, diabetes, loss of appetite-induced abrosia, dysentery and other gastrointestinal (GI) tract problems. Thus, for the past seven years, our laboratories have studied the anticancer activities of aqueous VA extracts, and we have observed and reported the following: Aqueous leaf extracts of native (VA) inhibit the proliferation of estrogen receptor positive (ER+) human breast carcinoma cells in vitro [8]; VA is relatively non-toxic as determined by MTT assays [8]; its mechanism of actions include the modulation of the Mitogen-Activated Protein Kinase (MAPK) pathway [9], and membrane potential disruption to increase efflux and subsequent cell death [10]. We and our collaborators have recently confirmed the therapeutic indexes of VA in vivo, using a xenograft mouse model with different tumoral cells. Solvents of varying polarities have been used to isolate multiple biologically-active fractions from VA extracts by HPLC, Thin Layer Chromatography (TLC), silica gel analysis, UV Spectrophotometer, and NMR.
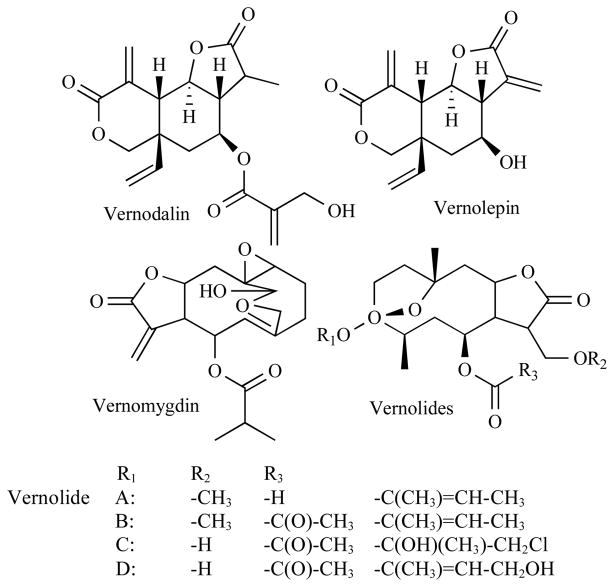
In the wild, Chimpanzees have been observed to instinctively ingest the VA plant leaves when suffering from parasitic infections [11]. The potential health benefits of VA were mostly anecdotal until 1969 when Kupchan and colleagues [12] first demonstrated that chloroform fractions of VA leaves contain some compounds that are toxic to some cancerous cells under in vitro conditions. Jisaka et al., 1993 [13] confirmed these findings. Thirty four years later, Izevbigie, 2003 [8] showed that additional anticancer compounds are present in the water-soluble fractions of VA leaf extracts. Chloroform and aqueous solvents have been used mainly to fractionate the anticancer component(s) of the VA extracts. Earlier investigators have shown that purified fractions of chloroform extracts of VA elicited anti-cancer effects in human carcinoma of the nasopharynx [12]. These molecules (vernomygdaline, vernolide, and vernodaline) belong to the family of sesquiterpene lactones (SLs). SLs, naturally occurring compounds, represent a class of potential antineoplastic/anticancer compounds currently being investigated in several laboratories. The VA and SLs research spans a period of more than thirty seven (37) years beginning in 1969 with the work of Kupchan and colleagues [12] who showed that chloroform fractions of VA leaves contain SLs that are toxic to human nasopharynx carcinoma cells under in vitro conditions. Since then, more recent studies not only corroborated, but have extended their reports by finding new active components and their modes of actions. After an exhaustive review of studies and reports, the following represent the VA and SLs’ anticancer activity literature: Many SLs (vernomydaline, vernolide, vernodaline, vernolepin, helenalin, elephantopin, eriofertopin, psilostachyins A, psilostachyins C, 2,3-dihydrohelenalin, bis-helenalinyl malonate, parthenolid, telekin, artemisinin, ergolide, Cynaropicrin and others) have been identified as anticancer agents [12–22]. (Fig. 1) shows the chemical structures of selected SLs previously isolated from VA extracts. Several derivatives of vernolide are known.
Fig. 1.
Chemical structures of selected SLs previously isolated from VA extracts.
The mechanisms of SLs anticancer actions include:
SLs (PUTATIVE COMPONENTS OF VA EXTRACTS) INDUCE APOPTOSIS
Apoptosis is the orchestrated cellular events characterized by membrane depolarization, cells shrinkage, chromatin condensation and fragmentation, and engulfment of dead cells by neighboring cells. In other words, apoptosis is a programmed cell death designed to maintain cellular balance. Treatment of Jurkat T cells with Ergolide (SL) from Britannica var chinensis caused the induction of apoptosis, DNA fragmentation, Caspase-3 activation, cleavage of poly ADP-ribose polymerase, mitochondria dysfunction, bax translocation and cytochrome c release. The NFκB expression inhibition was accompanied by down-regulation of cell pro-survival molecule (Bcl-2) by ergolide [22]. Parthenolide shows anticancer activity in vitro which correlates to its ability to inhibit DNA binding of the anti-apoptotic transcription factor (NF kappa B) and activation of the c-Jun NH2 terminal kinase (JNK). The study further investigated the chemosensitizing activity of Parthenolid in vitro as well as in MDA-MB-231 cell-derived xenograft metastasis model of breast cancer. Parthenolide was effective either alone or in combination with docetaxel in reducing colony formation, inducing apoptosis, and reducing the expression of prometastasis gene IL-8 and anti-apoptotic gene GADD 45 β-1 in vitro. In adjuvant settings, animals treated with parthenolide and docetaxel combined showed significantly enhanced survival rate compared to untreated animals or animals treated with either drug alone [20]. These data do not only corroborate our preliminary data that, the concentration of Taxol required to completely eliminate breast tumors was reduced by 50 % in the presence of VA extracts (unpublished data), but also support our hypothesis of synergism between VA or its component(s) and conventional drugs. If this phenomenon holds true in humans, it may mean reducing the therapeutic dosage for Taxol. Thus, fewer side effects and better quality of life (QOL) for the patients, is an expected outcome.
SLs (PUTATIVE COMPONENT OF VA EXTRACTS) INHIBIT MITOGEN-ACTIVATED PROTEIN KINASES (MAPKS) ACTIVITIES
Mitogen-activated proteins kinases (MAPKs) are also known as extracellular signal-regulated kinases (ERKs). MAPKs or (ERKs), are serine/threonine protein kinases that are rapidly activated upon stimulation by a variety of cell surface receptors [23–24]. They function to convert extracellular stimuli to intracellular signals regulating the expression of genes important for many cellular processes, including cell growth and differentiation [23]. Atanaskova and co-workers [25] showed that expression of constitutively activated MAPK’s activator (MEK1) in ER-positive MCF-7 cells (MEK1/MCF-7) increased ER-α-stimulated transcriptional activation and tumor growth. Thus, suggesting that activation of ER-α by MAPK was not only crucial for breast tumor growth, but represents a key regulatory point for tumor growth. SLs inhibit the MAPKs signaling pathways [26].
SLs (PUTATIVE COMPONENTS OF VA EXTRACTS) DOWN-REGULATE PRO-METASTASIS MOLECULE NUCLEAR FACTOR KAPPA B (NFκB) EXPRESSION AND ACTIVATION
NFκB is an anti-apoptotic transcription factors important for inflammatory responses, aberrant cell growth and carcinogesis, and metastasis [17]. Song et al., 2005 [22] demonstrated that treatment of Jurkat T cells with Ergolide (a SL) inhibit NFκB expression. In a xenograft mouse model, Sweeney et al., [20] showed that NFκB levels were lower in residual tumors and lung metastasis of animals treated with parthenolide, docetaxel, or both [20]. These results reveal in vivo chemosensitizing properties of parthenolide in the metastasis of breast cancer and support the contention that metastasis is very reliant on the activation of NFκB. The NFκB inhibition and anti-inflammatory activities of SLs are also supported by others [17,26].
SLs (PUTATIVE COMPONENTS OF VA) MODULATE GLUTATHIONE BIOSYNTHESIS
Glutathione (GSH; γ-Glutamylcysteinylglycine) is a tripeptide that contains an unusual γ-amide bond, and it participates in detoxification, transport, and metabolic activities. The balance between its reduced (GSH) and oxidized (GSSG) form helps maintain the sulfhydryl groups of intracellular proteins in their correct oxidation states. In 1983, Arrick et al., [14] showed that incubation of P815 mastocytoma cells with four SLs resulted in a 70–90% depletion of glutathione (GSH). Co-treatment of cells with SL and buthionine sulfoximine (BSO), a selective inhibitor of GSH synthesis, reduces SLs’ IC50 (more potent) by 6–34 fold, suggesting P815 mastocytoma cells susceptibility to low levels of GSH. Treatment of cells with BSO alone depleted GSH but not cell viability, suggesting that GSH depletion may be required but not sufficient for SLs anticancer activities. Interestingly, the anticancer activity of a GSH-depleting, conventional drug, Jatrophone, was enhanced 21-fold by BSO [14].
SLs (PUTATIVE COMPONENTS OF VA) INHIBIT IMP DEHYGROGENASE ACTIVITY
Inosine Monophosphate (IMP) dehydrogenase has been previously reported as a likely target enzyme for SLs’ anticancer activities. IMP is used in the synthesis of adenine and guanine; IMP differs from AMP and the GMP only in the replacement of its 6-keto group by an amino group, and the addition of an amino group respectively. Obviously, adenine and guanine are required for the biosynthesis of nucleotides. Limitation of adenine or guanine may affect DNA replication and subsequent cell growth. Using a purified IMP dehydrogenase from P-388 lympholytic leukemia tumor cell line, Page et al., 1987 [15], studied the binding kinetics (dissociation and rate constants) of nine different SLs against the inhibition of IMP dehydrogenase and found that SLs bind and inhibit IMP dehydrogenase activity. Five years later (1992), Helenin, another SL, was shown to inhibit the growth of L1210 lymphoid leukemia cells. The mode of actions of helenin include: the inhibition of DNA, RNA, and protein synthesis. In addition, thiol prosthetic group-bearing enzymes of nucleic acid metabolism such as: DNA polymerase-α, IMP dehydrogenase, and ribonucleoside reductase activities were also inhibited [16]. In 1994, Woerdenbag and colleagues [27] studied the cytotoxicities of 21 favonoids and 5 SLs, as present in Arnica species, in human colorectal cancer (COLO 320) and Non-small cell lung carcinoma cell lines (GLC4), using the MTT assay. Following continuous incubation, most flavonoids showed low to moderate cytotoxicity compared to the reference compound, cisplantin (IC50 = 1.1 μM) in GLC4 and 2.9 μM in COLO 320). Of the SLs tested, helenin, possessing both the reactive alpha-methylene-gamma-lactone (α-methylene-γ-lactone) moiety and a reactive alpha, beta-unsubstituted cyclopentanone ring, displayed the strongest cytotoxicity, suggesting that these two functional groups are important for activity [27].
SLs (PUTATIVE COMPONENTS OF VA) INHIBIT AROMATASE ACTIVITY
The involvement of elevated blood estrogen level in the etiology of estrogen receptor (ER) positive breast cancer has been widely reported [28]. An additional source of estrogen production in humans, besides the ovary and adrenal gland, is the conversion of testosterone to estrogen in a reaction catalyzed by Aromatase. Many studies have shown positive correlations between elevated blood estrogen levels and breast cancer risks [28]. Therefore, compounds that inhibit Aromatase activity are used for the treatment of breast cancer [19]. A group of SLs inhibit Aromatase activity and subsequently elicit cytotoxic actions of cancerous cells through their α-methylene-γ-lactone moieties. Using the α-methylene-γ-lactone group of 10-epi-8-deoxycumanbrin B (an Aromatase inhibitor) as a reference, a modified α-methylene-γ-lactone moiety compound (11β,1,3-dihydro-10epi-8-deoxycum anbrin B) revealed that α-methylene-γ-lactome is important for cytotoxicity and not Aromatase inhibition [29].
SLs (PUTATIVE COMPONENTS OF VA) POSSESS IMMUNOSTIMULATORY ACTIVITIES
Cynaropicrin, SL, from Saussurea lappa has been shown to possess immunomodulatory effects on cytokine release, nitric oxide production and immunosuppressive effects. The effects of cynaropicrin studied in several types of tumor cell lines such as: Macrophages, eosinophils, fibroblasts and lymphocytes reveal cynaropicrin potently inhibits the proliferation of leukocytes cancer cell line such as: U937, EOI-1 and Jurkat T cells, but not other cells such as Chang liver cells and human fibroblast cells lines. Thus, suggesting that these components of VA extracts (SLs) elicit some specificity for cancerous cells. The cytotoxic activity is due to increased apoptosis, cell cycle arrest at G1/G2 phase, and DNA fragmentation [18].
SLs (PUTATIVE COMPONENTS OF VA) AS NOVEL CHECK POINT INHIBITORS
A phenotypic cell-based assay for the inhibitors of the G2 DNA damage check point was carried out to screen plant extracts from NCI Natural Products Repository. Results show that methonolic fractions of Ragweed Ambrosia artemisiifolia were active. Assay-guided fractionation of the extracts led to the isolation of two SLs (Psilostachyins A and C) as novel check point inhibitors. Psilostachyins A and C also block cells in mitosis and cause formation of aberrant microtubule spindles [21]. Another assessment study of crude ethanolic extracts from 61 plants species used in the Russian ethnomedicine for the treatment of symptoms of diseases in the cancer patients was conducted in cultured human lymphoblastoid Raji cells. The extracts from Chelidonium majus, Potenlilla erecta, Chamaenerium angustfolium, Filipendula ulmaria, and Inula helenium possess marked cytotoxicity, suppressing cell growth at 10–50 Microgram /ml concentrations. The purified active compounds from selected plants (helenin, telekin, artemisinin, aromatic polyacetylene Capillin, and alkaloid preparation, Sanguirythrine) suppress growth at 1–2 μg/ml concentrations which exceed the cytotoxicities of pharmaceutical anti-neoplastic drugs, cyclophosphomide and fluorouracil [19].
V. AMYGDALINA EXTRACTION
A series of extraction of organic compounds from VA and the instrumental analysis of chemical components in the extracted fractions were carried out in order to identify the biologically-active chemical components against human breast cancerous cell line (MCF-7). First, the soxhlet apparatus was used with a mixture of ethanol and water (85:15), since we have previously reported that aqueous extracts of VA retard the proliferative activity of MCF-7 cells in vitro [8–9]. The evaporation of solvent from the extracts gave a dark-greenish black powder with similar potency as the aqueous extracts. Next, the powder (lyophilized extracts) were re-suspended in water followed by liquid-liquid extraction (hexane, chloroform, 1-butanol) for the second separation. The resulting fractions were tested for anti-proliferative activities; the chloroform fraction showed the most potency. The UV spectrum of this fraction showed a λmax value at 433 nm and 665 nm. These absorptions were later used for the HPLC analysis. The TLC of the fraction revealed at least four spots, based on the TLC chromatogram, a column chromatograph with silica gel was carried out for further separation. The eluent was a mixture of chloroform and methanol (8:2). The fractions from the column chromatograph were divided into four groups based on color and the TLC chromatogram. The first two fractions showed activities at microgram /ml concentrations, and were further analyzed by HPLC. Their retention times (rt) were slightly different, less than 10 seconds. 1H and 13C NMR were also taken for the two fractions. The spectra showed that the two fractions have similar chemical shifts, but the second fraction had more resonances around the olefin region in the proton NMR. 13C NMR spectra of the two fractions were also very similar to each other, although the second fraction had a peak of 88 ppm. In summation, various active components of VA, fractionated with a wide range of solvent polarities, have been investigated. Results include anticancer activities of SLs, Edotides, and VA by multiple mechanisms. For example: Ergolide down-regulates NFκB expression [22]; Parthenolide promotes JNK activation and NFκB expression down-regulation [20]; Edotide inhibits MAPK activity [9] etc. In addition VA extracts may also contain other health beneficial phytochemicals already found in other extracts [30–44]. Cynaropicrin promotes immunomodulatory activity, apoptosis, and GI/G2 phase arrest etc. Taken together, this review provides a compelling and convincing case for the potential anti-cancer benefits of VA, and it is timely for a translational or clinical evaluation.
Acknowledgments
This research was supported in part by the Research Centers in Minority Institutions (RCMI)/ NIH grant # G122RR13459-07S1; National Center for Minority Health Disparities (NCMHD)/NIH grant # P20MD000534-01; and the JSU Center for University Scholars Program.
References
- 1.Anonymous. In: The Emergence of Folklore in Everyday Life. Shcoemaker, editor. Bloomington, Indiana: Trickster Press: Indiana; 1990. pp. 51–57.pp. 59–72. [Google Scholar]
- 2.Fisher PS. West Virginia Hist Soc Quar. 1997:10,4, 11,1. [Google Scholar]
- 3.Richardson MA, Sanders T, Palmer JL, Anthony Greisinger A, Singletary SE. J Clin Oncol. 2000;18:2505. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 4.Richardson MA. J Nutr. 2001;131(11S):3037S. doi: 10.1093/jn/131.11.3037S. [DOI] [PubMed] [Google Scholar]
- 5.Kieu A. Proc of UCLA Healthcare. 2002;6:36. [Google Scholar]
- 6.Brevoort P. Herbalgram. 1998;44:33. [Google Scholar]
- 7.Hufford DJ. Newfolk New Directions in Folklore. 1997 Jul;(1) Archive. [Google Scholar]
- 8.Izevbigie EB. Exp Biol Med. 2003;228:293. doi: 10.1177/153537020322800308. [DOI] [PubMed] [Google Scholar]
- 9.Izevbigie EB, Bryant JL, Walker A. Exp Biol Med. 2004;229:163. doi: 10.1177/153537020422900205. [DOI] [PubMed] [Google Scholar]
- 10.Opata MM, Izevbigie EB. Int J Environ Res Public Health. 2006;3(2):174. doi: 10.3390/ijerph2006030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huffman MA, Seifu M. Primates. 1989;30:51. [Google Scholar]
- 12.Kupchan SM, Hemingway RJ, Karim A, Werner D. J Org Chem. 1969;34(12):3908. doi: 10.1021/jo01264a035. [DOI] [PubMed] [Google Scholar]
- 13.Jisaka M, Ohigashi H, Takegawa K, Huffman MA, Koshimizu K. Biosci Biotechnol Biochem. 1993;57(5):833. doi: 10.1271/bbb.57.833. [DOI] [PubMed] [Google Scholar]
- 14.Arrick BA, Nathan CF, Cohn ZA. J Clin Invest. 1983;71(2):258. doi: 10.1172/JCI110766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page JD, Chaney SG, Hall IH, Lee KH, Holbrook DJ. Biochim Biophys Acta. 1987;962(2):186. doi: 10.1016/0304-4165(87)90236-4. [DOI] [PubMed] [Google Scholar]
- 16.Grippo AA, Hall IH, Kiyokawa H, Muroaka O, Shen YC, Lee KH. Drug Des Discov. 1992;8(3):191. [PubMed] [Google Scholar]
- 17.Quintero A, Pelcastre A, Solano JD. Antitumoral activity of pyrimidine derivatives of seequiterpene lactones. J Pharm Sci. 1999;2(3):108. [PubMed] [Google Scholar]
- 18.Cho JY, Kim AR, Jung JH, Chun T, Rhee MH, Yoo ES. Eur J Parmacol. 2004;492(2–3):85. doi: 10.1016/j.ejphar.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Spiridonov NA, Konovalov DA, Arkhipov VV. Phytother Res. 2005;19(5):428. doi: 10.1002/ptr.1616. [DOI] [PubMed] [Google Scholar]
- 20.Sweeney CJ, Mehrotra S, Sadaria MR, Kumar S, Shortle NH, Roman Y, Sheridan C, Campbell RA, Murray DJ, Badve S, Nakshatri H. Mole Cancer Ther. 2005;4(6):1004. doi: 10.1158/1535-7163.MCT-05-0030. [DOI] [PubMed] [Google Scholar]
- 21.Sturgeon CM, Craig K, Brown C, Rundle NT, Andersen RJ, Roberge M. Planta Med. 2005;71(10):938. doi: 10.1055/s-2005-873109. [DOI] [PubMed] [Google Scholar]
- 22.Song YJ, Lee DY, Kim SN, Lee KR, Lee HW, Han JW, Kang DW, Lee HY, Kim YK. J Pharmacol. 2005;57(12):1591. doi: 10.1211/jpp.57.12.0009. [DOI] [PubMed] [Google Scholar]
- 23.Cobb MH, Boulton TG, Robbins DJ. Cell Regul. 1991;2:965. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ray LB, Sturgill TW. Proc Natl Acad Sci USA. 1987;84:1502. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atanaskova N, Keshamouni VG, Krueger JS, Schwartz JA, Miller F, Reddy KB. Oncogene. 2002;21(25):4000. doi: 10.1038/sj.onc.1205506. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Won YK, Ong CN, Shen HM. Curr Med Chem Anticancer Agents. 2005;5(3):239. doi: 10.2174/1568011053765976. [DOI] [PubMed] [Google Scholar]
- 27.Woedenbag HJ, Merfort I, Passreiter CM, Schmidt TJ, Willhn G, van Uden W, Pras N, Kampinga HH, Konings AW. Planta Med. 1994;60(5):434. doi: 10.1055/s-2006-959526. [DOI] [PubMed] [Google Scholar]
- 28.Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Sampler MJ, Henneckens C, Rosner B, Spiezer FE. N Engl J Med. 1995;332(24):1589. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- 29.Blanco JG, Gil RR, Bocco JL, Meragelman TL, Genti-Raimondi S, Flurry A. J Pharmacol Exp Ther. 2001;297(3):1099. [PubMed] [Google Scholar]
- 30.Pezzuto JM. Biochem Pharmacol. 1997;53(2):121. doi: 10.1016/s0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- 31.Zeegers MP, Goldbohm RA, Van den Brandt PA. Cancer Epidemiol Biomarkers Prev. 2001;10(11):1121. [PubMed] [Google Scholar]
- 32.Kobayashi T, Nakata T, Kuzumaki T. Effect of flavonaids on cell cycle progression in prostate cancer cells. Cancer Lett. 2000;176(1):17. doi: 10.1016/s0304-3835(01)00738-8. [DOI] [PubMed] [Google Scholar]
- 33.Block G, Patterson B, Subar A. Nutr Cancer. 2002;18(1):1. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 34.Fahey JW, Zhang Y, Talalay P. Broccoli Spouts. Proc Natl Acad Sci USA. 1997;94(19):10367. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singletary K. J Nutr. 2000;130:465. doi: 10.1093/jn/130.2.465S. [DOI] [PubMed] [Google Scholar]
- 36.Maoka T, Mochida K, Kozuka M, Ito Y, Fujiwara Y, Hashimoto K, Enjo F, Ogata M, Nobukuni Y, Tokuda H, Nishino H. Cancer Lett. 2001;172(2):103. doi: 10.1016/s0304-3835(01)00635-8. [DOI] [PubMed] [Google Scholar]
- 37.Littman AJ, Beresford SA, White E. Cancer Causes Control. 2001;12(8):691. doi: 10.1023/a:1011292003586. [DOI] [PubMed] [Google Scholar]
- 38.Terry P, Lagergren J, Hansen H, Wolk A, Nyren O. Eur J Cancer Prev. 2001;10(4):365. doi: 10.1097/00008469-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Heber D, Bowerman S. J Nutr. 2001;131(11 Suppl):3078S. doi: 10.1093/jn/131.11.3078S. [DOI] [PubMed] [Google Scholar]
- 40.Tatman D, Mo H. Cancer Lett. 2002;175(2):129. doi: 10.1016/s0304-3835(01)00723-6. [DOI] [PubMed] [Google Scholar]
- 41.Yu R, Hebbar V, Kim DW, Mandlekar S, Pezzuto JM, Kong AN. Mol Pharmacol. 2001;60(1):217. doi: 10.1124/mol.60.1.217. [DOI] [PubMed] [Google Scholar]
- 42.Meta-Greenwood E, Ito A, Westenburg H, Cui B, Mehta RG, Kinghorn AD, Pezzuto JM. Anticancer Res. 2001;21(3B):1763. [PubMed] [Google Scholar]
- 43.Talalay P, Fahey JW, Holtzclaw WD, Prestera T, Zhang Y. Toxicol Lett. 1995;82:173. doi: 10.1016/0378-4274(95)03553-2. [DOI] [PubMed] [Google Scholar]
- 44.Talalay P. Biofactors. 2000;12(1–4):5. doi: 10.1002/biof.5520120102. [DOI] [PubMed] [Google Scholar]