Abstract
BACKGROUND
Arterial stiffness measures are emerging tools for risk assessment and stratification for hypertension and cardiovascular disease (CVD). Carotid-femoral pulse wave velocity (cfPWV) is an established measure of central arterial stiffness. Other measures of PWV include femoral-ankle (faPWV), a measure of peripheral stiffness, and brachial-ankle PWV (baPWV), a composite measure of central and peripheral stiffness. Repeatability of central, peripheral, and composite PWV measures has not been adequately examined or compared.
METHODS
Participants (n = 79; mean age 75.7 years; USA) from a repeatability study nested within the Atherosclerosis Risk in Communities (ARIC) Study visit 5 (2011–2013) underwent 2 standardized visits, 4–8 weeks apart. Trained technicians obtained 2 PWV measurements at each visit using the VP-1000 Plus system. We calculated the intraclass correlation coefficient (ICC), SE of measurement, and minimal detectable change (MDC95; 95% confidence interval) and difference (MDD).
RESULTS
The ICCs and 95% confidence intervals (95% CIs) were 0.70 (0.59, 0.81) for cfPWV, 0.84 (0.78, 0.90) for baPWV, and 0.69 (0.59, 0.79) for faPWV. The MDC95 between repeat measures within an individual was 411.0cm/s for cfPWV, 370.6cm/s for baPWV, and 301.4cm/s for faPWV. The MDD for 2 independent samples of 100 per group was 139.3cm/s for cfPWV, 172.3cm/s for baPWV, and 100.4cm/s for faPWV.
CONCLUSIONS
Repeatability was acceptable for all PWV measures in a multicenter, population-based study of older adults and supports its use in epidemiologic studies. Quantifying PWV measurement variation is critical for applications to risk assessment and stratification and eventual translation to clinical practice.
Keywords: arterial stiffness, blood pressure, central arterial stiffness, hypertension, peripheral arterial stiffness, reliability, reproducibility, subclinical cardiovascular disease.
Pulse wave velocity (PWV), a measure of arterial stiffness, is an emerging screening tool for risk assessment in hypertension1 and for cardiovascular disease (CVD).2 Carotid-femoral PWV (cfPWV) represents central arterial stiffness and is considered the reference standard measure of aortic stiffness.2 Additional measures of PWV include brachial-ankle PWV (baPWV) that represents both central and peripheral arterial stiffness, and femoral-ankle (faPWV) that represents peripheral arterial stiffness. The latter 2 measures are being evaluated for risk assessment, specifically baPWV, because of the ease of measurement technique compared with cfPWV.
Despite the interest in arterial stiffness, the repeatability of segment-specific PWV has not been fully examined, especially in a multicenter, population-based setting. Previous studies have shown acceptable repeatability of cfPWV using various devices, such as the Complior (Alam Medical, Vincennes, France) and SphygmoCor (Atcor Medical, Sydney, Australia),3–5 but there is limited information for segment-specific measures of PWV.6 Moreover, the current literature primarily reports statistics that do not speak to repeatability, such as the correlation coefficient that indicates the strength of a linear relationship between 2 variables7,8 and the coefficient of variation that is a marker of precision.9,10
The aim of this study, therefore, was to characterize the repeatability of central, lower-extremity, and composite measures of PWV from an automated waveform analyzer (VP-1000 Plus) in a multicenter, population-based study of older adults. Accurate assessment of the reproducibility of PWV measures is critical to their analysis and interpretation, and for applications to risk assessment and stratification, and translation to clinical practice.
METHODS
Study population
This analysis included members of the Atherosclerosis Risk in Communities (ARIC) Study that were invited to participate in a repeatability study in 2011–2013. The ARIC Study is a population-based, longitudinal study of 15,792 participants aged 45–64 years enrolled between 1987 and 1989 from the following 4 US communities: Forsyth County, North Carolina; the city of Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland. Details of the initial visit have been previously described.11
At the most recent ARIC clinic exam (visit 5), staff at each of 4 field centers asked the first eligible participant seen each month if he/she was willing to return 4–8 weeks later to repeat the study visit. The mean follow-up time was 40.3 days (SD 9.5 days; range 27.9 to 71.5 days). If the participant was not interested, the staff recruited the next eligible and willing participant for the repeatability study. The repeatability study included 82 participants representing the 4 field centers (20 Forsyth County, NC; 23 Jackson, MS; 19 Minneapolis, MN; 20 Washington County, MD). Participants were asked to fast for 8 hours, refrain from tobacco, caffeinated beverages, and vigorous physical activity in the morning of the visit, and bring all prescription and nonprescription medications taken within 2 weeks of the visit. We excluded participants with evidence of a major arrhythmia on a 12-lead electrocardiogram (n = 1; Minnesota code 8-3-1), aortic stenosis (n = 1), or body mass index ≥40kg/m2 (n = 1) due to concerns about interference with PWV measurements. After exclusions, our analytic sample included 79 participants. Participants provided written informed consent, and the study was approved by the Institutional Review Boards at the field centers, coordinating center, and central labs and reading centers.
Pulse wave velocity
Trained technicians obtained 2 PWV measurements following the same standardized protocol at each exam 4–8 weeks apart. Details of the PWV methodology for ARIC have been reported elsewhere.12 Briefly, technicians measured PWV using the automated waveform analyzer VP-1000 Plus (Omron, Kyoto, Japan)13 after participants were supine for 5–10 minutes. Carotid and femoral arterial pressure waveforms were acquired by applanation tonometry sensors on the left common carotid artery and left common femoral artery. Bilateral brachial and posterior-tibial arterial pressure waveforms were detected by plethysmographic and an oscillometric pressure sensor wrapped on both arms and ankles. The electrodes and sensors were not removed when repeating measurements within-visit.
PWV was calculated as distance divided by transit time. Distance for cfPWV was measured with a segmometer (Rosscraft, Surray, Canada) and calculated as the distance between the suprasternal notch to carotid minus the carotid to femoral distance. Distances for baPWV and faPWV were automatically calculated by the VP-1000 Plus using height-based formulas, as previously described.14 The right baPWV and faPWV measurements were used for this analysis, but left and right PWV measurements are included in Supplementary Tables 1–4). Outliers, defined as PWV values 3 SDs above or below the mean, were excluded from the analyses.
Quality control procedures for the parent study (≥4,900 PWV measurements) included central training and recertification, quarterly equipment calibration, and ongoing quality control reviews by one of the authors (H.T.) on a random sample of 40 records per month stratified by center with feedback provided to technicians.12 Approximately 78% of records were considered optimal quality, 17% were good quality, 3% were acceptable, and none were poor or unacceptable.
Statistical analysis
Within- and between-visit summary measures of PWV were estimated as means and SDs. We calculated the average and absolute difference between pairs of measurements within-visit and between-visit.
We used a nested random-effects analysis of variance model to estimate the between-participant (σ 2 p), between-visit (σ 2 bv), and within-visit (σ 2 wv) variation. The model was as follows: Y ijk = µ + Personi + Visit(Person)j(i) + Errork(ij), where Y is the PWV index, µ is the intercept, i is 1, 2, 3 to the 79th participant, j is visit 1 or visit 2, and k is the first or second measurement. Our assumptions were that the between-visit variation is the same for all participants and the within-visit variation is the same for all visits and all participants.
To estimate the repeatability of PWV measures, the intraclass correlation coefficient (ICC) was calculated by dividing the between-participant variance by the total variance (). We also calculated the standard error of measurement (SEM) that represents the amount of measurement error, or repeatability, within an individual, SEM = . Additionally, we estimated changes in PWV beyond measurement error measures based on the variance and sample size for 1- and 2-sample study designs. We calculated the minimal detectable change with 95% confidence (minimal detectable change, MDC95) between 2 time points for an individual that reflects true change above measurement error . For a 2-sample study design, we calculated the minimal detectable difference (MDD) between 2 measurements that reflects true change above measurement error between 2 independent samples, MDD =, where df = 2 × n − 2. We performed a sensitivity analysis adjusting variance estimates and the ICC for heart rate, to account for the strong association between PWV and heart rate. Analyses were performed using SAS, version 9.2 (SAS Institute, Cary, NC) and all statistical tests are 2-sided with nominal statistical significance level of P < 0.05.
RESULTS
The mean age of the participants was 75.7 years and the mean body mass index was 29.6kg/m2 (Table 1). There were 46 (58.2%) females and 26 (32.9%) African Americans. The mean values for cfPWV, baPWV, and faPWV were fairly consistent within- and between-visits (Table 2). The average mean difference between-visit was higher than the average mean difference within-visit for all PWV measures except for cfPWV. The absolute mean difference between visit was higher than the absolute mean difference within-visit for all PWV measures.
Table 1.
Participant characteristics of the PWV repeatability study (N = 79)
| Characteristic | Mean ± SD or n (%) |
|---|---|
| Age, years | 75.7±4.6 |
| Body mass index, kg/m2 | 29.6±4.0 |
| Female, n (%) | 46 (58.2) |
| Non-Caucasian, n (%) | 26 (32.9) |
| Current smoker, n (%) | 1 (1.3) |
| Diabetes, n (%) | 34 (43.6) |
| Hypertension, n (%) | 58 (74.4) |
| Medication use, n (%) | |
| Beta-blocker, n (%) | 28 (37.8) |
| Alpha-blocker, n (%) | 3 (4.1) |
| Diuretic, n (%) | 33 (44.6) |
| Angiotensin-converting enzyme inhibitor, n (%) | 23 (31.1) |
| Calcium channel blocker, n (%) | 16 (21.6) |
| Angiotensin receptor blocker, n (%) | 18 (24.3) |
Abbreviation: PWV, pulse wave velocity.
Table 2.
Descriptive statistics for PWV measures by visit and differences within- and between-visit
| PWV measure | Visit 1 | Visit 2 | Within visita | Between visitb | ||||
|---|---|---|---|---|---|---|---|---|
| PWV 1 Mean ± SD |
PWV 2 Mean ± SD |
PWV 3 Mean ± SD |
PWV 4 Mean ± SD |
Average mean difference ± SD |
Absolute mean difference ± SD |
Average mean difference ± SD |
Absolute mean difference ± SD | |
| cfPWV (cm/s) | 1,259.0±318.6 | 1,163.0±246.7 | 1,189.8±241.9 | 1,185.1±259.5 | −35.2±92.3 | 64.9±78.3 | 11.3±151.1 | 115.7±96.8 |
| n | 65 | 53 | 64 | 57 | ||||
| baPWV (cm/s) | 1,758.0±335.5 | 1,740.6±309.7 | 1,744.8±348.5 | 1,724.6±324.0 | −6.3±63.7 | 52.7±44.8 | −26.2±179.4 | 140.9±115.8 |
| n | 78 | 77 | 78 | 77 | ||||
| faPWV (cm/s) | 1,084.1±197.5 | 1,059.7±178.6 | 1,042.7±187.7 | 1,066.1±195.0 | 3.5±76.5 | 69.3±82.5 | −15.5±113.8 | 105.4±88.4 |
| n | 75 | 76 | 79 | 74 | ||||
PWV 1 is the first measurement at visit 1, PWV 2 is the second measurement at visit 1, PWV 3 is the first measurement at visit 2, and PWV 4 is the second measurement at visit 2.
Abbreviations: PWV, pulse wave velocity; cfPWV, carotid-femoral PWV; baPWV, brachial-ankle PWV; faPWV, femoral-ankle PWV.
aPWV 2 – PWV 1 and PWV 4 – PWV 3. bPWV 3 – PWV 1 and PWV 4 – PWV 2.
Between-participant variation accounted for the majority of the total variation among all PWV measures (Table 3). The between-visit variation was higher than the within-visit variation for baPWV, but not for cfPWV and faPWV. The ICC (95% confidence interval) was 0.70 (0.59, 0.81) for cfPWV, 0.84 (0.78, 0.90) for baPWV, and 0.69 (0.59, 0.79) for faPWV (Table 4). The number of replicates to achieve an ICC of 0.90 was 6 for cfPWV and faPWV and 3 for baPWV, calculated with the Spearman-Brown formula.15
Table 3.
Components of measurement variation for PWV measures
| Source of variation | cfPWV | baPWV | faPWV | |||
|---|---|---|---|---|---|---|
| SD | % Total | SD | % Total | SD | % Total | |
| Between-participant | 227.8 | 70.2 | 308.4 | 84.2 | 162.8 | 69.2 |
| Between-visit | 96.2 | 12.5 | 122.4 | 13.2 | 58.5 | 8.9 |
| Within-visit | 112.8 | 17.2 | 53.9 | 2.6 | 91.6 | 21.9 |
| Total | 271.8 | 100.0 | 336.1 | 100.0 | 195.8 | 100.0 |
Abbreviations: PWV, pulse wave velocity; % total, percent of total variation; cfPWV, carotid-femoral PWV; baPWV, brachial-ankle PWV; faPWV, femoral-ankle PWV.
Table 4.
Repeatability estimates and the minimal detectable change (MDC95) for PWV measures
| PWV measure | ICC (95% CI) | SEM (cm/s) | MDC95 (cm/s) |
|---|---|---|---|
| cfPWV | 0.70 (0.59, 0.81) | 148.3 | 411.0 |
| baPWV | 0.84 (0.78, 0.90) | 133.7 | 370.6 |
| faPWV | 0.69 (0.59, 0.79) | 108.7 | 301.4 |
Abbreviations: ICC, intraclass correlation coefficient, CIs, confidence intervals; PWV, pulse wave velocity; MDC95, minimal detectable change; cfPWV, carotid-femoral PWV; baPWV, brachial-ankle PWV; faPWV, femoral-ankle PWV.
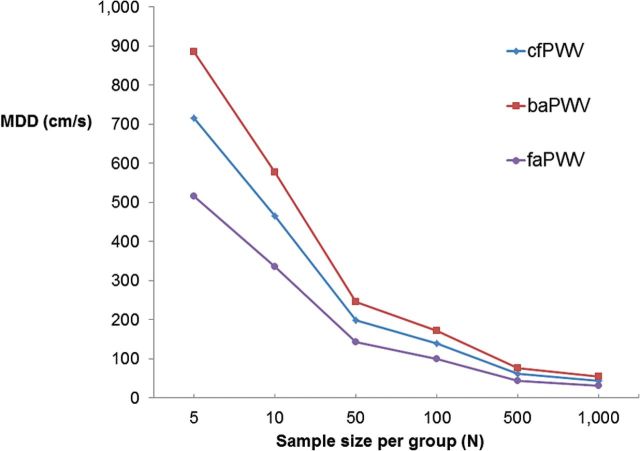
The MDC95 was 411cm/s for cfPWV, 371cm/s for baPWV, and 301cm/s for faPWV (Table 4). The MDC95 between repeat measures within an individual was ≈1 SD for baPWV and ≈1.5 SDs for cfPWV and faPWV (Table 4). The MDD for 2 independent samples of 100 people per group was 139cm/s for cfPWV, 172cm/s for baPWV, and 100cm/s for faPWV (Figure 1; Supplementary Table 4), which is approximately 0.5 SD for cfPWV, baPWV, and faPWV. Since differences in heart rate could affect the repeatability of PWV, we adjusted for heart rate in a sensitivity analysis and obtained similar results.
Figure 1.
Minimal detectable difference (MDD) in PWV between 2 independent samples, each of size N. Abbreviations: MDD, minimal detectable difference; PWV, pulse wave velocity; cfPWV, carotid-femoral PWV; baPWV, brachial-ankle PWV; faPWV, femoral-ankle PWV.
DISCUSSION
To our knowledge, this study is the first to report the short-term repeatability of segment-specific PWV measures in a population-based cohort study. Our analysis showed that the repeatability of baPWV is excellent and that of cfPWV and faPWV is fair, according to the ICC interpretation recommended by Fleiss.16 As expected, the majority of the variation was between-participant. Within-visit variation was smaller than the between-visit variation for baPWV, but was higher for faPWV and similar for cfPWV.
The between-visit variation estimated in our models represents the additional variation that is added when the PWV measurements are repeated 4–8 weeks apart, above that of the variation that occurs when the PWV measurements are repeated within minutes. For cfPWV, the within- and between-visit variation was similar. For baPWV, the within-visit variation was lower than the between-visit variation, suggesting that more variation was added between-visits. For faPWV, the within-visit variation was higher than the between-visit variation suggesting that little variation was added when relating 2 faPWV measurements from different visits compared to relating faPWV from the same visit.
Repeatability has been reported primarily for cfPWV using the Complior and SphygmoCor devices, although most studies presented statistics that do not reflect repeatability, focused on within- and between-observer reproducibility, and were limited to measurements taken on the same day. There are, however, a few higher quality reports of cfPWV repeatability. For measurements taken on the same day at a single site, the ICC for pairs of cfPWV measurements ranged from 0.94 to 0.98 taken 3 times with the same technician using the Complior.17 The mean difference between any 3 pairs of measurements ranged from 3 to 10cm/s, which is lower than the observed within-visit mean difference of −35cm/s in cfPWV in the current study.
Similar to our results for cfPWV measured in repeat visits, the repeatability of cfPWV was fair for the Complior (ICC = 0.62, SEM = 69cm/s) and SphygmoCor (ICC = 0.56, SEM = 69cm/s) among 20 healthy men and women (mean age 25) who came for 3 visits on separate days with the same technician.5 The mean difference between the first 2 measurements was 13±115cm/s for the Complior and 11±84cm/s for the SphygmoCor. These estimates are comparable to the median intra-individual difference of 8cm/s in cfPWV among 125 participants that had repeat PWV measures within 60 days using SphygmoCor in the Whitehall II Study.18 These between-visit differences and ICCs correspond to the between-visit difference of 11cm/s in cfPWV and ICC of 0.70 for cfPWV observed in the current study.
Unlike cfPWV, there are fewer repeatability studies of baPWV. The ICC for baPWV was 0.90 (0.87–0.94) in a random sample of participants from a multicenter cohort study who had PWV measured twice in 2 visits within 90 days; the estimated sources of variation were 80.1% for between-subject, 8.1% for technician, 7.8% for between-visit, and 4.0% for measurement error using the VaSera VS-1500N (Fukuda Denshi, Tokyo, Japan).19 This is similar to the ICC of 0.84 for baPWV in this study, and that 84% of the variation was due to between-subject and 13% was due to between-visit variation. The average between-visit difference in baPWV was −26.2cm/s in our study, which is similar to a reported mean difference of 29±202cm/s in baPWV between measurements taken 4 weeks apart in a study consisting of a select group of participants with essential hypertension using the Colin device (the same device as the Omron used in this study).20
Only one study has reported the repeatability for central, lower-extremity, and composite measures of PWV obtained simultaneously. Using the same device as the current study, the ICC of repeat measures of PWV on the same day was 0.76 for cfPWV, 0.97 for baPWV, and 0.96 for faPWV within technicians. Between technicians, the ICC was 0.73 for cfPWV, 0.91 for baPWV, and 0.87 for faPWV.6 This analysis, however, was limited to repeat measurements taken on the same day at a single site.
Although the repeatability of PWV is similar across devices, slight variations exist due to the differences in how sensors capture arterial waveforms, the algorithm used to calculate PWV, and the path length measurements. The Omron VP-1000 Plus and Complior simultaneously measure waveforms oscillometrically with circular pulse wave sensors placed on the skin that may allow for a more reproducible determination of the arterial wave signals than with handheld applanation tonometers used in other PWV methods, such as the SphygmoCor.
In our study baPWV had the highest repeatability, which is partly accounted for by the greater measurement dispersion and between-subject variability since more heterogeneous measures inherently give higher ICC estimates due to the nature of the calculation.21 The between-participant, between-visit, and within-visit variation we observed in PWV measures could be attributed to biological variation in hemodynamics and arterial anatomy, technician error, technical factors, sensor placement, and environmental factors. Differences in path length measurement would have contributed to the between-visit variation in cfPWV. Variability in PWV could also differ by populations with various CVD risk factors and by age.17 Options to increase the reproducibility of PWV measures include averaging measures, standardizing procedures, and assembling larger sample sizes. Replicate measures are recommended,22 and the average of 2 cfPWV measurements was shown to be comparable to the average of 3.17
The estimates of the MDC95 and MDD for PWV measures we report can aid in estimating study sizes and in evaluating whether PWV differences within participants or between-participant groups are above measurement variation. For example, the MDC95 for cfPWV was 411cm/s, suggesting that a change of more than 411cm/s may be necessary in order to determine whether a change in cfPWV exceeds measurement variability. A 411cm/s change in cfPWV may be clinically relevant as a cut point of 1,200cm/s1,23 for cfPWV (using the straight distance from carotid to femoral artery) have been recommended for the risk of CVD.
Current recommendations focus on cfPWV as the established measure of central arterial stiffness.2,24 In contrast, baPWV is widely used in East Asian countries, particularly in Japan, presumably due to the greater ease of measurement given that a carotid and femoral sensor are not required.25 Although baPWV is a composite measure of central and peripheral arterial stiffness representing heterogeneous arterial territories, it is proposed to have utility in CVD risk stratification.26–28
Our goal was to estimate short-term (4–8 week) repeatability, for which we measured PWV at 2 time points. Most studies, in contrast, evaluated repeatability during a single visit. A limitation of our study is that it was not designed to estimate variability due to technicians. As such, it is not a comprehensive evaluation of the main sources of measurement variability, but incorporates variability due to technician(s) into process variability. In that sense our study mimics typical clinical practice and research settings where there are multiple technicians, or the technicians work in pairs. As a further limitation of our study, although all examinations were conducted in the morning, using a standardized protocol and study procedures designed to minimize measurement variability, it is possible that conditions were not identical. As above, this type of variability is included in our estimates as part of process variability. Lastly, it is important to note that the calculations for the SEM and MDC assume that the measurement error and detectable change is constant across the range of values.
PWV is a widely used measure of arterial stiffness and has implications for clinical research and risk stratification. Repeatability was acceptable for all PWV measures in a multicenter, population-based study of older adults and supports its use in epidemiologic studies. Further investigation is needed to discern whether changes in cfPWV, baPWV, and faPWV are clinically meaningful. Toward this aim, we offer estimates to aid in designing and interpreting such studies. An understanding of PWV measurement variability is critical for applications to risk assessment and stratification and for translation to clinical practice.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
The Atherosclerosis Risk in Communities Study was carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). We thank the staff and participants of the ARIC Study for their important contributions. M.L.M. was supported by the NHLBI T32 training grant HL-007055. S.C. was supported by the Ellison Foundation and NHLBI grant R00HL107642.
REFERENCES
- 1. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B; Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 2. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 3. Calabia J, Torguet P, Garcia M, Garcia I, Martin N, Guasch B, Faur D, Vallés M. Doppler ultrasound in the measurement of pulse wave velocity: agreement with the Complior method. Cardiovasc Ultrasound 2011; 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magda SL, Ciobanu AO, Florescu M, Vinereanu D. Comparative reproducibility of the noninvasive ultrasound methods for the assessment of vascular function. Heart Vessels 2012. [DOI] [PubMed] [Google Scholar]
- 5. Huck CJ, Bronas UG, Williamson EB, Draheim CC, Duprez DA, Dengel DR. Noninvasive measurements of arterial stiffness: repeatability and interrelationships with endothelial function and arterial morphology measures. Vasc Health Risk Manag 2007; 3:343–349. [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper JN, Tepper P, Barinas-Mitchell E, Woodard GA, Sutton-Tyrrell K. Serum aldosterone is associated with inflammation and aortic stiffness in normotensive overweight and obese young adults. Clin Exp Hypertens 2012; 34:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bland JM, Altman DG. Comparing two methods of clinical measurement: a personal history. Int J Epidemiol 1995; 24(Suppl 1):S7–S14. [DOI] [PubMed] [Google Scholar]
- 8. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. International Journal of Nursing Studies. 2010;47:931–936 [Google Scholar]
- 9. Atkinson G, Nevill AM. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 1998; 26:217–238. [DOI] [PubMed] [Google Scholar]
- 10. Yao L, Sayre JW. Statistical concepts in the interpretation of serial bone densitometry. Invest Radiol 1994; 29:928–932. [DOI] [PubMed] [Google Scholar]
- 11. The Atherosclerosis Risk in Communities (ARIC) study: Design and objectives. The ARIC investigators. Am J Epidemiol 1989; 129:687–702. [PubMed] [Google Scholar]
- 12. Meyer ML, Tanaka H, Palta P, Cheng S, Gouskova N, Aguilar D, Heiss G. Correlates of Segmental Pulse Wave Velocity in Older Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens 2015; pii: hpv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 2003; 91:1519–1522, A9. [DOI] [PubMed] [Google Scholar]
- 14. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens 2009; 27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 15. Lord FM, Novick MR. Statistical Theories of Mental Test Scores. Addison-Wesley: Reading, MA,1968. [Google Scholar]
- 16. Fleiss JL. The Design and Analysis of Clinical Experiments. Wiley: New York, 1986. [Google Scholar]
- 17. Papaioannou TG, Protogerou AD, Nasothimiou EG, Tzamouranis D, Skliros N, Achimastos A, Papadogiannis D, Stefanadis CI. Assessment of differences between repeated pulse wave velocity measurements in terms of ‘bias’ in the extrapolated cardiovascular risk and the classification of aortic stiffness: is a single PWV measurement enough? J Hum Hypertens 2012; 26:594–602. [DOI] [PubMed] [Google Scholar]
- 18. Brunner EJ, Shipley MJ, Witte DR, Singh-Manoux A, Britton AR, Tabak AG, McEniery CM, Wilkinson IB, Kivimaki M. Arterial stiffness, physical function, and functional limitation: the Whitehall II Study. Hypertension 2011; 57:1003–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Endes S, Caviezel S, Dratva J, Schaffner E, Schindler C, Rothe T, Rochat T, Kunzli N, Probst-Hensch N, Schmidt-Trucksass A. Reproducibility of oscillometrically measured arterial stiffness indices: Results of the sapaldia 3 cohort study. Scand J Clin Lab Invest 2015; 75:170–176:1–7. [DOI] [PubMed] [Google Scholar]
- 20. Matsui Y, Kario K, Ishikawa J, Eguchi K, Hoshide S, Shimada K. Reproducibility of arterial stiffness indices (pulse wave velocity and augmentation index) simultaneously assessed by automated pulse wave analysis and their associated risk factors in essential hypertensive patients. Hypertens Res 2004; 27:851–857. [DOI] [PubMed] [Google Scholar]
- 21. Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res 2005; 19:231–240. [DOI] [PubMed] [Google Scholar]
- 22. Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, Jacobson M, Mahoney L, Mietus-Snyder M, Rocchini A, Steinberger J, McCrindle B; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 2009; 54:919–950. [DOI] [PubMed] [Google Scholar]
- 23. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 24. O’Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens 2002; 15:426–444. [DOI] [PubMed] [Google Scholar]
- 25. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25:359–364. [DOI] [PubMed] [Google Scholar]
- 26. Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: a systematic review and meta-analysis. Hypertension 2012; 60:556–562. [DOI] [PubMed] [Google Scholar]
- 27. Turin TC, Kita Y, Rumana N, Takashima N, Kadota A, Matsui K, Sugihara H, Morita Y, Nakamura Y, Miura K, Ueshima H. Brachial-ankle pulse wave velocity predicts all-cause mortality in the general population: findings from the Takashima study, Japan. Hypertens Res 2010; 33:922–925. [DOI] [PubMed] [Google Scholar]
- 28. Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Imai Y, Imaizumi T, Ishimitsu T, Ito M, Ito S, Itoh H, Iwao H, Kai H, Kario K, Kashihara N, Kawano Y, Kim-Mitsuyama S, Kimura G, Kohara K, Komuro I, Kumagai H, Matsuura H, Miura K, Morishita R, Naruse M, Node K, Ohya Y, Rakugi H, Saito I, Saitoh S, Shimada K, Shimosawa T, Suzuki H, Tamura K, Tanahashi N, Tsuchihashi T, Uchiyama M, Ueda S, Umemura S; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res 2014; 37:253–390. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.