Abstract
BACKGROUND
Arterial stiffness is an important marker of vascular aging that is increased in sedentary, obese older adults. Weight loss induced by caloric restriction (CR) can improve arterial stiffness in this population; however, the effects of resistance training (RT) are not clear. This pilot study determined the effects of RT with and without CR on arterial stiffness in overweight and obese older adults.
METHODS
Participants (mean age = 68±3 years, mean body mass index = 31.1±2.7kg/m2, 56% female, 13% Black) were randomly assigned to 3 days/week of supervised moderate-intensity RT (n = 16) or RT+CR (n = 16) for 5 months. Three indices of arterial stiffness were measured: brachial-ankle pulse wave velocity, large artery elasticity, and small artery elasticity.
RESULTS
Body mass was significantly reduced in the RT+CR group compared to the RT group (−6.2±4.8 vs. 0.2±1.2kg, P = 0.0006). Within-group analyses showed that none of the arterial stiffness measures changed with RT or RT+CR. There were also no significant between-group differences, though median changes in large artery elasticity were slightly greater with RT+CR: 0.7 (−2.5, 5.1) vs. 0.3 (−2.6, 0.9) ml/mm Hg × 10, P = 0.07. Changes in large artery elasticity were negatively correlated with changes in waist circumference (r = −0.36, P < 0.05), systolic blood pressure (r = −0.38, P = 0.03), and diastolic blood pressure (r = −0.41, P = 0.02).
CONCLUSIONS
The combination of RT and CR, leading to a modest amount of weight loss (7%), tended to increase large artery elasticity more than RT alone. Our data suggest that reductions in waist circumference and blood pressure may promote improvements in elasticity.
CLINICAL TRIALS REGISTRATION
Trial Number NCT01049698
Key words: aging, arterial elasticity, blood pressure, exercise, hypertension, obesity, pulse wave velocity, weight loss.
Arterial stiffness is an important cardiovascular disease risk factor and a strong predictor of adverse health outcomes.1 Aging is a major contributor to the adverse structural and functional changes in blood vessels that lead to arterial stiffness.2 Stiffer vessels increase systolic blood pressure (BP), decrease diastolic BP, and widen pulse pressure, which can have damaging effects on the brain, heart, and kidneys.3,4 Obesity may accelerate vascular aging and has been associated with increased arterial stiffness.5,6 Weight loss may be beneficial for improving arterial stiffness in obese older adults, due in part to reductions in total and abdominal fat.7 However, weight loss also reduces lean mass, and low muscle mass has been associated with increased arterial stiffness.8,9 Thus, optimizing body composition changes during weight loss will likely promote favorable changes in arterial stiffness in older adults.
A sedentary lifestyle is another factor that predisposes to increased arterial stiffness.10 Current guidelines recommend resistance training (RT) for the promotion of overall health, fitness, and well-being.11 Although RT may have beneficial effects on some cardiovascular disease risk factors, including body composition and BP,12,13 earlier reports suggest that RT may actually lead to increases in arterial stiffness.14–16 Recent meta-analyses indicate that the effects of RT are likely dependent on age, as well as the type and intensity of exercise.17–19 To our knowledge, no studies have specifically examined adults aged 65 years and older. Of the studies that included some older adults, over half reported nonsignificant increases in arterial stiffness after low-to-moderate intensity RT.20–24 Since RT is important for maintaining muscle mass, strength, and function, especially in obese older adults,25,26 it is essential to identify ways to prevent or ameliorate a potential increase in arterial stiffness with RT in this population. Therefore, the goal of this study was to determine the effects of adding caloric restriction (CR) for weight loss to a moderate-intensity RT intervention on arterial stiffness in overweight and obese older adults.
METHODS
Study participants
The present study includes a subset of participants who completed arterial stiffness measurements in the Improving Muscle for Functional Independence Trial (I’M FIT). I’M FIT was a 5-month randomized controlled trial comparing the effects of RT with and without CR (RT+CR vs. RT, respectively) on skeletal muscle function (NCT01049698). The main trial results reported beneficial effects of both RT and RT+CR on body composition, muscle strength, and physical function.27 Eligible participants were 65–79 years old, overweight or obese (body mass index = 27–34.9kg/m2), sedentary (no formal RT in the previous 6 months), weight-stable, and nonsmokers. Individuals with cardiovascular disease, uncontrolled hypertension, insulin-dependent or uncontrolled diabetes, kidney disease, liver disease, or chronic pulmonary disease were excluded from the study. All participants provided written informed consent prior to participation in this ancillary study and procedures were approved by the Institutional Review Board of Wake Forest School of Medicine.
RT intervention
All participants underwent a supervised, progressive, moderate-intensity RT program 3 days/week for 5 months. Each exercise session began and ended with 5 minutes of walking or cycling and light stretching. Upper and lower body exercises were performed using the following machines: seated leg curl, leg press, leg extension, seated calf raise, triceps press, biceps curl, incline press, and compound row. The maximal weight that could be lifted with correct form in 1 repetition (1-repetition maximum, 1RM) was used to prescribe intensity, with a target goal of 70%. The volume and intensity of exercise were gradually increased during the first month to allow participants to familiarize themselves with the equipment, minimize muscle soreness, and reduce potential for injury. Once participants were capable of completing 10 repetitions on the third set for 2 consecutive sessions the weight was increased. Every 4 weeks strength testing was repeated in order to adjust the training load as needed to maintain exercise intensity at 70%. Maximal knee extensor strength of the right leg was measured on a dynamometer (Biodex Medical Systems, Inc., Shirley, NY) in Newton-meters (Nm), as previously described.27
Dietary intervention
Participants randomized to RT+CR were instructed to reduce their energy intake by ~600 kcal below their estimated energy expenditure per day. This caloric deficit was designed to result in a weight loss of ~5–10%. Two meal replacements per day were provided in the form of Slim-Fast shakes or bars, with participants self-selecting their third meal according to recipes recommended by the study dietitian. These participants were asked to keep a diet log of all foods consumed, and the logs were monitored weekly to verify compliance with the weight loss intervention. Participants randomized to the RT group were instructed to continue their normal diet throughout the intervention. All participants were provided with daily calcium (1,200mg/day) and vitamin D (800 IU/day) supplements.
Arterial stiffness measures
Brachial-ankle pulse wave velocity (baPWV), large artery elasticity, and small artery elasticity were measured to assess arterial stiffness. Higher baPWV and lower elasticity values reflect stiffer arteries. Measurements were performed in the afternoon with participants in the supine position after resting for 5–10 minutes in a quiet, temperature-controlled room. Participants were fasted for at least 3 hours and refrained from strenuous activity for at least 24 hours.
Brachial-ankle PWV was assessed using a waveform analyzer (Colin VP-2000, Wave Nexus Corporation, San Antonio, TX). Oscillometric BP cuffs were placed around both arms and ankles, electrodes were placed on both wrists, and a microphone for detecting heart sounds was placed on the left side of the sternum. PWV was calculated as the distance between sampling points (using an equation based on participant height28) divided by the transit time (i.e., the time interval between the foot of the brachial waveform and the foot of the ankle waveform). The average of the left and right baPWV values was used in the analysis.
Measurements of large and small artery elasticity were obtained using the HDI/PulseWave CR-2000 (Hypertension Diagnostics Inc., Egan, MN). A piezoelectric, acoustic sensor was placed perpendicular to the left radial artery at the point of maximum pulsation in order to obtain radial artery waveforms, and a wrist stabilizer was placed on the forearm to minimize movement. Radial waveforms were calibrated to the brachial BP obtained by an oscillometric cuff placed on the upper right arm. This automated device uses pulse wave analysis to evaluate the diastolic portion of the waveform using a third-order, 4-element modified Windkessel model.29,30 Elasticity parameters are calculated as a change in volume for a given change in BP and reflect the ability of the large arteries (i.e., capacitive component) and small arteries (i.e., reflective component) to withstand distending pressures throughout the cardiac cycle.
Other vascular measures
Systolic and diastolic BP values were reported from the HDI device. This device also provides an estimate of systemic vascular resistance, which is calculated as the mean arterial pressure divided by the estimated cardiac output.29,30 Ankle-brachial index calculated as the ratio of the systolic BP in each ankle to the highest brachial systolic BP was obtained with the Colin device. The lower ankle-brachial index value was used in the analysis.
Anthropometric and body composition measurements
Body mass was measured using a digital scale with an attached stadiometer to measure height (SECA Model 703, Hamburg, Germany). Waist circumference was measured at the narrowest part of the torso below the ribcage and above the umbilicus, hip circumference was measured at the level of the maximal gluteal protuberance, and thigh circumference was measured midway between the inguinal crease and the proximal border of the patella. Percent body fat, total fat mass, and total lean mass were assessed using dual-energy x-ray absorptiometry (Hologic Delphi QDR, Bedford, MA).
Statistical analysis
All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC). The baPWV and large artery elasticity data were log-transformed for analysis. Chi-square frequency tests and analysis of variance were used to assess baseline differences. Paired t-tests were used to assess changes in the outcome variables within intervention groups. Two-sample paired t-tests were used to compare changes in the outcome variables between intervention groups. Pearson correlation coefficients were used to assess relationships among the adiposity, arterial stiffness, and other vascular measures. A P value ≤ 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
The study sample was mostly female (56%) and Caucasian (87%) with a mean age of 68±3 years and a mean body mass index of 31.1±2.7kg/m2 (Table 1). There were no significant differences in these variables between groups at baseline. Body mass, body composition, body fat distribution, and knee strength were also similar between groups at baseline (Table 2). Baseline baPWV was higher in the RT+CR group (P = 0.01); however, no other arterial stiffness or vascular measures were different between groups at baseline (Table 3). Adherence to the RT program was high and did not differ between groups (P = 0.65), with individuals in the RT and RT+CR groups attending 84% and 86% of the RT sessions, respectively.
Table 1.
Baseline characteristics by intervention group
| Variable | RT (n = 16) | RT+CR (n = 16) |
|---|---|---|
| Age (years) | 68±3 | 69±3 |
| Female | 8 (50%) | 10 (63%) |
| Black | 3 (19%) | 1 (6%) |
| Diabetes | 1 (6%) | 1 (6%) |
| Hypertension | 9 (56%) | 9 (56%) |
Table values are mean ± SD or N (%). There were no differences between groups.
Abbreviations: CR, caloric restriction; RT, resistance training.
Table 2.
Body mass, body composition, body fat distribution, and muscle strength by intervention group
| Variable | RT (n = 16) | RT+CR (n = 16) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| Body mass (kg) | 89.1±12.7 | 89.3±13.5 | 0.2±2.7 | 85.1±12.5 | 78.9±12.1 | −6.2±4.8a,b |
| BMI (kg/m2) | 31.0±2.7 | 31.1±3.4 | 0.08±0.9 | 30.7±2.5 | 28.5±3.0 | −2.2±1.7a,b |
| Waist circumference (cm) | 102.6±6.4 | 101.2±7.8 | −1.4±3.9 | 103.8±12.6 | 95.8±10.5 | −8.0±7.3a,b |
| Hip circumference (cm) | 107.5±7.8 | 105.8±9.5 | −1.7±4.9 | 107.0±7.0 | 100.9±6.8 | −6.1±3.0a,b |
| Waist-to-hip ratio | 0.96±0.06 | 0.96±0.06 | 0.002±0.04 | 0.97±0.09 | 0.95±0.07 | −0.02±0.05 |
| Thigh circumference (cm) | 52.4±5.4 | 52.3±4.7 | −0.1±2.0 | 50.8±2.5 | 49.0±2.8 | −1.9±1.8a,b |
| Body fat (%) | 38.2±6.9 | 38.0±7.3 | −0.2±1.3 | 40.8±5.6 | 37.7±6.9 | −3.1±2.2a,b |
| Total fat mass (kg) | 34.0±6.5 | 33.9±7.5 | −0.2±1.8 | 34.6±5.9 | 29.8±6.6 | −4.7±3.2a,b |
| Total lean mass (kg) | 55.9±12.0 | 55.8±11.5 | −0.1±1.5 | 50.7±9.9 | 49.6±9.6 | −1.1±1.5a,b |
| Knee strength (Nm) | 110.1±31.8 | 121.7±44.7 | 11.6±24.0 | 112.0±40.4 | 120.9±34.8 | 8.9±16.1a |
Table values are mean ± SD.
Abbreviations: BMI, body mass index; CR, caloric restriction; RT, resistance training.
aSignificant within-group change, P ≤ 0.05.
bSignificant between-group difference, P ≤ 0.05.
Table 3.
Arterial stiffness and other vascular measures by intervention group
| Variable | RT (n = 16) | RT+CR (n = 16) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |
| baPWV (cm/s) | 1,387 (1270, 1480) | 1360 (1176, 1550) | −37 (−158, 71) | 1504 (1431, 2019)a | 1492 (1409, 1707)a | −62 (−312, 98) |
| Large artery elasticity (ml/mm Hg × 10) | 14.5 (11.8, 20.1) | 15.1 (9.9, 18.5) | 0.3 (−2.6, 0.9) | 12.6 (10.4, 17.3) | 15.9 (10.8, 19.3) | 0.7 (−2.5, 5.1) |
| Small artery elasticity (ml/mm Hg × 100) | 4.3±2.3 | 4.8±2.2 | 0.5±2.0 | 4.4±2.2 | 4.4±2.8 | 0.05±3.5 |
| Systolic BP (mm Hg) | 135±20 | 131±16 | −3±16 | 127±14 | 129±12 | 2±12 |
| Diastolic BP (mm Hg) | 75±7 | 77±7 | 2±9 | 74±8 | 72±9 | −1±10 |
| Systemic vascular resistance (dyn·s/cm5) | 1522±192 | 1536±200 | 14±193 | 1467±271 | 1505±269 | 39±310 |
| Ankle-brachial index | 1.11±0.11 | 1.08±0.12 | −0.03±0.14 | 1.06±0.08 | 1.06±0.09 | −0.004±0.09 |
Table values are mean ± SD or median (interquartile range).
Abbreviations: baPWV, brachial-ankle pulse wave velocity; BP, blood pressure; CR, caloric restriction; RT, resistance training.
aSignificant between-group difference, P ≤ 0.05.
Changes in body mass, body composition, body fat distribution, and muscle strength
Body mass was significantly reduced in the RT+CR group compared to the RT group (P < 0.0006), corresponding to a −7.2±5.4% weight loss and 0.1±3.1% weight gain, respectively (Table 2). There were also significant reductions in body mass index, waist, hip, and thigh circumferences, percent body fat, fat mass, and lean mass in the RT+CR group (all P ≤ 0.05), but not in the RT group. Thus, between-group differences were significant for all of these measures (P ≤ 0.05). Both groups increased knee strength to a similar degree (P = 0.72); however, the change was only significant with RT+CR (P = 0.04).
Changes in arterial stiffness and other vascular measures
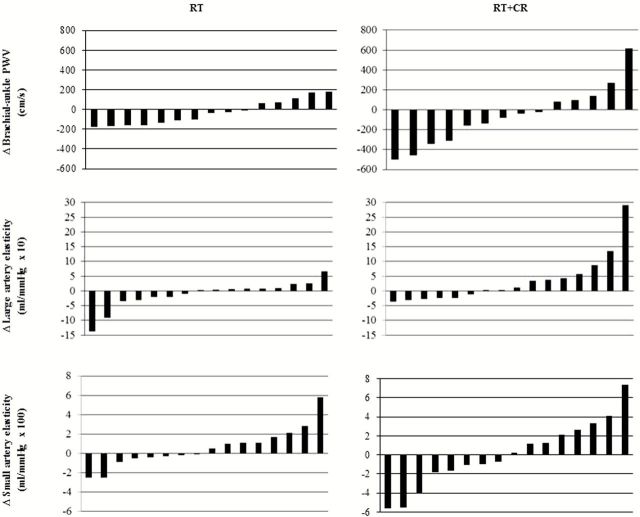
There were no significant within-group changes in baPWV, large or small arterial elasticity, BP, systemic vascular resistance, or ankle-brachial index in either group. Analyses of between-group differences showed a tendency for the RT+CR group to have a greater increase in large artery elasticity compared to the RT group (P = 0.07). At an individual level, the changes in arterial stiffness measures were highly variable in both intervention groups (Figure 1). Overall, changes ranged from −30% to +29% for baPWV, −73% to +411% for large artery elasticity, and −19% to +131% for small artery elasticity. Similarly, changes in systolic BP (−17% to +18%), diastolic BP (−25% to +26%), systemic vascular resistance (−29% to +34%), and ankle-brachial index (−36% to +21%) were highly variable, with significant overlap between the RT and RT+CR groups.
Figure 1.
Individual changes in arterial stiffness measures by intervention group. Note: Each bar represents the change in arterial stiffness in a single individual. A decrease in PWV and an increase in elasticity reflect an improvement in arterial stiffness. Abbreviations: CR, caloric restriction; PWV, pulse wave velocity; RT, resistance training.
Correlates of arterial stiffness
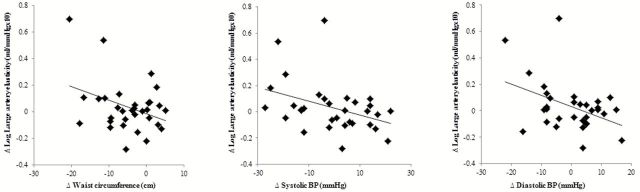
In the entire sample, individuals with higher arterial stiffness at baseline had greater reductions in arterial stiffness in response to the interventions. This was true for large artery elasticity (r = −0.60, P = 0.0003) and small artery elasticity (r = −0.54, P = 0.001), but not baPWV. No other variables at baseline including age, adiposity (i.e., body mass index, percent body fat, fat mass, waist circumference, and waist-to-hip ratio), and lean mass were associated with changes in the arterial stiffness measures. In response to the interventions, increases in large artery elasticity correlated with decreases in waist circumference (r = −0.36, P = 0.046, Figure 2). Changes in large artery elasticity also correlated negatively with changes in both systolic (r = −0.38, P = 0.03) and diastolic BP (r = −0.41, P = 0.02) (Figure 2). There was a borderline significant association between changes in baPWV and changes in thigh circumference (r = −0.34, P = 0.07). Changes in arterial stiffness measures were not significantly correlated with changes in body mass or lean mass.
Figure 2.
Association between changes in large artery elasticity and changes in waist circumference and blood pressure (BP).
DISCUSSION
The present study evaluated the effects of adding CR for weight loss to RT on arterial stiffness in overweight and obese older adults. We found that, although there were large interindividual changes in baPWV, large artery elasticity, and small artery elasticity with RT and RT+CR, overall there was no effect of either intervention on these 3 measures of arterial stiffness. However, greater reductions in central adiposity in the RT+CR group may have led to slightly greater improvements in large artery elasticity compared to RT alone.
In several recent reviews, arterial stiffness was shown to increase, decrease, or remain the same after RT,17–19 highlighting the need for more definitive studies. These reviews also underscore the fact that the effects of RT on arterial stiffness have not been adequately studied in individuals >65 years old. Consistent with our findings, prior studies in middle-aged and older adults found that RT at low, moderate, or high intensity had a minimal effect on several indices of arterial stiffness.20–24 Of note, baPWV increased nonsignificantly (13cm/s, 1.0%) with RT in obese postmenopausal women.21 In the present study, there was a small decrease in baPWV with RT (37cm/s, 2.7%). In addition, our study is the first to investigate the effects of RT on elasticity measures. On average, we found nonsignificant increases in both large (2.0%) and small (11.6%) artery elasticity.
Several studies demonstrate that weight loss is associated with reductions in arterial stiffness.7,21,31–33 However, to our knowledge, only one other study compared the effects of RT with and without CR on arterial stiffness. Figueroa and colleagues21 reported a significant reduction in baPWV (60cm/s, 4.3%) when RT was combined with a hypocaloric diet that led to a 5.5% weight loss; however, this was not different from RT alone. Similarly, the median change in baPWV with RT+CR in our study (62cm/s, 4.1%) did not differ from RT alone. Despite the fact that our study population was older, included men and diabetics, and had a higher prevalence of antihypertensive medication use, these findings suggest that adding CR to RT may mitigate potential RT-induced increases in baPWV. Changes in small artery elasticity were also similar between intervention groups in our study. The RT+CR group did, however, have slightly greater increases in large artery elasticity.
We observed large interindividual differences in the change in arterial stiffness measures in response to both interventions. The presence of such heterogeneity has been reported previously for other measures of arterial stiffness.34 While characteristics of both the participants and the RT intervention may influence the response to RT,17–19 baseline demographics and body composition were not predictive of changes in arterial stiffness in the current analysis. RT is also associated with significant reductions in BP and increases in peripheral blood flow.12,34,35 Although the mean change in BP was not significant in our study, changes in both systolic and diastolic BP were negatively correlated with changes in large artery elasticity. Increases in large artery elasticity were also related to improvements in waist circumference. Additionally, changes in baPWV tended to correlate negatively with changes in thigh circumference. A reduction in thigh circumference could reflect muscle loss and a potential increase in arterial stiffness;8,9 yet, this does not appear to explain the current observation, as changes in lean mass were not associated with changes in arterial stiffness. These data suggest that interventions that elicit significant reductions in BP and adiposity may help to improve arterial stiffness in overweight and obese older adults.
Strengths of this study include the randomized study design; the structured, well-controlled RT and CR interventions; the carefully characterized study participants; and the phenotypic measures of body composition. However, there were also some limitations. The sample size was small, albeit similar to previous studies on this topic.20–22 As a result, we had limited power to detect differences between intervention groups. For example, we would need at least 38 subjects per group to detect an effect size of 0.65 for changes in large artery elasticity. This also precluded a more extensive investigation into the sources of variation in the training responses (e.g., comparison of responders vs. nonresponders). In addition, although we did not assess the reproducibility of baPWV and elasticity in the current study, previous studies demonstrate high reproducibility for both measures.28,36 We did, however, follow the recommendations for arterial stiffness assessments in order to standardize the testing conditions.37 We also examined only systemic measures of arterial stiffness, which may reflect different aspects of the aging vasculature and respond differently to RT than local measures (e.g., distensibility, leg PWV).21,22
In conclusion, our study found that the combination of RT and CR tended to improve large artery elasticity more than RT alone, which may be partly due to greater decreases in waist circumference. Additionally, our data suggest that favorable changes in BP may also contribute to increases in large artery elasticity. Overall, RT was not associated with increases in arterial stiffness, as has been observed in some young and middle-aged populations. However, some participants did have adverse changes (i.e., increases) in arterial stiffness in response to the interventions, which highlights the need to better understand the clinical, physiological, and molecular mechanisms underlying changes in arterial stiffness with RT and CR. This knowledge will help to characterize individuals who are least likely to benefit from a given exercise intervention (i.e., nonresponders) and may identify adjuvant therapies that can be used to maximize the training response.
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants (R01-AG020583 and K01-AG033652), the American Heart Association, the Wake Forest Claude D. Pepper Older Americans Independence Center (P30-AG21332), and the Wake Forest Hypertension and Vascular Research Center.
REFERENCES
- 1. Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A; Health ABC Study. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation 2005; 111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 2. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 2007; 50:1–13. [DOI] [PubMed] [Google Scholar]
- 3. Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 4. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25:932–943. [DOI] [PubMed] [Google Scholar]
- 5. Sutton-Tyrrell K, Newman A, Simonsick EM, Havlik R, Pahor M, Lakatta E, Spurgeon H, Vaitkevicius P. Aortic stiffness is associated with visceral adiposity in older adults enrolled in the study of health, aging, and body composition. Hypertension 2001; 38:429–433. [DOI] [PubMed] [Google Scholar]
- 6. Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension 2003; 42:468–473. [DOI] [PubMed] [Google Scholar]
- 7. Dengo AL, Dennis EA, Orr JS, Marinik EL, Ehrlich E, Davy BM, Davy KP. Arterial destiffening with weight loss in overweight and obese middle-aged and older adults. Hypertension 2010; 55:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abbatecola AM, Chiodini P, Gallo C, Lakatta E, Sutton-Tyrrell K, Tylavsky FA, Goodpaster B, de Rekeneire N, Schwartz AV, Paolisso G, Harris T; Health ABC study. Pulse wave velocity is associated with muscle mass decline: Health ABC study. Age (Dordr) 2012; 34:469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sampaio RA, Sewo Sampaio PY, Yamada M, Yukutake T, Uchida MC, Tsuboyama T, Arai H. Arterial stiffness is associated with low skeletal muscle mass in Japanese community-dwelling older adults. Geriatr Gerontol Int 2014; 14(Suppl 1):109–114. [DOI] [PubMed] [Google Scholar]
- 10. Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH. Correlates of aortic stiffness in elderly individuals: a subgroup of the Cardiovascular Health Study. Am J Hypertens 2002; 15:16–23. [DOI] [PubMed] [Google Scholar]
- 11. Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Piña IL, Stein RA, Williams M, Bazzarre T. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: an advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation 2000; 101:828–833. [DOI] [PubMed] [Google Scholar]
- 12. Queiroz AC, Kanegusuku H, Forjaz CL. Effects of resistance training on blood pressure in the elderly. Arq Bras Cardiol 2010; 95:135–140. [DOI] [PubMed] [Google Scholar]
- 13. Ryan AS, Hurlbut DE, Lott ME, Ivey FM, Fleg J, Hurley BF, Goldberg AP. Insulin action after resistive training in insulin resistant older men and women. J Am Geriatr Soc 2001; 49:247–253. [DOI] [PubMed] [Google Scholar]
- 14. Collier SR, Kanaley JA, Carhart R, Jr, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J Hum Hypertens 2008; 22:678–686. [DOI] [PubMed] [Google Scholar]
- 15. Okamoto T, Masuhara M, Ikuta K. Effects of eccentric and concentric resistance training on arterial stiffness. J Hum Hypertens 2006; 20:348–354. [DOI] [PubMed] [Google Scholar]
- 16. Okamoto T, Masuhara M, Ikuta K. Upper but not lower limb resistance training increases arterial stiffness in humans. Eur J Appl Physiol 2009; 107:127–134. [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Hanssen H, Cordes M, Rossmeissl A, Endes S, Schmidt-Trucksäss A. Aerobic, resistance and combined exercise training on arterial stiffness in normotensive and hypertensive adults: a review. Eur J Sport Sci 2015; 15:443–457. [DOI] [PubMed] [Google Scholar]
- 18. Miyachi M. Effects of resistance training on arterial stiffness: a meta-analysis. Br J Sports Med 2013; 47:393–396. [DOI] [PubMed] [Google Scholar]
- 19. Ashor AW, Lara J, Siervo M, Celis-Morales C, Mathers JC. Effects of exercise modalities on arterial stiffness and wave reflection: a systematic review and meta-analysis of randomized controlled trials. PLoS One 2014; 9:e110034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cortez-Cooper MY, Anton MM, Devan AE, Neidre DB, Cook JN, Tanaka H. The effects of strength training on central arterial compliance in middle-aged and older adults. Eur J Cardiovasc Prev Rehabil 2008; 15:149–155. [DOI] [PubMed] [Google Scholar]
- 21. Figueroa A, Vicil F, Sanchez-Gonzalez MA, Wong A, Ormsbee MJ, Hooshmand S, Daggy B. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am J Hypertens 2013; 26:416–423. [DOI] [PubMed] [Google Scholar]
- 22. Maeda S, Otsuki T, Iemitsu M, Kamioka M, Sugawara J, Kuno S, Ajisaka R, Tanaka H. Effects of leg resistance training on arterial function in older men. Br J Sports Med 2006; 40:867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heffernan KS, Yoon ES, Sharman JE, Davies JE, Shih YT, Chen CH, Fernhall B, Jae SY. Resistance exercise training reduces arterial reservoir pressure in older adults with prehypertension and hypertension. Hypertens Res 2013; 36:422–427. [DOI] [PubMed] [Google Scholar]
- 24. Ho SS, Radavelli-Bagatini S, Dhaliwal SS, Hills AP, Pal S. Resistance, aerobic, and combination training on vascular function in overweight and obese adults. J Clin Hypertens (Greenwich) 2012; 14:848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nelson ME, Rejeski WJ, Blair SN, Duncan PW, Judge JO, King AC, Macera CA, Castaneda-Sceppa C; American College of Sports Medicine; American Heart Association. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116:1094–1105. [DOI] [PubMed] [Google Scholar]
- 26. Simons R, Andel R. The effects of resistance training and walking on functional fitness in advanced old age. J Aging Health 2006; 18:91–105. [DOI] [PubMed] [Google Scholar]
- 27. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr 2015; 101:991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, Koji Y, Hori S, Yamamoto Y. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002; 25:359–364. [DOI] [PubMed] [Google Scholar]
- 29. Cohn JN, Finkelstein S, McVeigh G, Morgan D, LeMay L, Robinson J, Mock J. Noninvasive pulse wave analysis for the early detection of vascular disease. Hypertension 1995; 26:503–508. [DOI] [PubMed] [Google Scholar]
- 30. McVeigh GE, Bratteli CW, Morgan DJ, Alinder CM, Glasser SP, Finkelstein SM, Cohn JN. Age-related abnormalities in arterial compliance identified by pressure pulse contour analysis: aging and arterial compliance. Hypertension 1999; 33:1392–1398. [DOI] [PubMed] [Google Scholar]
- 31. Hughes TM, Althouse AD, Niemczyk NA, Hawkins MS, Kuipers AL, Sutton-Tyrrell K. Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol 2012; 11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goldberg Y, Boaz M, Matas Z, Goldberg I, Shargorodsky M. Weight loss induced by nutritional and exercise intervention decreases arterial stiffness in obese subjects. Clin Nutr 2009; 28:21–25. [DOI] [PubMed] [Google Scholar]
- 33. Miyaki A, Maeda S, Yoshizawa M, Misono M, Saito Y, Sasai H, Endo T, Nakata Y, Tanaka K, Ajisaka R. Effect of weight reduction with dietary intervention on arterial distensibility and endothelial function in obese men. Angiology 2009; 60:351–357. [DOI] [PubMed] [Google Scholar]
- 34. Rossow LM, Fahs CA, Thiebaud RS, Loenneke JP, Kim D, Mouser JG, Shore EA, Beck TW, Bemben DA, Bemben MG. Arterial stiffness and blood flow adaptations following eight weeks of resistance exercise training in young and older women. Exp Gerontol 2014; 53:48–56. [DOI] [PubMed] [Google Scholar]
- 35. Anton MM, Cortez-Cooper MY, DeVan AE, Neidre DB, Cook JN, Tanaka H. Resistance training increases basal limb blood flow and vascular conductance in aging humans. J Appl Physiol (1985) 2006; 101:1351–1355. [DOI] [PubMed] [Google Scholar]
- 36. Zimlichman R, Shargorodsky M, Boaz M, Duprez D, Rahn KH, Rizzoni D, Payeras AC, Hamm C, McVeigh G. Determination of arterial compliance using blood pressure waveform analysis with the CR-2000 system: reliability, repeatability, and establishment of normal values for healthy European population–the seven European sites study (SESS). Am J Hypertens 2005; 18:65–71. [DOI] [PubMed] [Google Scholar]
- 37. Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens 2002; 15:445–452. [DOI] [PubMed] [Google Scholar]